Abstract
Sanghuangporus baumii (Pilát) L.W. Zhou & Y.C. Dai, a popular medicinal fungus, has been used to treat several diseases by its triterpenoids bioactive ingredient for many years. However, the triterpenoids content of S. baumii is low and their biosynthesis mechanisms are not clearly understood. In order to reveal the regulation mechanism of triterpenoids biosynthesis in S. baumii, the cDNA encoding isopentenyl diphosphate isomerase (IDI) involved in triterpenoids biosynthesis was cloned and named as SbIDI (GenBank number MK955885). The open reading frame of the SbIDI gene cDNA comprised 792 bp and encoded a polypeptide of 263 amino acids with a predicted protein molecular weight of 29.80 kDa. The transcript level of the SbIDI gene and triterpenoids content of S. baumii were detected at different developmental stages. The results showed that the transcript level of the SbIDI gene and triterpenoids content had almost the same trend of change, increased first and then decreased dynamically. The highest transcript level of the SbIDI gene occurred earlier than the highest triterpenoids content, indicating that the SbIDI gene may play an important role in triterpenoids biosynthesis in S. baumii. Moreover, the SbIDI gene cDNA was successfully expressed in Escherichia coli BL21 (DE3), and the protein bands were consistent with the prediction.The results will lay a foundation for further study of the function of the SbIDI gene.
Introduction
Sanghuangporus baumii (Pilát) is a traditional medicinal fungus that grows on the stem bark of Syringa reticulata (Bl.) Hara. This fungus has been widely used to treat several disorders as herbal medicine through its various bioactive ingredients [Citation1–3]. So far, many effective components have been isolated and purified from S. baumii, including triterpenoids, polysaccharides, flavonoids, organic acids and polyphenols. It is worth mentioning that the triterpenoids have been shown to possess effective medicinal activities such as anti-cancer, anti-tumor, anti-inflammatory, anti-oxidative, and anti-Alzheimer’s disease activities [Citation4–6], and the content of triterpenoids is an important index to evaluate the medicinal value of S. baumii. However, since the yield of triterpenoids is naturally relatively low in S. baumii [Citation7], it is difficult to meet the demand for medicinal use. Therefore, a method to increase the yield of triterpenoids is urgently needed. The wild resources of S. baumii are gradually becoming scarce and the artificial cultivation of S. baumii is still in the initial stage. Meanwhile, as the chemical structure of triterpenoids is very complicated, it is very difficult to synthesize triterpenoids using chemical methods. Therefore, one way to solve this problem would be manipulation of the regulation of the key enzyme genes to promote their expression and increase the triterpenoids product.
As secondary metabolites, triterpenoids are synthesized via the classic mevalonate pathway (MVA) in fungi; the common precursor of triterpenoids is dimethylallyl diphosphate (DMAPP) [Citation8]. Thus, the improvement of DMAPP content is the key to ensure the high yield of triterpenoids [Citation9,Citation10]. The precursor substance isopentenyl diphosphate (IPP) is converted into DMAPP, catalyzed by isopentenyl diphosphate isomerase (IDI), and over-expression of IDI gene has been recently reported to significantly increase triterpenoids production [Citation11,Citation12]. Therefore, IDI gene is considered as an important regulator in the process of triterpenoids biosynthesis.
As a key gene in the biosynthesis of triterpenoids, the regulatory mechanism of the SbIDI gene is still poorly understood in S. baumii. In this work, a SbIDI gene cDNA from S.baumii was cloned and characterized, and the SbIDI gene transcript level and triterpenoids content were explored during different developmental stages. Moreover, recombinant protein was successfully observed in Escherichia coli BL21(DE3). Our study could provide a reference for better understanding the function of the SbIDI gene in the triterpenoids biosynthesis of S. baumii.
