Abstract
This paper presents results from the first application of molecular-genetic analysis combined with conventional light microscopy (LM) for identification of cylindrospermopsin (CYN) producers in field phytoplankton samples from Bulgaria. In total 68 cyanoprokaryotes were found by LM, out of which 18 were considered potential CYN-producers according to the literature. They occurred in different abundance (0-40%) in five waterbodies. The presence of CYN-producers was sought by application of the primer pair cynsulfF/cylnamR specific for the sulfotransferase gene (cyrJ) considered the best genetic marker for detection of CYN-toxigenic strains. Although CYN was detected by enzyme-linked immunosorbent assay (ELISA) in two waterbodies (lake Vaya and reservoir Mandra), we did not find cyanoprokaryotes with cyrJ gene. Therefore, Raphidiopsis raciborskii, despite being identified by LM in both waterbodies where CYN occurred, was not the producer of this toxin and belonged to the European nontoxic population of the species. The same was true for the less widely spread Raphidiopsis mediterranea and Chrysosporum bergii (both found in small amounts only in lake Vaya). Our results on CYN occurrence without the assignment of its producer in the collected samples suggest that: 1) CYN remains stable in water after disappearance of the toxigenic species, or, more probably, 2) besides the already known species, there are others that are associated with the production of CYN. Because revealing of novel CYN producers is of ultimate importance for health risk assessment and water management, we propose more analyses to be performed in future studies.
Introduction
Cyanoprokaryota/Cyanobacteria is a group of microscopic photoautotrophs widely distributed in different habitats. However, mainly in aquatic environments they have become of great concern for both human and ecosystem health, and for drinking water management in particular because of their ability to form dense blooms, which decrease the water quality, and to produce different toxins. These toxins, named cyanotoxins, are commonly classified as hepatotoxins (microcystins, nodularins), neurotoxins (anatoxins, saxitoxins), cytotoxins (cylindrospermopsin) and dermotoxins [Citation1, Citation2]. In the past two decades, the knowledge on cyanotoxins has advanced significantly, with increasingly documented data on their findings and threats as enhanced by the climate changes with global warming, rapidly growing human population and eutrophication [Citation3, Citation4]. So far, only the specific group of microcystins (MCs) can be outlined as routinely analyzed in the monitoring of recreational and drinking waters [Citation5]. However, another toxin, cylindrospermopsin (CYN), is gaining strong attention currently. The growing interest in CYN is explainable by its high liberation to surrounding water, which poses more hazards to water users and is important for water management [Citation6]. Moreover, CYN is an alkaloid with cyto-, hepato-, neuro- and genotoxic properties and potential carcinogenic effects [Citation7–10], which has also been linked to death of domestic animals [Citation11]. Besides the liver, CYN can affect other organs, such as the eyes, kidneys, lung, heart, thymus, spleen, adrenal glands and the intestine [Citation12–19]. Probably, because of its genotoxic and mutagenic properties, CYN is even more hazardous to human and animal health than MCs [Citation18, Citation20]. The toxicity of CYN has been associated mainly with protein synthesis inhibition and oxidative stress [Citation10] but other mechanisms could be involved, including indirect effects after some metabolic modifications. Human exposure to CYN may occur during recreational activities (by dermal contact during bathing) and by consumption of contaminated drinking water or food (vegetables, fish, crustaceans, etc.) (for details see [Citation10]). Therefore, a provisional Tolerable Daily Intake (TDI) of 0.03 µg kg−1 body weight (b.w.) and a guideline value of 1 µg L−1 in drinking water were proposed for CYN [Citation21]. At present, five analogues of CYN are known [Citation22].
In Bulgaria, there have been records of the occurrence of potentially toxic cyanoprokaryotes and their blooms in inland and coastal wetlands since the beginning of the twentieth century (for details see [Citation23, Citation24]), but it was in 2006 when the presence of cyanotoxins (mainly MCs) was proved [Citation25]. Much later, in 2018, CYN was detected for the first time in Bulgaria in two large, but shallow waterbodies situated at the Black Sea coastline: reservoir Mandra and lake Vaya [Citation26]. The purposive analysis for this cyanotoxin was based on the knowledge of the increased spread in the country of the first species reported as a CYN-producer [Citation7]: Raphidiopsis raciborskii (Woloszynska) Aguilera, Berrendero Gómez, Kastovsky, Echenique & Salerno (Syn. Cylindrospermopsis raciborskii (Woloszynska) Seenayya & Subba Raju) [Citation23, Citation24, Citation27–36]. It is broadly accepted that this tropical species benefited from climate changes and became globally invasive with actual multi-continental spread [Citation37–42], which strongly suggested its current classification as a cosmopolitan [Citation42]. Therefore, R. raciborskii has been long considered as a major problem for water management on a global scale [Citation40].
In the context of climate changes with global warming, more studies of the distribution and toxicity of both native and invasive species became necessary worldwide [Citation43] and by years more cyanoprokaryotes capable of CYN-production were identified: Anabaena lapponica Borge, Aphanizomenon flos-aquae Ralfs ex Bornet & Flahault, Aphanizomenon gracile Lemmermann, Chrysosporum bergii (Ostenfeld) E. Zapomelová, O. Skácelová, P. Pumann, R. Kopp & E. Janecek (Syn. Anabaena bergii Ostenfeld), Chrysosporum ovalisporum (Forti) E. Zapomelová, O. Skácelová, P. Pumann, R. Kopp & E. Janecek (Syn. Aphanizomenon ovalisporum Forti), Dolichospermum mendotae (W. Trelease) Wacklin, L. Hoffmann & Komárek, Dolichospermum planctonicum (Brunnthaler) Wacklin, L. Hoffmann & Komárek (Syn. Anabaena planctonica Brunnthaler), Microseira wollei (Farlow ex Gomont) G. B. McGregor & Sendall ex Kenins (Syn. Lyngbya wollei (Farlow ex Gomont) Speziale & Dyck), Oscillatoria sp. PCC6506, Raphidiopsis curvata F. E. Fritsch & M. F. Rich, Raphidiopsis mediterranea Skuja, and Umezakia natans M. Watanabe (for details see [Citation5, Citation22, Citation44–46]). A proposal to focus on Aphanizomenon klebahnii Elenkin ex Pechar as a putative CYN producer was also published [Citation47]. Besides the above-mentioned species, a few other potential CYN-producers have remained identified only at the genus level, e.g. Aphanizomenon sp., Anabaena sp. [Citation48, Citation49]. Similarly to R. raciborskii, some of these potential CYN-producers were broadly distributed in the phytoplankton of more than 100 Bulgarian waterbodies during the period 2000-2015: Aphanizomenon flos-aquae, Aphanizomenon gracile, Aphanizomenon klebahni, and R. mediterrannea [Citation23]. However, up to now, there has been no molecular-genetic investigation to prove the production of CYN by any of these species. Such a study is necessary because most of the known CYN-producing species have both toxic and nontoxic strains [Citation22]. There are a growing number of reports of CYN occurrence worldwide without assignment of the causative agent. Thus, it is possible to suggest that, besides the species already known, there are others that are associated with CYN production [Citation9, Citation48]. The suggestion that the list of potential CYN-releasing cyanoprokaryotes probably still remains incomplete also comes from the accumulating evidence that the production of CYN is not species specific [Citation22].
