Abstract
SARS-CoV-2 virus is responsible for the current COVID-19 pandemic, which has resulted in more than 2 million confirmed cases and 139,515 deaths in 213 countries, areas and territories as of 20th April 2020. Although reverse transcription polymerase chain reaction is the diagnostic test, screening studies for SARS-CoV-2 antibodies are essential for our extensive knowledge of the viral spread, formation of collective immunity, prophylaxis and treatment algorithms for the infection. We investigated 586 outpatients, for IgM and IgG antibodies, by their request in Varna and the region of northeastern Bulgaria. All of them were patients of medical diagnostic laboratory “STATUS”, Varna city. We used serological, immunochromatographic tests (rapid tests) at least seven days after suspected viral infection. Twenty-eight of the serum samples were SARS-CoV-2 Ab positive: 4.8% (95% CI: 3.2–6.9%, n = 28). IgM Ab only were detected in 1.0% (95% CI: 0.4–2.2%, n = 6), IgG Ab only in 1.2% (95% CI: 0.5–2.5%, n = 7) and both IgM/IgG Ab in 2.6% (95% CI: 1.5–4.2%, n = 15), from all of the tested individuals (n = 586). In order to understand how many people have contracted the virus, to strengthen our collective immunity and to be able to assess the risk, in the aftermath, it is essential to investigate (IgM/IgG) antibody titers.
Introduction
In Bulgaria, from the beginning of March 2020 up to 20th April, there were 929 officially reported COVID-19 cases in 26 out of 28 provinces, with 43 deaths and 167 recoveries [Citation1]. Historically, human coronaviruses have been circulating in the human populations for hundreds of years, although officially identified in the 1960s. In general, coronaviruses can be classified into four genera (Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus) and can be detected in a wide range of animal species, including humans. There are six previously known human coronaviruses that can be transmitted between humans. Human alphacoronaviruses, 229E and NL63, and betacoronaviruses, OC43 and HKU1, are common respiratory viruses usually causing mild upper respiratory tract diseases. In contrast to these, another two human beta-coronaviruses, SARS-CoV (Severe Acute Respiratory Syndrome) and MERS-CoV (Middle East Respiratory Syndrome) coronaviruses, are highly pathogenic in humans [Citation2]. Much has been learned about highly pathogenic coronaviruses over the past years [Citation3]. Since the outbreak of severe acute respiratory syndrome SARS in 2003, a large number of SARS-related coronaviruses have been discovered in their natural reservoir host, bats (bat SARS-like coronaviruses) [Citation4–6]. SARS-CoV-2 is a novel positive-sense single-stranded RNA coronavirus, about 80 nanometers in diameter, with coronavirus spike (S) glycoproteins promoting entry into cells. The glycoprotein spikes are the main target of antibodies. Different pathogenic mechanisms in viral action are deliberated, which result in different forms and outcome of the infection: asymptomatic, milder or severe forms, and even death. As reviewed by, Yuen et al. [Citation7], asymptomatic and presymptomatic virus shedding posts a big challenge to infection control. Patients with mild and unspecific symptoms are also difficult to identify and quarantine [Citation7, Citation8]. The clinical manifestations of COVID-19 include flu-like symptoms, dry cough, high temperature, runny nose, headache, shortness of breath pneumonia, diarrhea, dyspnea and multiple organ failure. SARS-CoV-2 can be highly stable in a favourable environment, but it is also susceptible to standard disinfection methods [Citation9]. The virus is responsible for the current pandemic, which has resulted in more than 2 million confirmed cases and 278 993 deaths in 215 countries, areas and territories, as per WHO data on 11th May 2020 (1). The epidemic started in Wuhan, China, on 12th December 2019. Full-length genome sequences of SARS-CoV-2 were obtained from five patients at an early stage of the outbreak. The sequence is 96% identical at the whole-genome level to a bat coronavirus, which made it possible to investigate the virus via molecular and serology testing [Citation6, Citation10]. Being an emerging pathogen, therapies and/or vaccination strategies against SARS-CoV-2 are in early stage of development [Citation3]. This emphasizes the major role of screening studies for antibodies, which could transform our knowledge of the viral spread, collective immunity, prophylaxis and treatment algorithms. This study reports the current state of the seroepidemiological distribution of SARS-CoV-2 Ab (IgM and IgG) in northeastern Bulgaria.