Materials and methods
Strain and culture conditions
S. baumii strain DL101 was preserved in the protection laboratory of the Northeast Forestry University. The strain of S. baumii was maintained on potato dextrose agar plates for 10 d. One part of mycelia were inoculated into polypropylene bags filled with substrate (77% sawdust, 20% wheat bran, 2% corn flour, 0.5% gypsum and 0.5% lime, based on the dry weight of ingredients) after autoclaving at 121 °C for 2 h, and then the bags were cultured at 25 °C and 60%–70% relative humidity in the dark. The other part of the mycelia were inoculated into 500 mL glass bottles containing potato dextrose (PD) medium and grown in a shaking incubator at 170 rpm and 25 °C for 6, 8, 10, 12, and 14 d.
Cloning of the SbIDI gene from S. baumii
The total RNA of S. baumii was extracted using an RNAprep pure Plant Kit (Tiangen Biotech, Beijing, China), and then the total RNA was reverse-transcribed using a PrimeScript TM 1st strand cDNA synthesis kit (Takara, Dalian, China).The cDNA sequence of the SbIDI gene was amplified from the S. baumii transcriptome using primers SbIDI-F1 and SbIDI-R1 (). The PCR reactions were carried out under the following conditions: 94 °C for 5 min; followed by 35 cycles of 94 °C for 30 s, 65 °C for 30 s, and 72 °C for 40 s; and a final extension at 72 °C for 7 min (Agilent Technologies, Santa Clara, CA, USA). All the primer synthesis and PCR products sequencing were done by TSINGKE (Harbin, China).
Table 1. Primers used in the present study.
Bioinformatics analysis of the SbIDI gene
The open reading frame (ORF) of the SbIDI gene cDNA and the structural regions of the deduced protein sequence were identified by searches at National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/). The physical and chemical properties were estimated using ProtParam software (http://www.expasy.ch/tools/protparam.html). The presence of a signal peptide was predicted using SignalP software (http://www.cbs.dtu.dk/services/SignalP/). The transmembrane regions were predicted using TMHMM Server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/). The secondary structure analysis was achieved using the software SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/secpred_sopma.pl). The three-dimensional structure of the SbIDI protein was predicted using the homologous modeling software Swiss model (http://www.swissmodel.expasy.org/). Similar SbIDI protein sequences from different species were downloaded via the BLAST algorithm at GenBank. Using MEGA 6.0 software for IDI sequence alignment, a phylogenetic tree was constructed using the Neighbor-Joining method, with the repeated sampling frequency set as 1000 replicates to ensure the stability of the branches of the tree [Citation13].
Quantification of triterpenoids
Samples of S. baumii at different developmental stages were harvested (including mycelia, primordia and fruiting body), one part of each sample was frozen at −80 °C until RNA extraction, and the other part was dried at 45 °C for triterpenoids extraction. To extract the triterpenoids from S. baumii, 0.1 g of sample from different developmental stages were dried at 45 °C to a constant weight. The extraction process of triterpenoids and the drawing of the standard curve followed previously described methods [Citation7].
Measurement of the SbIDI gene transcript level by quantitative real time polymerase chain reaction
Total RNA extraction was determined as described earlier, and then it was reverse-transcribed into cDNA using a PrimeScriptRT reagent Kit (Takara). Then, the synthesized cDNA was used as the template for the quantitative real time polymerase chain reaction (qRT-PCR) experiment. Primers SbIDI-F2 and SbIDI-R2 were used to evaluate the transcript level of the SbIDI gene, and α-tubulin and β-tubulin transcripts were amplified as internal controls (). After initial denaturation at 95 °C for 3 min, amplification occurred in three steps: 30 s of denaturation at 95 °C, 30 s of annealing at 57 °C, and 1 min of extension at 72 °C, for a total of 40 cycles. The mycelia harvested at the 6th day were defined as the reference sample and their SbIDI gene expression was set as 1.0, against which the data for different developmental stages were normalized. The transcript level calculations were performed using the 2−ΔΔCt method described by Livak and Schmittgen [Citation14].