The importance of revealing all species responsible for CYN production is strongly related to the risk management, especially considering the fact that the blooms of R. raciborskii generally appeared to be non-toxic in Europe [Citation40, Citation50–52]. It was even assumed that CYN-producing strains of this species do not appear in water bodies of temperate climate [Citation53]. Data on species phylogeography, spreading routes and toxicity are quite challenging [Citation42] because R. raciborskii can produce at least two types of potent toxins, CYN or saxitoxins (STX), with biogeographical differences between strains [Citation54]. There is a general indication that American strains are able to produce STX but not CYN, Australian strains produce CYN but not STX, only some Asian strains are CYN-positive, whereas African strains are non-toxic (for details see [Citation54]). In Europe, SXT production by R. raciborskii was also reported [Citation55]. In addition, a possibility for this species to produce a third type of toxin, MC, could be supposed because its MCs-positive strain was isolated from Greece [Citation56]. Although abundant data have accumulated, in some way the occurrence of the toxic strains of R. raciborskii has remained underestimated [Citation42]. It is believed that at present state-of-art studies of this species related to CYN production, especially in Europe, are essential [Citation57]. Therefore, in this study, a purposive molecular-genetic analysis based on the specific cynsulfF/cylnamR primer pair (with cyrJ gene as a genetic marker for detection of CYN-producing strains [Citation22, Citation58]) was conducted on the phytoplankton material from nine shallow Bulgarian waterbodies considered as threatened by harmful algal blooms (HABs) [Citation23, Citation25, Citation59].
Materials and methods
Sites and sampling
The study was carried-out in June 2018 in nine shallow waterbodies situated in Central and Eastern Bulgaria (, ). Detailed descriptions on the morphometry, historical development, usage, conservational status and biodiversity of each of the waterbodies are provided in the Inventory of Bulgarian wetlands and their biodiversity [Citation60]. Therefore, in the unique inventory number of each waterbody from this database (IBWXXXX) is provided.
Figure 1. Map of Bulgaria showing the sampling sites (modified after http://www.ginkgomaps.com and Google Maps, accessed 6 November 2019).
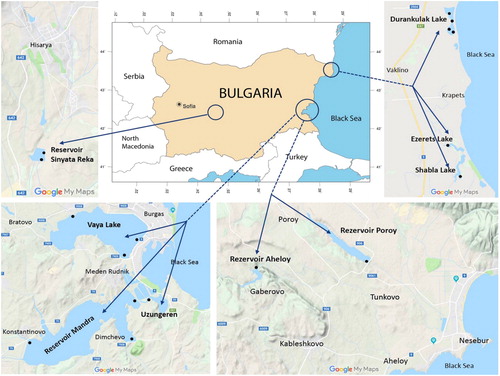
Table 1. Sampling sites and their environmental parameters with types of cyanotoxins found in Bulgarian waterbodies (WBs) (after [Citation26,Citation59]): WBN – name of the water body; IBW – number in the Inventory of Bulgarian Wetlands [Citation60]; SAN – site abbreviation and number; Alt – altitude; WT – water temperature (° C), SD – Secchi depth (m); TTB – total transparent to bottom; CND – conductivity (µS); TDS – total dissolved solids (µg L-1); DO – oxygen concentration (mg L-1); TP – total phosphorus (mg L-1); TN – total nitrogen (mg L-1); CT - cyanotoxins, MCs – microcystins, CYN – cylindrospermopsin, SXT – STXitoxins.
The sampling was preceded by sending a drone (DJI Mavic Pro, Model: M1P GL200A) supplied with a photo camera to observe and document the whole waterbody and potential hot spots with visible differences in the color as indicators of cyanoblooms. The spots/areas of different color were chosen for sampling or, in case of visible water homogeneity, the sites from our previous studies were repeated for each waterbody (for details see [Citation26]). All the chosen 17 sites were reached by inflatable boats. The site coordinates, altitude, water temperature, pH, water hardness (TDS), oxygen content (DO) and conductivity were measured in situ by Aquameter AM-200 and Aquaprobe AP-2000 from Aquaread water monitoring instruments, 2012 Aquaread Ltd. Total nitrogen (TN) and phosphorus (TP) were measured ex situ using Aqualytic AL410 Photometer from AQUALYTIC®, Dortmund, Germany. The water transparency was measured using Secchi disk. All results, together with detailed data on cyanotoxins found were published [Citation26, Citation59].
Phytoplankton samples for taxonomic identification and for molecular-genetic studies (each in a volume of 0.5 L) were collected from the water surface (0–20 cm). The samples for light microscopy processing were fixed immediately with 2% formalin and thus transported to the lab, where they were further processed by sedimentation method [Citation26, Citation59]. The samples for polymerase chain reaction (PCR) studies were filtered within several hours after collection, and the obtained filters, kept in sterile plastic tubes, were transported to the lab in a box with dry ice [Citation26, Citation59].