Subjects and methods
The prospective study included five-hundred and eighty-six (n = 586) outpatients (general population, policemen, medical workers). They were investigated for IgM and IgG, by their request, in Varna city and the region of northeastern Bulgaria from 26th March to 20th April 2020 in Medical Diagnostic Laboratory “STATUS”, Varna city. Serological, immunochromatographic tests (rapid cassette test) were used at least seven days after suspected viral infection. We used whole blood (intravenous or capillary), serum or plasma. Patients who had been tested in the same laboratory within the previous 6 h, did not have to give blood samples again.
Test procedure
Testing was done using the COVID-19 IgG/IgM Rapid test cassette (whole blood, serum/plasma), which is a lateral flow immunochromatographic assay (Zhejiang Orient Gene Biotech Co, Ltd), CE and IVD marked. The test procedure was done according to the manufacturer’s instructions. According to the test cassette specifications, the SARS-CoV-2 IgM/IgG rapid test cassette whole blood/serum/plasma was compared with leading commercial PCR tests and the results show that for IgM Ab has relative sensitivity: 85% (95% CI:62.1%-96,8%), relative specificity: 96% (95%CI: 86.3%-99.5%) and accuracy: 92.9% (95% CI:84.1%-97.6%). IgG Ab results- relative sensitivity: 100% (95% CI: 86%-100%), relative specificity: 98% (95% CI: 89.4-99.9%) and accuracy: 98.6% (95% CI: 92.3%-99.96%).
Ethics statement
This study was conducted in an ethical and responsible manner, in full compliance with all relevant codes of experimentation and legislation. All of the participants provided written informed consent for the use of their blood and blood components (such as sera and plasma).
Statistical analysis
Statistical analyses were performed using SPSS v.23. Quantitative variables were expressed as mean values with standard deviation (± SD) or median (range) as appropriate, and the qualitative variables were reported as a number, relative proportion (%) and confidence intervals (95% CI). Data were analyzed using t-test and Pearson’s χ2 test. Two-sided p-values of less than 0.05 were considered to indicate statistically significant differences.
Results and discussion
The results reported here are based on the testing of 586 patients from northeastern Bulgaria (Varna, Dobrich, Devnya, Targovishte, Dalgopol): 43.9% (95% CI: 39.8% − 48.0%, n = 257) of them men and 56.1% (95% CI: 52.0% − 60.2%, n = 329) women, from 3 years and 7 months to 92 years old. Twenty-eight of the patients were SARS-CoV-2 Ab positive: 4.8% (95% CI: 3.2% − 6.8%, n = 28). IgM/IgG Ab positive females were 64.3% (95% CI: 44.1% − 81.4%, n = 18) and males, 35.7% (95% CI: 18.6% − 55.9%, n = 10), with no statistically significant difference in the SARS-CoV-2 Ab status between males and females (χ2=0.791; p = 0.373). These 28 patients were aged from 21 to 68 years, mean age 45.0 years (SD ± 14.33), with no statistically significant difference in age between males and females (Pearson’s χ2 =22.91; p = 0.241).
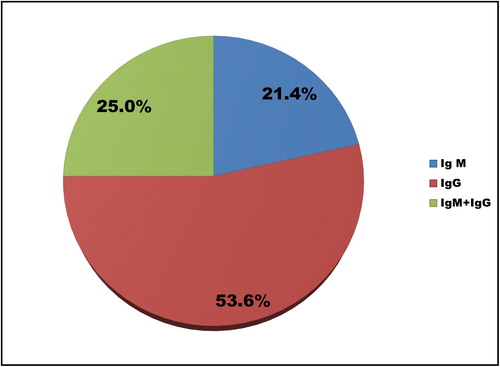
IgM Ab only were detected in 1.0% (95% CI: 0.4% − 2.2%, n = 6), IgG Ab only in 1.2% (95% CI: 0.5% − 2.5%, n = 7) and both IgM/IgG Ab in 2.6% (95% CI: 1.5% − 4.2%, n = 15), of all the individuals tested here (n = 586). More than half (53.6%) of the subjects positive for SARS-CoV-2 Ab COVID-19 () had already formed IgG.
