Abstract
White celery (Apium graveolens L.), a variety of common celery, is an important leafy vegetable in the Apiaceae family and is famous for its nutritional value. However, limited work has been devoted to quality formation and regulation in white celery. In this study, four white celery varieties, ‘Xuebaiqincai’, ‘Saixue’, ‘Baiganshiqin’ and ‘Ruixue’, were selected and analyzed for the comparison of ascorbic acid (AsA) and lignin levels, which are two important quality evaluation indicators in celery. The expression levels of the genes involved in AsA and lignin metabolic pathways were also detected. In the leaf blades, the AsA content was highest in ‘Xuebaiqincai’ compared with other varieties, whereas the most abundant AsA levels in the petioles were observed in ‘Baiganshiqin’ and ‘Ruixue’. The expression levels of AsA-related genes varied among the studied varieties. The highest level was detected in ‘Xuebaiqincai’, whereas other varieties exhibited relatively lower levels. The lignin content in the leaf blades was lower than that in the petioles. Correspondingly, the transcript profiles of genes involved in lignin biosynthesis were in accordance with the different levels in the petioles and blades. The results of this study provide potentially useful information for white celery breeding aimed at quality improvement and regulation.
Introduction
Celery, an important leafy vegetable in the Apiaceae family, is cultivated worldwide and is an important source of ascorbic acid (AsA) and lignin [Citation1, Citation2]. It is popular among the urban and rural residents for its rich nutrients and minerals. In addition to its nutraceutical functions, celery is also an excellent source of industrial materials [Citation3]. White celery (Baiqin) is a well-received vegetable in southern Jiangsu Province with crisp and tender taste, and with white petioles. However, there is not much research work on its quality formation and underlying mechanisms.
AsA is an acidic polyhydroxy compound containing six carbon atoms [Citation4, Citation5]. It is well recognized that AsA has a strong antioxidant effect, removing excess free radicals from plants [Citation6–8]. Further studies revealed that AsA is able to regulate plant growth, induce flowering, and delay aging through complex signal transduction networks [Citation4, Citation9]. In rice, the scarcity of GalLDH, an enzyme at the last step in the AsA synthesis pathway in plants, affects the number of tillers and accelerates premature senescence [Citation10]. In cotton, ascorbate was reported to play an important role in cell elongation [Citation11]. AsA acts as a coenzyme of many essential enzymes and is necessary for the synthesis of many metabolites [Citation12]. Despite the benefits mentioned above, the human body has lost the ability to synthesize AsA. Lack of AsA could lead to blood diseases [Citation13]. Therefore, it is of vital importance to ensure AsA accumulation and regulation within plants.
Combined with isotope tracing, biochemical analysis and early research results, the first pathway for the synthesis of AsA in plants was proposed by Wheeler and his co-workers, namely the d-mannose/l-galactose pathway [Citation14]. Other synthetic pathways including the d-galacturonic acid pathway [Citation15, Citation16], glucose pathway [Citation17] and inositol pathway [Citation18] have also been reported. The last synthetic step of the glucose pathway and the inositol pathway in plants is similar to that in animals. Previous studies indicated that ascorbic acid accumulation is dependent upon tissue and developmental stages [Citation19, Citation20]. This phenomenon is partly due to the changing expression levels of genes related to AsA metabolism [Citation21]. For instance, the transcript levels of GME, GGP and GalLDH in AsA synthesis, MDHAR and DHAR related to recycling, were well correlated with AsA accumulation [Citation21]. In a recent study, the transcription levels of GGP2, GalLDH, APX3, GR1 and DHAR1 were in accordance with AsA accumulation patterns in sweet cherry fruit [Citation22]. In tea leaves [Citation23], APX1 and DHAR2 proteins might also perform critical roles.
Lignin, one of the important agronomic indicators in celery, plays important roles in the maintenance of plant morphology, water transport and resistance to adverse conditions [Citation24]. It is reported that lignin can play a role in disease resistance and lodging resistance [Citation25]. Lignin has three monomer forms: p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) monolignols. It is worth noting that lignin also has antioxidant properties according to previous reports [Citation26]. As mentioned above, white celery has a crisp and tender taste, and it is not clear whether lignin contributes to its quality formation. Hence, comparing the content of lignin in different varieties helps to understand the quality of white celery more comprehensively.