Phytoplankton identification by conventional light microscopy (LM)
In the lab, the microscopic work was done mainly under magnification 100x and immersion on 52 non-permanent slides on a Motic BA 4000 microscope with a Moticam 2000 camera, and later on 22 non-permanent slides on a Motic B1 microscope with a Moticam 2.0 mp camera. Both cameras were supplied by Motic Images 2 and 3 Plus software program, respectively [Citation59].
Taxonomic identification of cyanoprokaryotes followed the standard European taxonomic literature [Citation61–65] with updates from AlgaeBase [Citation66], CyanoDB [Citation67] and relevant modern taxonomic papers. Traditional morphological features for distinguishing nostocalean taxa (to which most CYN-producers belong) include the cell form and size, heterocyte shape and size, akinete shape and size in combination with their position in the trichomes in relation to heterocytes, and facultative or obligatory occurrence of aerotopes [Citation61–65].
Quantitative contribution of the different species to the phytoplankton biomass was estimated using the Thoma-counting chamber and the method of the stereometrical approximations [Citation23, Citation68].
Molecular studies
The molecular study was conducted in order to detect CYN-producing strains or species based on the presence of the sulfotransferase gene (cylJ) using a primer set specific for this gene, cynsulfF/cylnamR [Citation58]. The targeted gene is a part of the gene cluster, 43 kb in size, which encodes amidinotransferase, peptide synthetase (PS), polyketide synthase (PKS) and tailoring enzymes involved in CYN production [Citation69]. The cluster has been described in R. raciborskii [Citation58], R. curvata [Citation70], Aphanizomenon sp. [Citation49] and Oscillatoria sp. [Citation44]. The primer set used in this study is specific for the strains of R. raciborskii, R. curvata, R. mediterranea and Microseira wollei. The expected length of amplified fragments is 585 bp [Citation58].
Water samples were collected from the nine water bodies described above and a few hours afterwards were filtered through 45 µm cellulose filters Whatman NC45 ST/Sterile EO. The filters were kept under -20 °C and used for total DNA extraction. The protocol of Genomic DNA Purification Mini Kit (Sigma) was followed. DNA was amplified using primer combination cynsulfF (5’ACTTCTCTCCTTTCCCTATC3’) x cylnamR (5′ GAGTGAAAATGCGTAGAACTTG3’) according to [Citation58]. The PCR amplification was performed in a 25 µL volume containing 10 pmol primers; 0.16 mmol L−1deoxynucleoside triphosphates dNTPs; 1.25 units of Taq polymerase, 0.75 mmol L−1 MgCI2 and 10xPCR buffer supplied by FastStart High Fidelity PCR System (Roche). The amplification of DNA was done in a thermal cycler QB-96 (Qianta Biotech) under the following PCR conditions: denaturation at 95 °C for 5 min, 35 cycles of denaturation (30 s at 95 °C), annealing at 55 °C for 30 s, extension at 72 °C for 40 s, and a final extension at 72 °C for 5 min. The resulting PCR products around 500 bp in size were purified using Sigma Gel GenElute Gel Extraction Kit (Sigma), following the manufacturer’s instructions. The amplification products obtained were subsequently cloned using a CloneJET PCR Cloning Kit (Thermo Scientific). Recombinant plasmids were isolated using Sigma Plasmid Miniprep Kit. For each sampling site six clones were selected and sequenced by Macrogen Inc. The obtained sequences were processed with Vector NTI 11.5 software and used for search by Basic Local Alignment Search Tool (BLAST) [Citation71] in the National Centre for Biotechnology Information (NCBI) data base [Citation72].
Results and discussion
Phytoplankton species composition, obtained by light microscopy (LM)
In total, more than 240 species from different taxonomic groups were identified using LM in the phytoplankton of all studied waterbodies. The reliably determined cyanoprokaryotes were 68, or 28% of all taxa. Considering CYN-producers as target of the study and the results from the PCR analysis provided below, here we report only the presence of genera and species known as toxigenic for CYN and their close relatives in the studied waterbodies.
Using traditional morphological diagnostic features [Citation61–65], we identified 18 taxa which could be possibly associated with CYN-production (). Among them, there were three species from the genera Raphidiopsis (2) and Chrysosporum (1) well-known for their ability to produce CYN. We found also one representative of the CYN-producing genus Raphidiopsis (unidentified to species level because of the lack of heterocytes and akinetes) and a representative of the genus Oscillatoria unidentified to the species level, from which a benthic strain PPC 6506 positive for CYN production was reported [Citation73]. The other fourteen taxa have not been listed among CYN-producers, but were taken into consideration and included in because of their close relations or belonging to the genera Anabaena/Dolichospermum and Aphanizomenon/Chrysosporum, from which CYN-positive strains were found, as already described in the Introduction.
Table 2. Distribution of potential CYN-producing taxa in the studied Bulgarian waterbodies: VA – lake Vaya, MN - reservoir Mandra, PR – reservoir Poroy, DK – lake Durankulak, UZ – lake Uzungeren, SR – reservoir Sinyata Reka; d – dominance (>25% of the biomass); a – abundance (5–25% of the biomass); x – occurrence (<5% of the biomass); r - rare occurrence in single trichomes. Bright grey lines indicate the species which have been reported as CYN producers (for details see the text). Taxa are enlisted in alphabetical order.
The differences in the distribution of potential CYN-producers by waterbodies are shown in . CYN-producers had different quantitative role in the total phytoplankton biomass: ca. 40% in lake Vaya and reservoir Poroy, ca. 20% in lake Durankulak and reservoir Mandra, 4% in lake Uzungeren, <1% in reservoir Sinyata Reka, and were not found in the lakes Shabla and Ezerets, and in reservoir Aheloy as well.