Two of the COVID-19 positive women were tested with a pair of serum samples: the first one with both IgM and IgG Ab(+). Ten days later, one of the women was still IgM and IgG positive, whereas the other one was IgG Ab positive only (2-nd sample). The data about all of the SARS-CoV-2 antibody positive subjects broken down into five age groups are shown in .
Table 1. Distribution of SARS-CoV-2 Ab (+) subjects in different age groups.
Of all SARS-CoV-2 Ab positive patients, 78.6% (95% CI: 59.0%-91.7%, n = 22) were asymptomatic. Clinical symptoms (dry cough, high temperature, runny nose, headache, sore throat, shortness of breath, fatigue) were reported by 6 people: 21.4% (95% CI: 8.3%-41.0%, n = 6) with no statistically significant differences between the symptomatic presentation in males and females (Pearson’s χ2= 3.187; p = 0.074). Of them, 66.7% (95% CI: 22.3%-95.7%, n = 4) had dry cough, as a major symptom. As per our results, four of these symptomatic individuals showed IgG Ab only, i.e. 66.7% (95% CI: 22.3%-95.7%, n = 4). According to the information provided by the patients, ten of them – 35.7% (95% CI:18.6%-55.9%, n = 10) of all of the SARS-CoV-2 antibody positive ones – had been abroad, to countries such as Austria, England, France.
Bulgaria is one of the European countries with the least COVID-19 affected population. During the ongoing pandemic, in Bulgaria from the beginning of March 2020 up to 20th April, there were 929 officially reported confirmed cases with SARS-CoV-2 in 26 out of 28 provinces, with 43 fatal cases and 167 recovered [Citation1]. As per WHO, for the 20th of April 2020, the information about confirmed cases (people) with COVID-19 in the countries neighboring Bulgaria was: in Turkey- 86300, in Romania-8700, Serbia-6300, Greece-2200, North Macedonia-1200 people. Although this places Bulgaria as one of the European countries with the least SARS-CoV-2 infected population, it is still too early to draw conclusions and to summarize the information, as the pandemic is ongoing and there are no sufficient data for broad screening and testing in Bulgaria. Against the backdrop of the recent COVID-19 outbreak, the opportunity to study the antibody response of individuals infected with SARS-CoV-2 provides valuable and still rapidly changing information about the regional prevalence of the virus.
Antibody responses to SARS-CoV-2 infection in humans and animal models have been reported in very recently published papers and non-peer reviewed preprints. These early studies suggest that the immune response to SARS-CoV-2 is similar to that for SARS-CoV and MERS-CoV. Most infected individuals (testing positive by real time polymerase chain reaction (RT-PCR)) seroconvert 10-14 days after symptom onset, but antibody levels in some mild cases can be low or undetectable [Citation10]. Combining an RNA test and an Ab test significantly raises the sensitivity for detecting patients. Reverse transcription PCR (RT-PCR) is used as the golden standard to detect viral nucleic acid worldwide and is used as a diagnostic test. SARS-CoV-2 can be isolated from various types of samples . Identification of patients with few or no symptoms and with modest levels of detectable viral RNA in the oropharynx for at least 5 days suggests that we need better data to determine the transmission dynamics and inform our screening practices [Citation11]. Testing with the COVID-19 RT-PCR test is intended for use by trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures. PCR testing success depends also on the proper sample collection procedures, transport and PCR protocol implementation. Early reports cited a possible test sensitivity rate of 70% [Citation12]. Even with sensitivity values as high as 90% the magnitude of risk from false-negative test results will be substantial as testing becomes more widespread and the prevalence of COVID-19 infection rises [Citation13]. The seroconversion rate and the antibody levels increase rapidly during the first two weeks and some patients with negative nucleic acid findings could be screened out through antibody testing. The Ab detection is an important supplement to RT-PCR detection during the infection course [Citation14]. In addition to viral RNA detection, measurement of IgM and IgG antibodies as well as antigens would be very helpful, as patients with mild and unspecific symptoms are also difficult to identify and quarantine [Citation7]. Viral activity is only one of various factors that might influence disease transmission. Epidemiology is the gold standard to measure the transmission potential of patients who recover from COVID-19 yet are still positive for SARS-CoV-2 RNA. Further effort is urgently needed to evaluate the basic reproductive number in these patients [Citation15].