Vector construction and prokaryotic expression of SbIDI gene
The SbIDI gene cDNA fragments were ligated into the vector pET-32a (linearized using EcoR I and Hind III restriction sites) to obtain the pET-32a-SbIDI construct using an In-Fusion HD Kit (Takara). The pET-32a-SbIDI construct was transformed into E. coli BL21(DE3) competent cells to obtain a recombinant expression strain. The positive clones were screened by PCR using the primers pET-32a-F and pET-32a-R (). A positive individual bacterial colony was transferred into 50 mL of Luria-Bertani (LB) medium containing 100 μg/mL ampicillin and cultured in a rotary shaker incubator (180 rpm) for 12 h at 37 °C. Next, 100 μL of the overnight culture was inoculated into 50 mL of fresh LB medium containing 100 μg/mL ampicillin and incubated at 37 °C, 180 rpm until the optical density at 600 nm (OD600) reached 0.6–0.8, representing an exponentially growing culture. Then, 1 mmol/L isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to the culture medium to induce SbIDI protein expression at 28 °C, 180 rpm. Different induction times (0, 2, 4, 6, 8, and 10 h) were assessed to optimize SbIDI protein expression. Finally, the thallus was dissolved in protein buffer (Solarbio, Beijing, China), and then the mixture was boiled for 10 min; 8 μL of the supernatant of the disintegrated cultures were subjected to 8% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE).
Statistical analysis
All data are the mean values of three independent sample measurements, including the determination of the transcript level of SbIDI gene and triterpenoids content at three developmental stages. Error bars in the figures show the standard deviation (±SD). The statistical significance was analyzed by t-test using the SPSS 17.0 software, and the differences were considered significant at p < 0.05.
Results and discussion
Cloning of the SbIDI gene from S. baumii
The cDNA sequence of the SbIDI gene was amplified using the first-strand cDNA of S. baumii as a template. A 792 bp ORF was obtained encoding a putative 263 amino-acid residue protein (). The cDNA sequence of the SbIDI gene was deposited in GenBank (Accession No. MK955885).
Bioinformatic analysis of the deduced protein sequence of the SbIDI gene
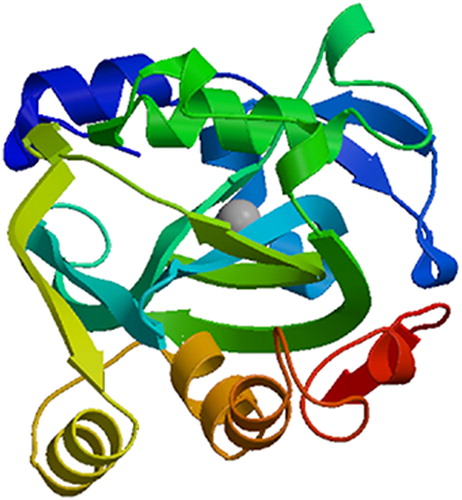
Searches at NCBI identified that SbIDI was a member of the NUDIX (Nucleoside Diphosphate linked to another moiety, X) Hydrolase superfamily and had conserved domains. ProtParam predictive analysis showed that the molecular weight of the protein was 29.80 kDa, with a theoretical pI of 5.24, a total number of negatively charged residues (Asp + Glu) of 42, a total number of positively charged residues (Arg + Lys) of 33, a GRAVY hydropathicity score of −0.381, and an instability index (II) of 28.63, indicating that SbIDI protein was a stable hydrophilic protein. No signal peptide or transmembrane region were predicted in the SbIDI protein, suggesting that the SbIDI protein was not a transmembrane or secretory protein, which was consistent with the IDI protein of Rabdosia rubescens [Citation15].The secondary structure analysis of the SbIDI protein using SOPMA showed that alpha-helices occupied 52.85%, extended strands occupied 11.41%, beta turns occupied 6.08%, and random coils occupied 29.66% of the protein structure. The three-dimensional structure of the SbIDI protein was predicted using Swiss model and the crystal structure of human type I isopentenyl-diphosphate delta-isomerase 1 protein (SMTL ID:2i6k.1.A) was selected as a template, the sequence identity between SbIDI protein and isopentenyl- diphosphate delta-isomerase 1 protein was 53.85% ().