Results from PCR analysis for cylindrospermopsin-producing strains
The sulfotransferase (cyrJ)-gene-specific primer pair was used to identify CYN-producing genotypes in the phytoplankton samples from 17 studied sites of nine Bulgarian waterbodies. As a result, PCR fragments ca. 500 bp in length were obtained from the lakes Durankulak (sites 1 and 2) and Vaya (sites 1 and 2), and from the reservoirs Mandra (site 2) and Poroy. Аlthough the amplified fragments were shorter than the expected length of 585 bp, all PCR products were extracted from the gel and cloned. From each site six individual clones were sequenced. The results obtained showed no homology with the annotated sequences from CYN-producing cyanobacteria available in the NCBI [Citation72].
Discussion on the results obtained by light microscophy and genetic-molecular methods
The sulfotransferase gene (cyrJ) required for tailoring the reaction to complete the biosynthesis of the CYN [Citation58] was applied to assess the toxigenic potential of 17 water samples collected in nine shallow Bulgarian lakes and reservoirs. In four of them (Vaya, Mandra, Durankulak and Uzungeren) three well-known potential CYN producers were identified by LM: Raphidiopsis raciborskii, R. mediterranea and Chrysosporum bergii (), and in two of them (Vaya and Mandra) the presence of CYN at the lowest detection limit (0.4 µg L−1) was discovered [Citation26]. However, in all investigated samples, no amplification of the sulfotransferase gene was found.
On the background of the results obtained by LM, this absence of the cyrJ gene, which is considered a good marker for CYN toxicity [Citation22, Citation45, Citation58, Citation74, Citation75], clearly shows that the first species shown to be a CYN-producer, Raphidiopsis raciborskii [Citation7], is practically incapable of CYN production in the studied Bulgarian waterbodies. This suggestion is additionally supported by the different distribution of this species in both waterbodies, Mandra and Vaya, where CYN was detected ([Citation26], ): it was never found in reservoir Mandra ([Citation34], ) but since 2000 it has been developing periodically forming water blooms in lake Vaya [Citation31, Citation33–35]. During this study it was found to occur there but not as a dominant or abundant species (). Moreover, R. raciborskii was more abundant in two other waterbodies, reservoir Poroy and lake Uzungeren (), where CYN was not detected ([Citation26], and ). The absence of cyrJ and hence of the ability to produce CYN is in accordance with the earlier opinion of Stoyneva [Citation34] that R. raciborskii appeared in Bulgaria from the European populations along the Danube and is in accordance with the generally accepted idea for geographically distinct strains of this species spread in the world.
During the studied period Chrysosporum ovalisporum was not identified in the samples either by LM, or by molecular-genetic methods. This species is regarded as a major source of CYN not only in Australia, the United States (Florida) and Israel, but also in Mediterranean Europe, where it can be considered the main CYN producer to date, which has a great spreading potential (for details see [Citation9]). We note especially the absence of this well-known CYN-producer in our samples because it once more confirms the need to identify the real CYN causative agents in the studied Bulgarian waterbodies. With the same consideration we have to point out also the absence of all other known CYN producers [Citation5, Citation22, Citation44–46]: Aphanizomenon flos-aquae, Aphanizomenon gracile, Anabaena lapponica, Dolichospermum mendotae, Dolichospermum planctonicum, Microseira wollei, Oscillatoria sp. PCC6506, Raphidiopsis curvata and Umezakia natans.
Besides the powerful cyr genes [Citation53, Citation58] the activity of other genera and species in the production of CYN was previously observed as based only on identification of aoaA gene encoding an amidinotransferase [Citation76], or cyrA/aoaA gene [Citation49], on ps gene [Citation48] or on pks/ps genes putatively associated with the CYN production [Citation58, Citation77–79]. Therefore, it is possible to suppose that the detected CYN production [Citation26] sought in the present study only by cyrJ, was caused by strains and species bearing other genes, so further PCR investigations have to be performed to reveal the real CYN producers.
At present, by the primer applied, we cannot assign to any species or strain the CYN recorded in low amounts in the coastal waterbodies Vaya and Mandra [Citation26]. Here, we have to mention also that still relatively little work has been done on CYN-detection methods [Citation22]. CYN in the studied waterbodies was discovered by ELISA [Citation26], which was confirmed as a good screening method for CYN with a sensitive level of detection [Citation47]. However, there are some opinions that ELISA is nonselective for CYN analogues and that cross-reactivity may exist [Citation45, Citation47, Citation77].
The discrepancy which at first glimpse occurs between the molecular-genetic results, which showed no presence of known CYN-producers, and our ELISA data, which indicated the presence of CYN in Mandra and Vaya [Citation26], needs more discussion. One possible explanation could be the effect of environmental conditions on CYN production. This effect has been only partly resolved, and optimal conditions for toxin production may not be optimal for the growth of the CYN producer [Citation22]. Although laboratory experiments have shown effects of temperature, nitrogen source, phosphate availability and light intensity on CYN production [Citation80–85], it was supposed that the influence of environmental conditions on CYN production may vary among different producing species and strains, especially those isolated from different geographical regions [Citation22, Citation86, Citation87]. Therefore, considering also the strong extracellular release of CYN (which can reach up to 90% of the total toxin) [Citation22], it is possible to suppose that the strains responsible for CYN production were developed in very low amounts during the sampling period. Taking into account the stability of CYN in waters [Citation22, Citation88, Citation89], we cannot completely exclude the possibility that the low amounts of CYN found by us in Mandra and Vaya [Citation26] represented remnants from former blooms.