The most precise and accurate test for antiviral antibodies testing is the virus neutralization test. However, using this test for SARS-CoV-2 requires work inside high containment laboratories using infectious virus. In the routine practice a surrogate neutralization assay is used, based on the principle of pseudotyped virus particles (PV) that bear the SARS-CoV-2 spike protein [Citation10]. The main target of neutralizing antibodies is the C-terminal domain of the spike (S) glycoproteins, which attach to a protein on the surface of cells, called ACE2 (angiotensin-converting enzyme 2). The C-terminus forms homotrimers protruding the viral surface and promotes entry into host cells. Eight monoclonal antibodies targeting SARS-CoV-2 S glycoprotein, and one, S309 from EBV- immortalized memory B cells of a SARS survivor infected in 2003, have been identified. Although S309 recognizes an epitope on the viral spike protein, it does not target the receptor-binding domain and is not predicted to block ACE2 [Citation16].
The variations of SARS-CoV-2 specific NAbs in recovered COVID-19 patients may raise concerns about the role of NAbs in disease progression and their usage afterwards for prevention or treatment [Citation17]. There are suggestions that convalescent plasma may have beneficial effects in the treatment of COVID-19. Treatment of 15 critically ill patients with convalescent plasma along with additional therapy (such as antivirals, antibiotics, corticosteroids) eventually led to clinical improvement and virus clearance [Citation18, Citation19]. However, since all these patients received at least one additional therapy, it is still unclear what impact convalescent plasma itself had in their treatment [Citation18, Citation19]. Studies in macaques with extracted antibodies from COVID-19 survivors showed that these antibodies were neutralizing and able to bind to the virus and block its entry into the host cell [Citation20]. In general, it is considered that convalescent plasma is able to provide short- to medium-term humoral immunity and could potentially improve the clinical outcomes through neutralizing viremia against SARS-CoV-2 [Citation18, Citation19].
Enzyme linked immunosorbent assays (ELISA) tests and immunochromatographic assays, do not measure the function of the antibody as they detect binding to a given antigen.
The immunochromatographic testing that we used in this study gives fast, reliable results for IgM antibodies at least one week after possible contact with the virus. The presence of IgM antibodies alone indicates a recent infection (most likely the patient is infected) so the person is treated as an infectious one (1.0% of the tested individuals in the study). RT-PCR gives the final result and algorithm of proceeding. At the end of the third week, IgG antibodies can also be detected.
The presence of IgG antibodies alone indicates encounter with the virus that has passed asymptomatically or with mild and transient symptoms. As per our results, IgG Ab were found in 1.2% of the samples, and four of the symptomatic individuals (66.7%) had IgG alone. Acute antibody responses to SARS-CoV-2 were reported in 285 patients with COVID-19; within 19 days after symptom onset, all of the patients tested positive for antiviral IgG [Citation21]. Such people are considered to be “safe” or at least in the low-risk group for viral spread. They are able to take care of infected patients (with personal protective clothing only), as well as to perform their professional responsibilities. SARS-CoV-2 IgG positive people can quickly become involved in the work process and increasing their numbers in the future can pull the global economy round. Several countries, such as Germany, the United Kingdom, Italy, as well as the director of the US National Institute of Allergy and Infectious Diseases (A.S.Fauci) proposed the idea of “immunity ID cards” to identify people who have recovered from the viral infection and gained immunity to it [Citation22]. It is also crucial to determine for how long the antibody levels last. The best possibility would be if neutralizing IgG antibodies can give life-long protection, as in the case of measles [Citation23]. Some reports about the 2003 SARS epidemic imply that these neutralizing antibodies lasted for up to three years and in certain cases up to seventeen years [Citation24, Citation25]. For the MERS epidemic, the levels of protective antibodies have been observed to fade after three years [Citation26]. Antibody responses can vary from person to person.