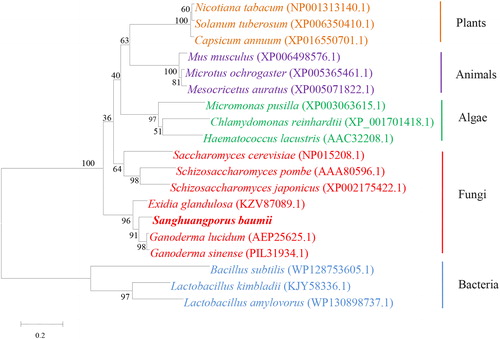
The IDI protein sequences of 18 species deposited in the GenBank database were selected for alignment with the SbIDI protein. The result of the phylogenetic analysis () showed that the SbIDI protein had a distant genetic relationship with bacterial IDI protein, and was most closely related with fungal IDI proteins. In particular, SbIDI protein showed the highest similarity (91%) with the IDI protein of Ganoderma, the result was consistent with known biological evolution, and it was speculated that the SbIDI protein had similar function in triterpenoids biosynthesis as those of G. lucidum and G. sinense.
Triterpenoids profile at different developmental stages of S. baumii
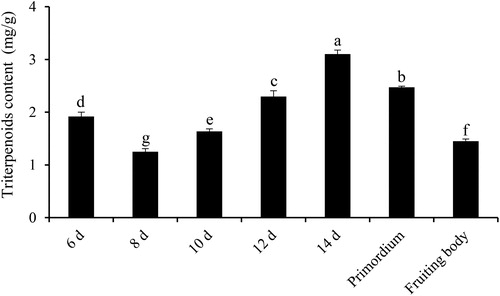
To detect the change in triterpenoids content at different developmental stages of S. baumii, the samples were collected and processed. The results showed that the triterpenoids content of S. baumii changed dynamically at different developmental stages (). The content of triterpenoids increased from the 6th day to the 14th day in the mycelium stage, before gradually declining during the 14th day of the mycelium stage to the fruiting body stage. The highest triterpenoids content was detected on the 14th day (3.10 mg/g dry weight), which was significantly different from that of other developmental stages.
Transcript level of SbIDI gene at different developmental stages of S. baumii
To investigate the transcript level of the SbIDI gene at different developmental stages of S. baumii, qRT-PCR was performed. The results showed that the transcript level of the SbIDI gene also changed dynamically at different developmental stages (). In general, the transcript level of the SbIDI gene increased from the 6th day to the 12th day in the mycelium stage, before gradually decreasing during the 12th day of the mycelium stage to the fruiting body stage. The results suggest that the SbIDI gene was most actively transcribed during the 12th day of the mycelium stage, at which point its transcript level was 13.09-fold higher than that in the fruiting body stage. At the same time, the transcript level of the SbIDI gene on the 12th day was significantly different from that of other developmental stages. The results were similar to those of the key gene HMGR in G. lucidum [Citation16].
Figure 5. The transcript level of the SbIDI gene at different developmental stages of S. baumii (p < 0.05).
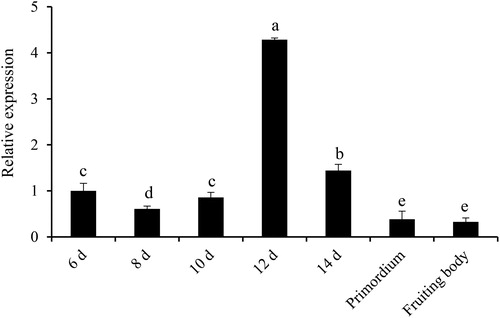
The change in the SbIDI gene transcript level was approximately consistent with the triterpenoids content of S. baumii at different developmental stages. The results from this study were similar to those in G. lucidum and S. baumii, in which the transcript level of a key gene was correlated positively with the triterpenoids content [Citation17,Citation18]. The highest transcript level of the SbIDI gene was detected on the 12th day, whereas the highest triterpenoids content was measured on the 14th day. The reason could be explained by the induced expression of the biosynthetic gene occurring much earlier than the accumulation of secondary metabolites, which was also the case for Ganoderic acids and triterpenoids ginsenoside production [Citation19,Citation20].