In the present work, four white celery varieties were selected, and their quality factors, AsA and lignin levels, in different tissues and varieties were detected and analyzed. Correspondingly, the transcript levels of genes related to AsA and lignin biosynthesis and metabolism were also determined to elucidate the mechanism for AsA and lignin accumulation. The results from the present work would improve our understanding of quality formation and regulation in white celery.
Materials and methods
Plant materials and growth conditions
Four white celery varieties were used as materials in this study. ‘Xuebaiqincai’ is a newly selected variety suitable for cultivation in most areas of China. This variety is crisp and tender, holding the compact plant architecture. ‘Saixue’ is a hybrid improved breeding variety, having good commercial properties. The plant is a medium to late maturing variety with more luxuriant leaves. ‘Baiganshiqin’ is mainly distributed in south and southwest China, and part of north China. This variety originates from China possessing the characteristics of fast growth, good fertility, wide adaptability and strong disease resistance. ‘Ruixue’ is a high-yield and solid white celery appropriate for planting in spring and autumn with a crisp taste and good quality.
Celery seeds were conserved in the State Key Laboratory of Corp Genetics and Germplasm Enhancement, Nanjing Agricultural University (32°02’N, 118°50’E). Seeds were sown in Huacheng vegetable professional cooperative in Lishui district (31°50’N, 118°99’E) in Nanjing. The samples were collected at 150 days after sowing. Leaf and petiole samples were stored at -80 °C for subsequent research.
Determination of AsA and lignin levels
AsA content in celery was measured by high-pressure liquid chromatography according to previous methods with slight modifications [Citation27]. The standard of ascorbic acid used in this study was purchased from Shanghai Yuanye Biotechnology co., Ltd. In brief, celery leaf blades or petioles were put into mortars and ground to powder with liquid nitrogen. Afterwards 2 mL of precooled 0.1% oxalic acid solution was added and the samples were centrifuged at 12,000×g for 10 min at 4 °C. Then the supernatant was aspirated and transferred into little clean brown bottles for determination of ascorbic acid. A total of 10 µL of fluid was injected into the chromatographic column using the UPLC (Ultra high performance liquid chromatograph) method. The mobile phase was set as 0.1% acetic acid at a flow rate of 1 mL min−1, the column temperature was 30 °C, and the elution absorbance was 245 nm. Three independent biological replicates of each variety were carried out.
Determination of lignin content was done mainly according to the references mentioned in the specification of the method with some modifications [Citation28, Citation29]. Briefly, approximately 2.0 g samples were ground with liquid nitrogen. The powder was added to a 10 mL tube containing 6 mL of absolute ethanol promptly and centrifuged for 20 min (12,000×g). After removal of the supernatant, the precipitate was allowed to dry at room temperature. About 10 mg of each dried sample were transferred into a 2 mL tube with 1 mL of 2 mol L−1 HCl and 100 μL of thioglycolic acid. The mixture was heated at 100 °C for 8 h. After cooling on ice, the samples were centrifuged at 12,000×g for 20 min. The sediment was washed with 1 mL of ddH2O and was added 1 mol L−1 NaOH. The mixed liquids were allowed to stand at 25 °C for 18 h. Finally, the absorbance of celery samples was recorded at 280 nm with 1 mol L−1 NaOH solution as the blank. Three independent biological replicates of each variety were performed.
Total RNA extraction and cDNA synthesis
Celery leaf blades and petioles were ground to powder, and then total RNA was extracted according to the instructions of the RNA extraction kit (Tiangen, Beijing, China). cDNA was synthesized using the PrimeScript RT reagent kit (TaKaRa, Dalian, China).
Gene expression analysis by RT-qPCR
The transcript abundance of genes involved in AsA and lignin metabolic pathways in celery were measured by Bio-Rad IQ5 real-time PCR System and calculated with the 2-ΔΔCt method [Citation30]. The primers were designed by Primer Premier 6.0 and SYBR Premix Ex Taq (TaKaRa, Dalian, China). AgTuB-B gene was selected as the internal standard to normalize the expression levels of genes related to AsA and lignin metabolism [Citation31, Citation32]. Each reaction contained a 20 μL volume and was performed three times. Each reaction system consisted of 7.2 μL of deionized H2O, 10 μL of SYBR Premix Ex Taq, 0.4 μL of forward and reverse quantification primers and 2.0 μL of cDNA. The reaction conditions were as follows: 95 °C for 30 s; 40 cycles of 95 °C for 5 s and 60 °C for 30 s; followed by 61 cycles at 65 °C for 10 s.