The detection of both IgM and IgG antibodies (2.6%) indicates infection with a limitation of at least 3 weeks before that. RT-PCR is recommended, even in the absence of clinical symptoms, as some asymptomatic individuals can potentially transmit the virus.
We investigated patients from northeastern Bulgaria: predominantly from Varna (71.4%), Dobrich (10.8%), Devnya (7.1%), Dalgopol (7.1%) and Targovishte (3.6%). The obtained results showed COVID-19 Ab in five different age groups. In the first two groups, there were positive subjects for IgM alone, IgG alone, as well as IgM and IgG. All of the tested patients in the 51 to 60 years age range were IgG positive. IgG and IgM Ab together were not detected in the last group.
In many countries, as well as in Bulgaria, the testing capacity has lagged behind the spread of the virus. Large numbers of people have developed COVID-19 symptoms but have not been tested, and the vast majority of people who had the virus but never developed symptoms are non-registered. The asymptomatic patients accounted for 78.6% of all SARS-CoV-2 Ab positive subjects in our survey. As per our results, SARS-CoV-2 Ab were found in 4.8% of all of the tested subjects. Various results about viral distribution and asymptomatic state have been reported. For example, a study conducted in Germany (Bonn University) based on serological samples of approximately 1000 inhabitants estimated a 14% infectious rate and 0.37% fatality rate (with 44 reported deaths in the town) [Citation27]. The systematic screening of mothers in Columbia University Irving Medical Center in New York (n = 214) showed that 13.7% were asymptomatic carriers of SARS-CoV-2 and 1.9% were symptomatic [Citation28]. A large diagnostic testing conducted in Iceland showed that 43% of all positive cases were without any symptoms at the time of testing, which is a lower relative share compared with our data [Citation29].
Rapid tests are used for epidemiological purposes in order to define the scale of distribution of SARS-CoV-2 in community and do not have such a crucial diagnostic value. If rapid testing shows a negative result for both types of antibodies, it does not mean that the person is not infected with SARS-CoV-2. The reasons behind this may be that the blood sample had been taken before the 7th–10th day from initial infection, as well as that there are differences in the time of formation of antibodies in the human body, after primary contact (lag time, or lag phase). The Ab formation starts as the lymphoid cells encounter the antigen. These cells divide repeatedly to form a clone of cells with similar reactivity, after that differentiate, and start to synthesize antibodies. The antibody levels rise gradually, reach a plateau, and then decline [Citation30]. A plausible scenario is that some people simply do not develop antibodies against the virus (similar to the so called “non-responders”) to vaccination (around 2–10% of the healthy individuals). Various factors could underlie that phenomenon: host genetics, immune status, age, health or nutritional status [Citation30, Citation31].
Conclusions
Despite the controversial information about the formation and duration of neutralizing antibodies against SARS-CoV-2, there is evidence that patients develop such antibodies (or at least some form of short-term immunity), as the human body does for other viruses, and that these antibodies are protective. In order to understand how many people have contracted the virus and in order to strengthen our collective immunity and to be able to assess the risk, in the aftermath, it is essential to investigate antibody titers (IgM/IgG). This can be achieved by testing as many people as possible on a large scale, which is still not reported in the official statistics worldwide, but is the only way to contain the virus and restore our normal life and economics, in shorter terms.
Disclosure statement
The author declares that there are no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The author confirms that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- WHO-World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Chu D, Pan Y, Cheng S, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66(4):549–555.
- Coleman C, Frieman MB. Coronaviruses: important emerging human pathogens. J Virol. 2014; 88(10):5209–5212.
- Yang L, et al. Novel SARS-like betacoronaviruses in bats, China. Emerg Infect Dis. 2011; 19:989–991.