Prokaryotic expression of the SbIDI gene
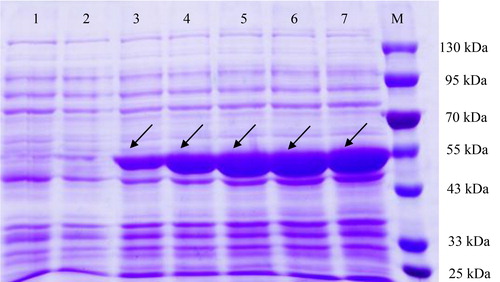
To validate the expression of the SbIDI gene cDNA in E. coli BL21(DE3), the whole SbIDI gene ORF was cloned into the expression vector pET-32a forming the pET-32a-SbIDI construct. The pET-32a-SbIDI construct was transformed into E. coli BL21(DE3) competent cells. The results of SDS-PAGE showed that the recombinant transformant produced clear protein bands with a molecular weight of approximately 50.8 kDa (including a 21.0-kDa fusion tag protein) (), which was consistent with other species, including Lycium chinense [Citation21], Camptotheca acuminata [Citation22] and Elaeagnus umbellata [Citation23]. The results indicated that the prokaryotic expression vector pET-32a-SbIDI was correctly constructed and the target protein was successfully expressed in E. coli BL21(DE3). In addition, we determined the effect of different induction times on the expression level of SbIDI gene. The results showed that IPTG-induced expression of SbIDI protein changed significantly from 2 h to 10 h, and the expression of the target protein increased visibly with increasing induction time. Meanwhile, the content of the target protein was markedly higher than that of other proteins. The results indicated that the SbIDI protein was successfully synthesized by the transcript of the SbIDI gene in E. coli.
Figure 6. Prokaryotic expression of SbIDI gene cDNA in E. coli BL21(DE3) with IPTG induction. Lane 1 is the blank control, E. coli BL21(DE3) harbouring pET-32a vector without IPTG induction; lanes 2–7 E. coli BL21(DE3) harbouring vector pET-32a-SbIDI induced by IPTG (1 mmol/L) at 0, 2, 4, 6, 8, and 10 h, respectively; lane M is the protein marker (Solarbio, Beijing, China).
All of the above results indicated that the SbIDI gene might have a key role in the triterpenoids biosynthesis of S. baumii, and the prokaryotic transformation system of the SbIDI gene was formed. The variation of the triterpenoids content was influenced by the transcript level of the key gene. Over-expression of the key gene LS in G. lucidum increased accumulation of triterpenoids, representing a 1.65-fold increase compared with that in the wild-type strain [Citation24]. However, whether over-expression of the SbIDI gene in S. baumii could improve the triterpenoids content requires further confirmation.
Conclusions
The present study is the first to demonstrate the response of triterpenoids content to the change of SbIDI gene transcript level in S. baumii. The study identified that the change in the SbIDI gene transcript level showed a similar trend to the triterpenoids content. Furthermore, the successful expression of the SbIDI gene in E. coli BL21(DE3) will provide a theoretical basis for obtaining S. baumii strains with high production of triterpenoids.
Acknowledgement
All the authors are grateful to Tingting Sun (Department of Food Science and Engineering, College of Food Engineering, Harbin University, Harbin, PR China) for her revision and polishing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Xue Q, Sun J, Zhao MW, et al. Immunostimulatory and anti-tumor activity of a water-soluble polysaccharide from Phellinus baumii mycelia. World J Microbiol Biotechnol. 2011;27(5):1017–1023.
- Ge Q, Mao JW, Zhang AQ, et al. Purification, chemical characterization, and antioxidant activity of a polysaccharide from the fruiting bodies of sanghuang mushroom (Phellinus baumii Pilat). Food Sci Biotechnol. 2013;22(2):301–307.
- Yayeh T, Lee WM, Ko D, et al. Phellinus baumii ethyl acetate extract alleviated collagen type II induced arthritis in DBA/1 mice. J Nat Med. 2013;67(4):807–813.
- Su HG, Zhou QM, Guo L, et al. Lanostane triterpenoids from Ganoderma luteomarginatum and their cytotoxicity against four human cancer cell lines. Phytochemistry. 2018;156:89–95.
- Song X, Xiao H, Luo SW, et al. Biosynthesis of squalene-type triterpenoids in Saccharomyces cerevisiae by expression of CYP505D13 from Ganoderma lucidum. Bioresour Bioprocess. 2019; 6:1–10.