Overall, our results are in accordance with other studies where the source of CYN remained uncertain due to still scarce knowledge of organisms able to produce it [Citation47, Citation57, Citation90–92]. CYN occurrence cannot always be clearly linked with the presence of its putative producing species in the waterbodies (e.g. [Citation77, Citation91–95]). For example, despite several indications of R. raciborskii occurrence in European lakes, its strains had a minor contribution to the CYN production [Citation47] or were not found to be involved in it [Citation92, Citation96, Citation97], with the exception of one strain from Hungary [Citation98]. Similar results were obtained for Aphanizomenon flos-aquae: despite being the only reliably identified CYN producer in German waters [Citation48, Citation94], its presence could not always explain the occurrence of the toxin [Citation94]. Therefore, an explanation was sought in the co-occurrence of CYN-producing and non-producing strains of the same taxon or in the presence of additional, so far unidentified CYN-producing species, most likely of the genera Aphanizomenon/Chrysosporum or Anabaena/Dolichospermum [Citation45, Citation94, Citation99]. Here we would like to underline also the occurrence of CYN in relation with Microcystis and Microcystis-dominated blooms in Greek freshwaters [Citation55, Citation100]. Considering these data, we have to note that seven Microcystis species were detected by our team in the studied waterbodies during the same investigation period and in the same samples [Citation59]. Therefore, in the proved absence of cyrJ-bearing cyanobacteria but detected CYN [Citation26], we should not exclude the possibility to find some link between presence of Microcystis and CYN-production in the coastal waterbodies Vaya and Mandra.
Conclusions
The results obtained from application of LM combined with molecular-genetic studies allowed us to determine that the most commonly suspected species, the invasive Raphidiopsis raciborskii, was not the CYN-producer in the studied shallow Bulgarian waterbodies. This shows its general affiliation to the European non-toxic gene pool of this species, confirming also the wide-spread opinion that CYN synthesis appears to be linked to biogeographic patterns. The lack of positive signal for the cyrJ gene demonstrated also the nontoxic character of the other potential CYN-producing species found during the study, Chrysosporum bergii, Raphidiopsis mediterranea and Raphidiopsis sp. By application of cyrJ (based on the single cynsulfF/cylnamR primer set) we could not identify any other species responsible for the presence of CYN in two of the waterbodies (Vaya and Mandra). The occurrence of fourteen other species closely related to the known CYN-producers and identified by LM shows that further more complex research based on genetic analyses and cyanobacterial cultures should be performed in order to reveal the CYN-producers in Bulgarian waterbodies.
Author contributions
Conceptualization and writing - MSG, GG and BU; sampling – BU, GG, MSG, MR; formal analysis and investigation of molecular genetic data - KS and MR; investigation by light microscopy - MSG, BU, and GG; funding acquisition – BU, MR, GG; project administration - BU.
Acknowledgments
The authors would like to acknowledge SRF-MESB for project granting and the European Cooperation in Science and Technology, COST Action ES 1105 ‘CYANOCOST - Cyanobacterial blooms and toxins in water resources: Occurrence, impacts and management’ for adding value to this paper through networking and knowledge sharing with European experts and researchers in the field.
Disclosure statement
No conflict of interest was reported by the author(s).
Additional information
Funding
References
- Carmichael WW, Li RH. Cyanobacterial toxins in the Salton Sea. Saline Syst. 2006;2(1):5.
- Buratti FM, Manganelli M, Vichi S, et al. Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch Toxicol. 2017;91(3):1049–1130.
- Codd GA, Meriluoto J, Metcalf JS. Introduction: cyanobacteria, cyanotoxins, their human impact, and risk management. In: Meriluoto J, Spoof L, Codd J, editors. Handbook of cyanobacterial monitoring and cyanotoxin analysis. Chichester, UK: John Wiley & Sons, Ltd.; 2017. p. 3–8.
- Svirčev Z, Lalić D, Savić GB, et al. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch Toxicol. 2019;93:2429–2481.
- Brient L, Lengronne M, Bormans M, et al. First occurrence of cylindrospermopsin in freshwater in France. Environ Toxicol. 2009;24(4):415–420.
- Akcaalan R, Köker L, Oğuz A, et al. First report of cylindrospermopsin production by two cyanobacteria (Dolichospermum mendotae and Chrysosporum ovalisporum) in Lake Iznik. Turkey. Toxins. 2014;6(11):3173–3186.
- Ohtani I, Moore RE, Runnegar MTC. Cylindrospermopsin: a potenthepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J Am Chem Soc. 1992;114(20):7941–7942.
- Pearson L, Mihali T, Moffitt M, et al. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, STXitoxin and cylindrospermopsin. Mar Drugs. 2010;8(5):1650–1680.
- Moreira C, Azevedo J, Antunes A, et al. Cylindrospermopsin: occurrence, methods of detection and toxicology. J Appl Microbiol. 2013;114(3):605–620.
- Pichardo S, Came_An AM, Jos A. In vitro toxicological assessment of cylindrospermopsin: a review. Toxins. 2017;9:402e443.
- Saker M, Thomas A, Norton J. Cattle mortality attributed to the toxic cyanobacterium Cylindrospermopsis raciborskii in an outback region of north queensland. Environ Toxicol. 1999;14(1):179–182.
- Terao K, Ohmori S, Igarashi K, et al. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon. 1994;32(7):833–843.(94)90008-6.
- Hawkins PR, Chandrasena NR, Jones GJ, et al. Isolation and toxicity of Cylindrospermopsis raciborskii from an ornamental lake. Toxicon. 1997;35(3):341–346.(96)00185-7.
- Falconer IR, Hardy SJ, Humpage AR, et al. Hepatic and renal toxicity of the blue-green alga (cyanobacterium) Cylindrospermopsis raciborskii in male Swiss albino mice. Environ Toxicol. 1999;14(1):143–150.
- Falconer IR. An overview of problems caused by toxic blue-green algae (cyanobacteria) in drinking and recreational water. Environ Toxicol. 1999;14(1):5–12.
- Carmichael WW. Health effects of toxin-producing cyanobacteria: “The Cyano Habs. Hum Ecol Risk Assess. 2001;7(5):1393–1407.
- van Apeldoorn ME, van Egmond HP, Speijers GJA, et al. Toxins of cyanobacteria. Mol Nutr Food Res. 2007;51(1):7–60.
- Žegura B, Štraser A, Filipič M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins – a review. Mutat Res-Rev Mutat. 2011;727(1–2):16–41.
- Guzmán-Guillén R, Prieto AI, Moyano R, et al. Dietary L-carnitine prevents histopathological changes in tilapia (Oreochromis niloticus) exposed to cylindrospermopsin. Environ Toxicol. 2017;32(1):241–254.