Statistical analysis
Duncan’s method was used to analyze the difference at the 0.05 significance level based on SPSS (SPSS Inc., Chicago, IL, USA) statistics software. Correlations between AsA and lignin accumulation and gene expression levels were analyzed by Pearson’s correlation coefficient (r) analysis.
Results and discussion
Celery growth analysis
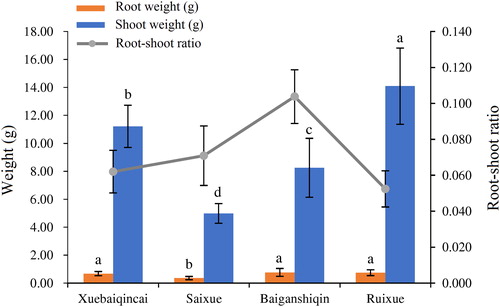
Celery, a herb vegetable of the Apiaceae family with a series of varieties, has long been cultivated around the world [Citation1, Citation33]. It is a green leafy vegetable that people like to consume because of its nutritional and medicinal value. In this study, the four white celery varieties planted in southern Jiangsu were selected as the experimental material. The morphological characteristics of ‘Xuebaiqincai’, ‘Saixue’, ‘Baiganshiqin’ and ‘Ruixue’ are shown in . The plant architecture of ‘Xuebaiqincai’, ‘Saixue’ and ‘Baiganshiqin’ is compact and their petioles are slender, whereas the celery plants of ‘Ruixue’ hold the highest height. The fresh weight values of ‘Saixue’ and ‘Baiganshiqin’ are relatively lower, consistent with their height. ‘Ruixue’ has the largest shoot weight with the least root/shoot ratio, whereas ‘Baiganshiqin’ possesses the highest root/shoot ratio (). There is little difference in the appearance in the four varieties of white celery except the larger size of ‘Ruixue’. This may contribute largely to the lowest root/shoot ratio of ‘Ruixue’.
AsA and lignin levels in white celery
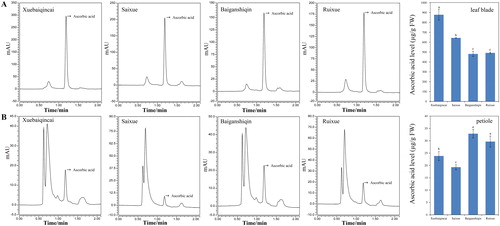
Many studies have shown that AsA content within the same species varies greatly depending on the varieties [Citation1, Citation20]. In this study, a similar result was also observed in celery. We found that the AsA content in celery leaf blades and petioles and different varieties differed (). The AsA levels in the leaf blades of the four white celery varieties were much higher than those in the petioles. In the leaf blades, the AsA content was the highest in ‘Xuebaiqincai’ among the four white celery varieties, whereas the lowest level was observed in ‘Baiganshiqin’ and ‘Ruixue’ (). In the petioles, ‘Baiganshiqin’ and ‘Ruixue’ had the highest content of AsA, whereas the AsA content in ‘Saixue’ was the lowest (). Compared to green celery, the leaf blades of white celery present higher levels of AsA, which can provide potential reference in celery plant and production [Citation1].
Figure 3. UPLC chromatogram of AsA and AsA levels in celery plants. (A) Leaf blades. (B) Petioles. Lowercase letters represent significant differences at p < 0.05.
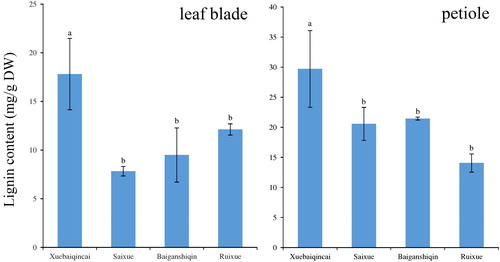
Given the rich dietary fibre, the lignin accumulation in celery attracted our attention [Citation34]. Its levels in the petioles were higher than those in the leaf blades, which was completely opposite to the patterns of AsA accumulation (). In the leaf blades, the content of lignin in ‘Xuebaiqincai’ was higher than that in the other white celeries. The lignin levels in the petioles showed similar patterns to that in the leaf blades. ‘Xuebaiqincai’ contained the highest amount of lignin, whereas other varieties showed relatively lower accumulation.