- Lu X, Sun LD, Zhang YX, et al. New barrigenol-type triterpenoids with anti-Alzheimer’s disease activity from Koelreuteria paniculata Laxm. J Funct Food. 2019;61:103459.
- Sun TT, Zou L, Zhang LF, et al. Methyl jasmonate induces triterpenoid biosynthesis in Inonotus baumii. Biotechnol Biotechnol Equip. 2017;31(2):312–317.
- Ye LY, Liu SR, Xie F, et al. Enhanced production of polysaccharides and triterpenoids in Ganoderma lucidum fruit bodies on induction with signal transduction during the fruiting stage. PloS One. 2018;13(4):e0196287.
- Potter D, Miziorko HM. Identification of catalytic residues in human mevalonate kinase. J Biol Chem. 1997;272(41):25449–25454.
- Lange BM, Croteau R. Isopentenyl diphosphate biosynthesis via a mevalonate-independent pathway: isopentenyl monophosphate kinase catalyzes the terminal enzymatic step. Proc Natl Acad Sci USA. 1999;96(24):13714–13719.
- Liao ZH, Chen M, Yang YJ, et al. A new isopentenyl diphosphate isomerase gene from sweet potato: cloning, characterization and color complementation. Biologia. 2008;63(2):221–226.
- Sun J, Zhang YY, Liu H, et al. A novel cytoplasmic isopentenyl diphosphate isomerase gene from tomato (Solanum lycopersicum): cloning, expression, and color complementation. Plant Mol Biol Rep. 2010;28(3):473–480.
- Sun J, Wang SX, Wang XT, et al. Cloning and expression analyses of a cellobiohydrolase gene from Auricularia heimuer. Biotechnol Biotechnol Equip. 2019;33(1):1327–1334.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408.
- Su XH, Yin L, Chen SQ. Cloning and expression of cDNA encoding key enzymes (isopentenyl diphosphate isomerase) in diterpenoids biosynthesis pathway from Rabdosia rubescens Henrsl. Hara. Northern Horticulture (Chinese). 2016;12:80–85.
- Shang CH, Zhu F, Li N, et al. Cloning and characterization of a gene encoding HMG-CoA Reductase from Ganoderma lucidum and its functional identification in yeast. Biosci Biotechnol Biochem. 2008;72(5):1333–1339.
- Shi L, Qin L, Xu YJ, et al. Molecular cloning, characterization, and function analysis of a mevalonate pyrophosphate decarboxylase gene from Ganoderma lucidum. Mol Biol Rep. 2012;39(5):6149–6159.
- Wang XT, Sun TT, Sun J, et al. Molecular cloning, characterisation and heterologous expression of farnesyl diphosphate synthase from Sanghuangporus baumii. Mol Biotechnol. 2020;62(2):132–141.
- Xu YN, Xia XX, Zhong JJ. Induction of ganoderic acid biosynthesis by Mn2+ in static liquid cultivation of Ganoderma Lucidum. Biotechnol Bioeng. 2014;111(11):2358–2365.
- Huang C, Zhong JJ. Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process Biochem. 2013;48(8):1227–1234.
- Li ZD, Ji J, Wang G, et al. Cloning and heterologous expression of isopentenyl diphosphate isomerase gene from Lycium chinense. J Plant Biochem Biotechnol. 2016;25(1):40–48.
- Pan XC, Chen M, Liu Y, et al. A new isopentenyl diphosphate isomerase gene from Camptotheca acuminata: cloning, characterization and functional expression in Escherichia coli. DNA Seq. 2008;19(2):98–105.
- Cheng ZX, Hu HT, Cheng LP, et al. Molecular characterisation and regulation of expression of a novel isopentenyl diphosphate isomerase 1 gene from autumn olive fruit (Elaeagnus umbellata). J Hortic Sci Biotech. 2015;90(6):635–642.
- Zhang DH, Li N, Yu XY, et al. Overexpression of the homologous lanosterol synthase gene in ganoderic acid biosynthesis in Ganoderma lingzhi. Phytochemistry. 2017;134:46–53.