- Puerto M, Prieto AI, Maisanaba S, et al. Mutagenic and genotoxic potential of pure Cylindrospermopsin by a battery of in vitro tests. Food Chem Toxicol. 2018; 121:413–e422.
- Humpage AR, Falconer IR. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male swiss albino mice: Determination of no observed adverse effect level for deriving a drinking water guideline value. Environ Toxicol. 2003;18(2):94–103.
- Kokociński M, Cameán AM, Carmeli S, et al. Handbook of cyanobacterial monitoring and cyanotoxin analysis. Meriluoto J, Spoof L, Codd J, Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017. pp. 127–137.
- Stoyneva-Gärtner MP, Descy J-P, Latli A, et al. Assessment of cyanoprokaryote blooms and of cyanotoxins in Bulgaria in a 15-years period (2000-2015). Adv Oceanogr Limnol. 2017;8:131–152.
- Descy J-P, Stoyneva-Gärtner MP, Uzunov BA, et al. Studies on cyanoprokaryotes of the water bodies along the Bulgarian Black Sea Coast (1890-2017): a review, with special reference to new, rare and harmful taxa. Acta Zool Bulgar. 2018;11:43–52.
- Pavlova V, Babica P, Todorova D, et al. Contamination of some reservoirs and lakes in Republic of Bulgaria by microcystins. Acta Hydrochim Hydrobiol. 2006;34(5):437–441.
- Stoyneva-Gärtner MP, Uzunov BA, Descy J-P, et al. Pilot application of drone-observations and pigment marker detection by HPLC in the studies of CyanoHABs in Bulgarian inland waters. Mar Freshwater Res. 2019. doi:10.1071/MF18383.
- Draganov St, Stoyneva M. Algal flora of the Danube river (Bulgarian sector) and adjoined water basins. III. Algae from some adjoined water basins. Ann Univ Sof. 1992;82:63–78.
- Stoyneva M. Algal flora of the Danube river (Bulgarian sector) and adjoined water basins. V. Algal flora of the water bodies adjacent to the Lake of Srebarna. Ann Univ Sof. 1995;88:5–19.
- Stoyneva, M. Algae. In: Michev TM, Georgiev BB, Petrova AV, Stoyneva MP, editors. Biodiversity of the Srebarna Biosphere Reserve. Checklist and bibliography. Sofia, Bulgaria: Co-publ. Context & Pensoft; 1998. pp. 10–37.
- Stoyneva MP. Development of the phytoplankton of the shallow Srebarna Lake (North-Eastern Bulgaria) across the trophic gradient. In: Alvarez-Cobelas M, Reynolds CS, Sanchez-Castillo P, Kristiansen J, editors. Phytoplankton and Trophic Gradients, Proceedings of the 10th Workshop of the International Association of Phytoplankton Taxonomy & Ecology. (IAP), Granada, Spain; 1996 Jun 21–29; Springer-Science + Business Media: Dordrecht, The Netherlands, Hydrobiologia; 1998. p. 259–267. DOI: 10.1007/978-94-017-2668-9.
- Stoyneva MP. Steady-state phytoplankton assemblages in shallow Bulgarian wetlands. In: Naselli-Flores L, Padisak J, Dokulil M, editors. Phytoplankton and Equilibrium Concept: The Ecology of Steady-State Assemblages, Proceedings of the 13th Workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP), Castelbuono, Italy, 1–8 September 2002; Springer-Science + Business Media: Dordrecht, The Netherlands, Hydrobiologia; 2003. p. 169–176. DOI: 10.1007/978-94-017-2666-5.
- Michev TM, Stoyneva MP. Editors’ afterword. In: Michev TM, Stoyneva MP, editors. Inventory of Bulgarian Wetlands and their Biodiversity. Part. 1: Non-Lotic Wetlands; Sofia, Bulgaria: Publ. House Elsi-M; 2007. p. 226–228.
- Dimitrova RE, Nenova EP, Uzunov BA, et al. Phytoplankton composition of Vaya Lake (2004–2006). Bulg J Agric Sci. 2014;20:165–172.
- Stoyneva M. Contribution to the studies of the biodiversity of hydro- and aerobiontic prokaryotic and eukaryotic algae in Bulgaria Dr. Sc. Thesis, Sofia University “St. Kliment Ohridski”, Bulgaria; 2014.
- Stoyneva MP. Allochtonous planctonic algae recorded in Bulgaria during the last 25 years and their possible dispersal agents. Hydrobiologia. 2016;764(1):53–64.
- Kokociński M, Akcaalan R, Salmaso N, Stoyneva-Gärtner MP, Sukenik A. Expansion of alien and invasive cyanobacteria. In: Meriluoto J, Spoof L, Codd J, editors. Handbook of cyanobacterial monitoring and cyanotoxin analysis. Chichester, UK: John Wiley & Sons, Ltd; 2017. pp. 28–40.
- Padisák J. Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju, an expanding, highly adaptative cyanobacterium: worldwide distribution and review of its ecology. Arch Hydrobiol. 1997; 107:563–593. http://real.mtak.hu/id/eprint/3229.
- Briand JF, Leboulanger C, Humbert JF, et al. Cylindrospermopsis raciborskii (Cyanobacteria) invasion at midlatitudes: selection, wide physiological tolerance, or global warming? J Phycol. 2004;40(2):231–238.
- Briand JF, Robillot C, Quiblier-Lloberas C, et al. Environmental context of Cylindrospermopsis raciborskii (Cyanobacteria) blooms in shallow pond in France. Water Res. 2002;36(13):3183–3192.
- Neilan B, Saker M, Fastner J, et al. Phylogeography of the invasive cyanobacterium Cylindrospermopsis raciborskii. Mol Ecol. 2002;12(1):133–140.
- Sinha R, Pearson LA, Davis TW, et al. Increased incidence of Cylindrospermopsis raciborskii in temperate zones - Is climate change responsible? Water Res. 2012;46(5):1408–1419.
- Antunes JT, LeãO PN, Vasconcelos VÃt. M. Cylindrospermopsis raciborskii: review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front Microbiol. 2015;6:473.