Expression profiles of genes involved in AsA and lignin metabolism in celery
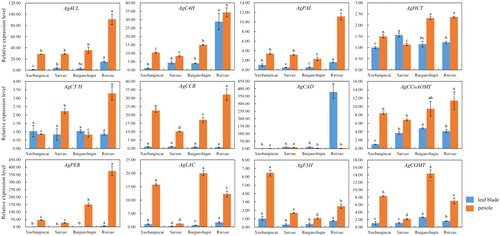
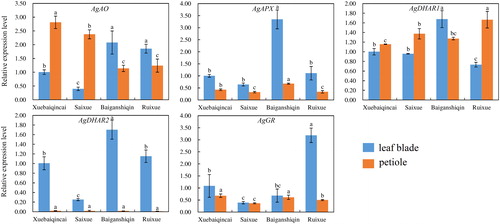
Genes involved in AsA metabolic pathways were analyzed to verify the potential regulation roles of AsA levels at the transcriptional profiles [Citation21]. A total of 11 genes involved in AsA biosynthesis were detected and analyzed in ‘Xuebaiqincai’, ‘Saixue’, ‘Baiganshiqin’ and ‘Ruixue’, respectively (). On the whole, the mRNA abundances of most genes in the leaf blades were higher than those in the petioles, coinciding with the AsA accumulation patterns. In the leaf blades, the transcription levels of AgPGI2, AgPMI, AgGMP, AgGGP1, AgGGP2 and AgGPP were the highest in ‘Xuebaiqincai’, whereas most genes showed lower expression in ‘Saixue’. In the petioles, most of the genes showed the highest mRNA abundance in ‘Xuebaiqincai’ (). The transcription levels of AgAO in the leaf blades of ‘Xuebaiqincai’ and ‘Saixue’ were considerably lower than those in the other two white celery varieties, whereas the opposite trend was determined in the petioles (). AgAPX gene displayed similar expression patterns in different varieties, highly expressed in ‘Baiganshiqin’. GME, as a key enzyme in the ascorbate biosynthesis pathway, has been shown to regulate the development of male gametophyte in Arabidopsis thaliana [Citation35, Citation36]. Here, the transcription levels of GME were extremely low in white celery varieties, which had trace expression in the petioles. The PMM gene has also been shown to be involved in the ascorbate biosynthesis pathway of Nicotiana benthamiana [Citation37]. Meanwhile, the expression level of PMM gene was high in the four varieties of white celery, indicating that it may show a similar function. AgDHAR1 was highly expressed in ‘Baiganshiqin’ and ‘Ruixue’ in the leaf blades and petioles, respectively. The transcript levels of AgDHAR2 in the leaf blades were highest in ‘Baiganshiqin’, whereas in the petioles the gene expression was maintained almost at the same level among all varieties. AgGR was highly expressed in ‘Ruixue’ in the leaf blades, and showed high amount of expression in ‘Xuebaiqincai’ ().
Figure 5. Transcriptional levels of genes involved in AsA biosynthesis in leaf blades and petioles of ‘Xuebaiqincai’, ‘Saixue’, ‘Baiganshiqin’ and ‘Ruixue’.
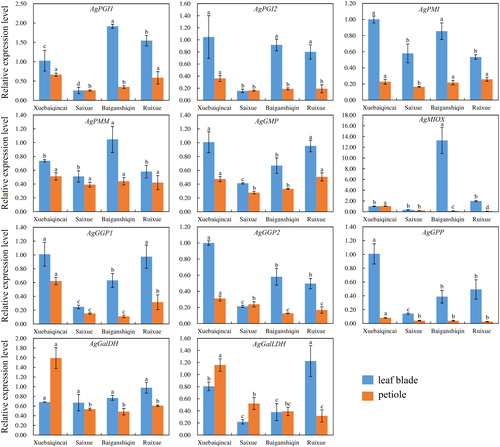
Figure 6. Transcriptional levels of genes involved in AsA recycling and degradation in leaf blades and petioles of ‘Xuebaiqincai’, ‘Saixue’, ‘Baiganshiqin’ and ‘Ruixue’.