- Hu B, et al. Discovery of a rich gene pool of bats SARS-related coronaviruses provides new insights into origin of SARS coronavirus. Plos Pathol. 2017; 13(11):e-1006698.
- Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probate bat origin. Nature. 2020;579(7798):270–273.
- Yuen K-S, Ye Z-W, Fung S-Y, et al. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10:40.
- Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406.
- Chin A, Chu TS, Perera MRA, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020.
- Kellam P, Barcley W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. Preprints (www.preprints.org). 2020.
- Zhou L, Ruan F, Huang M, еt al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. NEJM. 2020;382:1177–1179.
- Gallagher G. Dealing with false negative Covid-19 test results. Contagion Live.Infectious diseases today (article). 2020. https://www.contagionlive.com/news/dealing-with-false-negative-covid19-test-results.
- West CP, Montori VM, Sampathkumar P. COVID-19 testing: the threat of false-negative results. MCP: Mayo Clin Proc. 2020. https://doi.org/10.1016/j.mayocp.2020.04.004.
- Zhao J, Yuan Q, Wang H, et al. Antibody Responses to SARS-CoV-2 in Patients of Novel Coronavirus Disease 2019 (February 25, 2020). Available from: https://ssrn.com/abstract=3546052. http://dx.doi.org/10.2139/ssrn.3546052.
- Zhou F, Fan G, Liu Z, et al. SARS-CoV-2 shedding and infectivity – authors’ reply. The Lancet. 2020;395(10233):1340.
- Pinto D, Park YJ, Beltramello M, et al. Structural and functional analysis of a potent sarbecovirus neutralizing antibody. bioRxiv.2020.04.07.023903.
- Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. MedRXiV. 2020. doi: https://doi.org/10.1101/2020.03.30.20047365.
- Duan K, Lui B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020;117(17):9490–9496. http://doi.org.10.1073/pnas.2004168117.
- Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582.
- Bao L, Deng W, Gao H, et al. Reinfection could not occur in SARS-COV-2 infected rhesus macaques. bioRxiv. 2020; Preprint; doi:.
- Long Q, Lui BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020. http://doi.rg/10.1038/s41591-020-0897-1.
- Forgey Q. Politico magazine. 04/10/2020; https://www.politico.com/news/2020/04/10/fauci-coronavirus-immunity-cards-for-americans-are-being-discussed-178784.
- Griffin D. The immune response in measles: virus control, clearance and protective immunity. Viruses. 2016;8(10):282.
- Wu L-P, Wang N-C, Chang Y-H, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562–1564.
- Poh CM, Carissimo G, Wang B, et al. Potent neutralizing antibodies in the sera of convalescent COVID-19 patients are directed against conserved linear epitops on the SARS-COV-2 spike protein. bioRxiv. 2020; Preprint;.
- Alshukairi AN, et al. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis. 2016;22(6):1113–1115.
- Streck H, Hartmann G, Exner M, et al. Vorläufiges Ergebnis und Sshlussfolgerungen der COVID-19 case-cluster-study (Gemeinde Gangeit). 2020. University of Bonn. non-peer reviewed report. https://hallespektrum.de/nachrichten/bildung/erste-ergebnisse-der-corona-studie-von-prof-streek-in-heinsberg/373118/.
- Sutton D, Fuchs K, D’Alton M, et al. Correspondence. Universal screening for SARS-CoV-2 in women admitted for delivery. N Eng J Med. 2020. http://www.nejm.org/doi/full/10.1056/NEJMc2009316.
- Gudbjartsson D, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in Icelandic population. N Eng J Med. 2020. http://www.nejm.org/doi/full/10.1056/NEJMoa2006100.
- Galazka A, et al. The immunological basis for immunization series module 1: general immunology. WHO/EPI/GEN/93.11.1993:10; https://apps.who.int/iris/bitstream/handle/10665/58891/WHO_EPI_GEN_93.11_mod1.pdf?sequence=1&isAllowed=y.
- Wiedermann U, Garner-Spitzer E, Wagner A, et al. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother. 2016;12(1):239–243.