- Savadova K, Mazur-Marzec H, Karosiené J, et al. Effect of increased temperature on native and alien nuisance cyanobacteria from temperate lakes: An experimental approach. Toxins. 2018;10(11):445.
- Mazmouz R, Chapuis-Hugon F, Mann S, et al. Biosynthesis of cylindrospermopsin and 7-epicylindrospermopsin in Oscillatoria sp. strain PCC 6506: identification of the cyr gene cluster and toxin analysis. Appl Environ Microbiol. 2010;76(15):4943–4949.
- Kokociński M, Mankiewicz-Boczek J, Jurczak T, et al. Aphanizomenon gracile (Nostocales), a cylindrospermopsin-producing cyanobacterium in Polish lakes. Environ Sci Pollut Res. 2013;20(8):5243–5264.
- Cirés S, Wörmer L, Ballot A, et al. Phylogeography and paralytic shellfish toxin-producing nostocales cyanobacteria from Mediterranean Europe (Spain). Appl Environ Microbiol. 2014;80(4):1359–1370. DOI: 10.1128/AEM.03002-13
- Blahova L, Oravec M, Marsalek B, et al. The first occurence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods. Toxicon. 2009;53(5):519–524.
- Preußel K, Stüken A, Wiedner C, et al. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (Cyanobacteria) isolated from two German lakes. Toxicon. 2006;47(2):156–162.
- Stüken A, Jakobsen KS. The cylindrospermopsin gene cluster of Aphanizomenon sp. strain 10E6: organization and recombination. Microbiology. 2010;156(8):2438–2451. DOI: 10.1099/mic.0.036988-0.
- Haande S, Rohrlack T, Ballot A, et al. Genetic characterization of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) isolates from Africa and Europe. Harmful Algae. 2008;7(5):692–701.
- Antal O, Karisztl-Gácsi M, Farkas A, et al. Screening the toxic potential of Cylindrospermopsis raciborskii strains isolated from Lake Balaton. Hungary. Toxicon. 2011;57(6):831–840.
- Sukenik A, Hadas O, Kaplan A, et al. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes—physiological, regional, and global driving forces. Front Microbiol. 2012;3:86.
- Mankiewicz-Boczek J, Kokociński M, Gagała I, et al. Preliminary molecular identification of cylindrospermopsin producing Cyanobacteria in two Polish lakes (Central Europe). FEMS Microbiol Lett. 2012;326(2):173–179.
- Piccini C, Aubriot L, D’Alessandro B, et al. Revealing toxin signatures in cyanobacteria: report of genes involved in cylindrospermopsin synthesis from STXitoxin-producing Cylindrospermopsis raciborskii. AiM. 2013;3(3):289–296.
- Gkelis S, Zaoutsos N. Cyanotoxin occurrence and potentially toxin producing cyanobacteria in freshwaters of Greece: a multi-disciplinary approach. Toxicon. 2014;78:1–9.
- Panou M, Zervou S-K, Christophoridis C, et al. First record of microcystin-producing Cylindrospermopsis raciborskii strain isolated from Greece. In Proceedings of the 10th International Conference on Toxic Cyanobacteria, Wuhan, China, p. 23–28. October 2016.
- Kokociński M, Dziga D, Spoof L, et al. First report of the cyanobacterial toxin cylindrospermopsin in the shallow, eutrophic lakes of western Poland. Chemosphere. 2009;74(5):669–675.
- Mihali TK, Kellmann R, Muenchhoff J, et al. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. AEM. 2008;74(3):716–722. 01988–07.
- Radkova M, Stefanova K, Uzunov B, et al. Morphological and molecular identification of microcystin-producing cyanobacteria in nine shallow Bulgarian water bodies. Toxins. 2020;12(1):39.
- Michev T, Stoyneva M, Eds. Inventory of Bulgarian wetlands and their biodiversity; Elsi-M: Sofia, Bulgaria, 2007.
- Geitler L. Cyanophyceae. In: Rabenhorst L, editors. Kryptogamen-Flora von Deutschland, Österreich und der Schweiz. 2nd ed. Leipzig, Germany: Akademische Verlagsgesellschaft; 1931. Vol. 14. pp. 289–672.
- Geitler L. Schizophyta: Klasse Schizophyceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien, Sweite Auflage. Leipzig, Germany: Wilhelm Engelmann; 1942. Vol. 1b. pp. 1–232.
- Gollerbakh MM, Kossinskaya EK, Polyanskiy VI. Manual of freshwater algae of the USSR. Volume 2. Blue-green algae. Moscow, Russia: Sovetskaya Nauka; 1953.
- Starmach K. Cyanophyta-Sinice. Glaucophyta-Glaukofity. Warszawa, Poland: Państwowe Wydawnictwo Naukowe; 1966.
- Komárek J. Cyanoprokaryota. 3. Heterocytous genera. In: Büdel B, Gärtner G, Krienitz L, Schagerl M, editors. Süswasserflora von Mitteleuropa/Freshwater Flora of Central Europe. Berlin, Germany: Springer Spektrum; 2013. p. 1–1130.
- AlgaeBase. http://www.algaebase.org/. accessed on 18 September 2019.
- CyanoDB 2.0. http://www.cyanodb.cz. accessed on 19 September 2019.
- Rott E. Some results from phytoplankton counting intercalibration. Schweiz Z Hydrologie. 1981;43(1):34–62.
- Jiang Y, Xiao P, Yu G, et al. Sporadic distribution and distinctive variations of cylindrospermopsin genes in cyanobacterial strains and environmental samples from Chinese freshwater bodies. Appl Environ Microbiol. 2014;80(17):5219–5230.
- Jiang Y, Xiao P, Yu G, et al. Molecular basis and phylogenetic implications of deoxycylindrospermopsin biosynthesis in the cyanobacterium Raphidiopsis curvata. Appl Environ Microbiol. 2012;78(7):2256–2263. DOI: 10.1128/AEM.07321-11
- Basic Local Alignment Search Tool (BLAST). https://blast.ncbi.nlm.nih.gov/Blast.cgi. accessed 24 October 2019.