Our research showed that the content of lignin in the petiole of celery was significantly higher than that in the leaf blades, which showed an opposite trend to the AsA content in celery, exhibiting obvious variety differences in white celery [Citation29, Citation38]. A total of 12 genes involved in lignin biosynthesis were identified to analyze the expression profiles (). The expression levels of genes in the petioles were significantly higher than those in the leaf blades, in consistence with lignin accumulation in the petioles and leaf blades, which was similar to a previous study [Citation34]. In the leaf blades, Ag4CL, AgC4H, AgPAL, AgCAD, AgPER and AgLAC were highly expressed in ‘Ruixue’, whereas AgF5H showed high expression in ‘Xuebaiqincai’. In the petioles, Ag4CL, AgC4H, AgPAL, AgHCT, AgC3’H, AgCCR, AgCCoAOMT and AgPER were all highly expressed in ‘Ruixue’. 4CL and PAL, as branch point enzymes in the phenylpropanoid pathway, have been shown to play important developmental roles in Juglans regia L. microshoots [Citation39]. Given its high expression, it may also play an important role in regulating the growth and development of white celery. AgLAC and AgCOMT exhibited higher expression in ‘Baiganshiqin’, whereas abundant expression of AgF5H was observed in ‘Xuebaiqincai’ ().
Correlation analysis
The correlations of AsA content and the relative expression level are shown in . AgPGI1, AgPGI2, AgPMI, AgPMM, AgGMP, AgMIOX, AgGGP1, AgGGP2, AgGPP, AgGalDH, AgGalLDH, AgAO, AgAPX, AgAPX3, AgDHAR1, AgDHAR2 and AgGR were selected to examine the relationship between the AsA content and the expression profiles of the genes in AsA pathways in white celery. The results showed that the transcript abundance of AgMIOX displayed significant positive correlation with AsA content (p < 0.01; r = 0.874) and AgDHAR2 with some level of correlation (p < 0.05; r = 0.681). Previous studies have shown that higher transcription levels of DHAR genes were associated with higher AsA accumulation in blueberry [Citation21], and our results were similar in white celery. Bodra et al. [Citation40] also reported DHAR2’s flexibility in catalytic reactions. Endres and Tenhaken [Citation41] found that MIOX enzymes can only regulate the level of inositol in A. thaliana and not the content of AsA. The content of AsA may be consistent with the relative expression of AgMIOX, which may be due to differences in species. Our data demonstrated that the transcript abundance of some genes (AgPMI, AgGMP, AgGGP1, AgGGP2 and AgGPP) was higher as the content of AsA in leaf blades of ‘Xuebaiqincai’ . However, the transcription levels of AgPGI1, AgPMM, AgMIOX, AgAO, AgAPX, AgDHAR1 and AgDHAR2 were higher in petioles of ‘Baiganshiqin’ in comparison with other celeries. Ren et al. [Citation38] found that the AsA content decreased with the increase in the expression levels of AO in Chinese cabbage, which was consistent with our research.
Table 1. Correlations between AsA content and relative expression levels of genes involved in the AsA biosynthesis.
In this study, the lignin content was closely associated with the expression levels of genes encoding the enzymes in the biosynthetic pathway of lignin. The correlations between the lignin content and transcriptional profiles of lignin-related genes are presented in . Our results showed that AgCCoAOMT and AgCOMT transcription levels illustrated strong positive correlation with lignin level (p < 0.05; r = 0.773, 0.697). It can be seen that CCoAOMT may also be a key gene for lignin synthesis in celery [Citation42].
Table 2. Correlations between lignin content and relative expression levels of genes involved in the AsA biosynthesis.
Conclusions
In this study, four white celery varieties were selected and analyzed with regard to the AsA and lignin levels. The transcript abundance of genes involved in AsA and lignin metabolism also were determined. The AsA levels in leaf blades were higher than those in petioles, and the patterns of lignin content showed the opposite trend. The expression levels of AsA and lignin-related genes varied in different varieties and tissues. This study will provide potential useful information for the quality improvement and molecular breeding of white celery.
Author contribution statement
XAS and YL initiated and designed the research, YL, LJX, FK and DX performed the experiments; YL, XGM, SS and LJX analyzed the data; XAS contributed reagents/materials/analysis tools; YL wrote the paper; WGL and XAS revised the paper. All authors read and approved the final manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
| Abbreviations | ||
| 4CL | = | 4-Coumarate-CoA ligase |
| AO | = | Ascorbate oxidase |
| APX | = | Ascorbate peroxidase |
| AsA | = | Ascorbic acid |
| C3’H | = | p-Coumaroyl shikimate/quinate 3’-hydroxylase |
| C4H | = | Cinnamate 4-hydroxylase |
| CAD | = | Cinnamyl alcohol dehydrogenase |
| CCoAOMT | = | Caffeoyl-CoA O-methyltransferase |
| CCR | = | Cinnamoyl-CoA reductase |
| COMT | = | Caffeic acid O-methyltransferase |
| DHAR | = | Dehydroascorbate reductase |
| F5H | = | Ferulate 5-hydroxylase |
| GalDH | = | l-galactose dehydrogenase |
| GalLDH | = | l-galactono-1,4-lactone dehydrogenase |
| GalUR | = | d-galacturonate reductase |
| GGP | = | GDP-l-galactose phosphorylase |
| GME | = | GDP-d-mannose-3′,5′-epimerase |
| GMP | = | GDP-d-mannose pyrophosphorylase |
| GPP | = | l-galactose-1-phosphate phosphatase |
| GR | = | Glutathione reductase |
| HCT | = | Hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase |
| LAC | = | Laccase |
| MDHAR | = | Monodehydroascorbate reductase |
| MIOX | = | Myo-inositol oxygenase |
| PAL | = | Phenylalanine ammonia lyase |
| PER | = | Peroxidase |
| PMI | = | Phosphomannose isomerase |
| PMM | = | Phosphomannose mutase |
| PGI | = | Phosphoglucose isomerase |
| RT-qPCR | = | Quantitative real-time polymerase chain reaction |
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Li MY, Hou XL, Wang F, et al. Advances in the research of celery, an important Apiaceae vegetable crop. Crit Rev Biotechnol. 2018;38(2):172–183.
- Li MY, Wang F, Jiang Q, et al. Identification of SSRs and differentially expressed genes in two cultivars of celery (Apium graveolens L.) by deep transcriptome sequencing. Hortic Res. 2014;1:10.
- Sowbhagya HB. Chemistry, technology, and nutraceutical functions of celery (Apium graveolens L.): an overview. Crit Rev Food Sci Nutr. 2014;54(3):389–398.
- De Tullio MC, Arrigoni O. Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci. 2004;61(2):209–219.
- Stevens R, Buret M, Duffé P, et al. Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol. 2007;143(4):1943–1953.
- Wang HS, Yu C, Zhu ZJ, et al. Overexpression in tobacco of a tomato GMPase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity. Plant Cell Rep. 2011;30(6):1029–1040.
- Wang HS, Zhu ZJ, Feng Z, et al. Antisense-mediated depletion of GMPase gene expression in tobacco decreases plant tolerance to temperature stresses and alters plant development. Mol Biol Rep. 2012;39(12):10413–10420.
- Yabuta Y, Motoki T, Yoshimura K, et al. Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 2002;32(6):915–925.
- Gallie DR. The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Exp Bot. 2013;64(2):433–443.
- Liu Y, Yu L, Tong J, et al. Tiller number is altered in the ascorbic acid-deficient rice suppressed for l-galactono-1,4-lactone dehydrogenase. J Plant Physiol. 2013;170(4):389–396.
- Song W, Wang F, Chen L, et al. GhVTC1, the key gene for ascorbate biosynthesis in Gossypium hirsutum, involves in cell elongation under control of ethylene. Cells. 2019;8(9):E1039.
- Jung HI, Kong MS, Lee BR, et al. Exogenous glutathione increases arsenic translocation into shoots and alleviates arsenic-induced oxidative stress by sustaining ascorbate-glutathione homeostasis in rice seedlings. Front Plant Sci. 2019;10:1089
- Firth N, Marvan E. Oral lesions in scurvy. Aust Dent J. 2001;46(4):298–300.
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393(6683):365–369.
- Davey MW, Gilot C, Persiau G, et al. Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol. 1999;121(2):535–543.
- Agius F, González-Lamothe R, Caballero JL, et al. Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nat Biotechnol. 2003; 21(2):177–181.
- Maruta T, Ichikawa Y, Mieda T, et al. The contribution of Arabidopsis homologs of l-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Biosci Biotechnol Biochem. 2010;74(7):1494–1497.