- National Centre for Biotechnology Information (NCBI). https://www.ncbi.nlm.nih.gov/. accessed 24 October 2019.
- Bormans M, Lengronne M, Brient L, et al. Cylindrospermopsin accumulation and release by the benthic cyanobacterium Oscillatoria sp. PCCC 6506 under different light conditions and growth phases. Bull Environ Contam Toxicol. 2014;92(2):243–247.
- Ballot A, Ramm G, Rundberget T, et al. Occurrence of non-cylindrospermopsin-producing Aphanizomenon ovalisporum and Anabaena bergii in Lake Kinneret (Israel). J Plankton Res. 2011;33(11):1736–1746.
- Lorenzi AC, Chia MA, Piccin-Santos V, et al. Microcystins and cylindrospermopsins molecular markers for the detection of toxic cyanobacteria: a case study of northeastern Brazilian reservoirs. Limnetica. 2015;34(2):269–282.
- Shalev-Alon G, Sukenik A, Livnah O, et al. A novel gene encoding amidinotransferase in the cylindrospermopsin producing cyanobacterium Aphanizomenon ovalisporum. FEMS Microbiol Lett. 2002;209(1):87–91.
- Yilmaz M, Phlips EJ, Szabo NJ, et al. A comparative study of Florida strains of Cylindrospermopsis and Aphanizomenon for cylindrospermopsin production. Toxicon. 2008;51(1):130–139.
- Schembri MA, Neilan BA, Saint CP. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ Toxicol. 2001;16(5):413–421.
- Fergusson KM, Saint CP. Multiplex PCR assay for Cylindrospermopsis raciborskii and cylindrospermopsin-producing cyanobacteria. Environ Toxicol. 2003;18(2):120–125. DOI: 10.1002/tox.10108.
- Burford M, Davis TW. Physical and chemical processes promoting dominance of the toxic cyanobacterium Cylindrospermopsis raciborskii. Chin J Ocean Limnol. 2011;29(4):883–891.
- Cirés S, Wörmer L, Timón J, et al. Cylindrospermopsin production and release by the potentially invasive cyanobacterium Aphanizomenon ovalisporum under temperature and light gradients. Harmful Algae. 2011;10(6):668–675.
- Neilan BA, Pearson LA, Muenchhoff J, et al. Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environ Microbiol. 2013;15(5):1239–1253.
- Saker ML, Neilan BA. Variable diazotrophies, morphologies and toxicities of genetically similar isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from Northern Australia. Appl Environ Microbiol. 2001;67(4):1839–1845.
- Dyble J, Tester PA, Litaker RW. Effects of light on cylindrospermopsin production in the cyanobacterial HAB species Cylindrospermopsis raciborskii. Afr J Mar Sci. 2006;28(2):309–312.
- Bã¡Csi Ián, Vasas Gáb, SuráNyi G, et al. Alteration of cylindrospermopsin production in sulfate‐ or phosphate-starved cyanobacterium Aphanizomenon ovalisporum. FEMS Microbiol Lett. 2006;259(2):303–310.
- Kokociński M, Soininen J. Environmental factors related to the occurrence of Cylindrospermopsis raciborskii (Nostocales, Cyanophyta) at the north-eastern limit of its geographical range. Eur J Phycol. 2012;47(1):12–21.
- Mantzouki E, Lürling M, Fastner J, et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins. 2018;10(4):156. Toxins pii: E156.
- Chiswell RK, Shaw GR, Eaglesham G, Smith MJ, Norris RL, Seawright AA, Moore MR. Stability of cylindrospermopsin, the toxin from the cyanobacterium, Cylindrospermopsis raciborskii: Effect of pH, temperature, and sunlight on decomposition. Environ Toxicol. 1999;14:155–161. 1999.
- Adamski M, Żmudzki P, Chrapusta E, et al. Effect of pH and temperature on the stability of cylindrospermopsin. Characterization of decomposition products. Algal Res. 2016;15:129–134.
- Stirling DJ, Quilliam MA. First report of the cyanobacterial toxin cylindrospermopsin in New Zealand. Toxicon. 2001;39(8):1219–1222.(00)00266-X.
- Spoof L, Berg KA, Rapala J, et al. First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environ Toxicol. 2006;21(6):552–560.
- Fastner J, Heinze R, Humpage AR, et al. Chorus, I. Cylindrospermopsin occurrence in two German lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii (cyanobacteria) isolates. Toxicon. 2003;42(3):313–321.
- Carmichael WW, Azevedo SM, An JS, et al. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ Health Perspect. 2001;109(7):663–668.
- Rücker J, Stüken A, Nixdorf B, et al. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon. 2007;50(6):800–809.
- Stüken A, Campbell RJ, Quesada A, et al. Genetic and morphologic characterization of four putative cylindrospermopsin producing species of the cyanobacterial genera Anabaena and Aphanizomenon. J Plankton Res. 2009;31(5):465–480.
- Bernard C, Harvey M, Briand JF, et al. Toxicological comparison of diverse Cylindrospermopsis raciborskii strains: evidence of liver damage caused by a French C. raciborskii strain. Environ Toxicol. 2003;18(3):176–186.
- Saker ML, Nogueira ICG, Vasconcelos VM, et al. First report and toxicological assessment of the cyanobacterium Cylindrospermopsis raciborskii from Portuguese freshwaters. Ecotoxicol Environ Saf. 2003;55(2):243–250.
- Kiss T, Vehovszky Á, Hiripi L, et al. Membrane effects of toxins isolated from a cyanobacterium, Cylindrospermopsis raciborskii, on identified molluscan neurones. Comp Biochem Phys C. 2002;131(2):167–176.
- Fastner J, Rücker J, Stüken A, et al. Occurrence of the cyanobacterial toxin cylindrospermopsin in the Northeast Germany. Environ Toxicol. 2007;22(1):26–32.
- Gkelis S, Tussy PF, Zaoutsos N. Isolation and preliminary characterization of cyanobacteria strains from freshwaters of Greece. Open Life Sci. 2015;10:52–60.