- Gillaspy GE, Keddie JS, Oda K, et al. Plant inositol monophosphatase is a lithium-sensitive enzyme encoded by a multigene family. Plant Cell. 1995;7(12):2175–2185.
- Wang GL, Xu ZS, Wang F, et al. Regulation of ascorbic acid biosynthesis and recycling during root development in carrot (Daucus carota L.). Plant Physiol Biochem. 2015;94:10–18.
- Huang W, Wang GL, Li H, et al. Transcriptional profiling of genes involved in ascorbic acid biosynthesis, recycling, and degradation during three leaf developmental stages in celery. Mol Genet Genomics. 2016;291(6):2131–2143.
- Liu F, Wang L, Gu L, et al. Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem. 2015;188:399–405.
- Liang D, Zhu T, Ni Z, et al. Ascorbic acid metabolism during sweet cherry (Prunus avium) fruit development. PLoS One. 2017;12(2):e0172818
- Li H, Liu ZW, Wu ZJ, et al. Differentially expressed protein and gene analysis revealed the effects of temperature on changes in ascorbic acid metabolism in harvested tea leaves. Hortic Res. 2018;5:65.
- Kumar M, Campbell L, Turner S. Secondary cell walls: biosynthesis and manipulation. J Exp Bot. 2016;67(2):515–531.
- Liu Q, Luo L, Zheng L. Lignins: Biosynthesis and Biological Functions in Plants. Int J Mol Sci. 2018;19(2):335.
- Azadfar M, Gao AH, Bule MV, et al. Structural characterization of lignin: a potential source of antioxidants guaiacol and 4-vinylguaiacol. Int J Biol Macromol. 2015;75:58–66.
- Attia TZ. Simultaneous determination of rutin and ascorbic acid mixture in their pure forms and combined dosage form. Spectrochim Acta A Mol Biomol Spectrosc. 2016;169:82–86.
- Cai C, Xu CJ, Li X, et al. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol Technol. 2006;40(2):163–169.
- Duan AQ, Feng K, Wang GL, et al. Elevated gibberellin enhances lignin accumulation in celery (Apium graveolens L.) leaves. Protoplasma. 2019;256(3):777–788.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
- Li MY, Wang F, Jiang Q, et al. Validation and comparison of reference genes for qPCR normalization of celery (Apium graveolens) at different development stages. Front Plant Sci. 2016;7:313
- Feng K, Liu JX, Xing GM, et al. Selection of appropriate reference genes for RT-qPCR analysis under abiotic stress and hormone treatment in celery. Peer J. 2019;7:e7925.
- Que F, Hou XL, Wang GL, et al. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic Res. 2019;6:69.
- Liu JX, Feng K, Wang GL, et al. Elevated CO2 induces alteration in lignin accumulation in celery (Apium graveolens L.). Plant Physiol Biochem. 2018;127:310–319.
- Tao J, Wu H, Li Z, et al. Molecular Evolution of GDP-d-Mannose Epimerase (GME), a Key Gene in Plant Ascorbic Acid Biosynthesis. Front Plant Sci. 2018;9:1293.
- Qi T, Liu Z, Fan M, et al. GDP-d-mannose epimerase regulates male gametophyte development, plant growth and leaf senescence in Arabidopsis. Sci Rep. 2017;7(1):10309.
- Qian W, Yu C, Qin H, et al. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. Plant J. 2007;49(3):399–413.
- Ren J, Chen Z, Duan W, et al. Comparison of ascorbic acid biosynthesis in different tissues of three non-heading Chinese cabbage cultivars. Plant Physiol Biochem. 2013;73:229–236.
- Cheniany M, Ganjeali A. Developmental role of phenylalanine-ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) genes during adventitious rooting of Juglans regia L. microshoots. Acta Biol Hung. 2016;67(4):379–392.
- Bodra N, Young D, Astolfi Rosado L, et al. Arabidopsis thaliana dehydroascorbate reductase 2: Conformational flexibility during catalysis. Sci Rep. 2017;7:42494.
- Endres S, Tenhaken R. Myoinositol oxygenase controls the level of myoinositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiol. 2009;149(2):1042–1049.
- Pang SL, Ong SS, Lee HH, et al. Isolation and characterization of CCoAOMT in interspecific hybrid of Acacia auriculiformis x Acacia mangium-a key gene in lignin biosynthesis. Genet Mol Res. 2014;13(3):7217–7238.