Abstract
Here we report the phycosynthesis of silver nanoparticles (Ag-NPs), using Codium capitatum extract, and the synthesis of fungal chitosan nanoparticles (FC-NPs), using extracted chitosan from Aspergillus niger mycelia. Then nanoconjugates from FC/Ag-NPs were produced and evaluated. The synthesized NPs had mean particles’ size diameters of 37.2, 68.4 and 79.6 nm for Ag-NPs, FC-NPs and FC/Ag-NPs, respectively. The FTIR (Fourier-transform infrared spectroscopy) analysis of synthesized NPs indicated their cross-linkage and interaction. The antibacterial activity of each type of NPs was assayed against drug resistant pathogens of Salmonella Typhimurium and Staphylococcus aureus. All NPs had powerful inhibitory effect and FC/Ag nanoconjugates had stronger activity than the other types. Scanning micrographs of FC/Ag-NPs treated S. Typhimurium elucidated vigorous alterations in cell surfaces and lethal damage to bacterial structure after 8 h of treatment. The nanoconjugates form FC-NPs and Ag-NPs had minute particle size with increasing bioactivity as antimicrobial agents to control drug resistant bacterial pathogens, which recommends their further exploration for topical applications in biomedical sectors.
Introduction
The synthesis and applications of nanomaterials (NM) are among the hottest topics in biomedical and health-related fields, because of the NM augmented bioactivity and applicability [Citation1]. Nanoparticles (NPs) of metals have been extensively assayed as effective microbicidal agents against a wide range of pathogens. Metal NPs could be safely applied into human systems for inhibiting pathogens’ growth without harmful effects on contacted tissues [Citation2].
Silver (Ag) salts have been proved historically to have strong antimicrobial actions, which increased their applications for treatment of numerous infections (including burn wounds, dental work and catheters) [Citation3]. The Ag-NPs have bioactivities suitable for usage as antimicrobial, anticancer and antioxidant agents [Citation4–6]. The successful green synthesis of Ag-NPs has been reported in several investigations, using biological compounds from plant, algal and biopolymer origins [Citation7–11]. This biosynthesis generated biologically-effective NPs with particle size (PS) and structures comparable to chemically synthesized particles.
The application of algal biomass/extracts as precursors in Ag-NPs synthesis (phycosynthesis) has currently become widespread due to their high biosafety and efficiency in generating NPs with favorable bioactive attributes [Citation12–16]. However, the algal biomolecules responsible for the biosynthesis of metals NPs are comparatively less exploited than other related sources from plants or microorganisms [Citation2].
Chitosan (Cts) is the chitin-derived biopolymer obtained after deacetylation; Cts has various applications in biomedical, nutritional, environmental, health-care and biotechnological fields [Citation17]. Cts has very promising antimicrobial action toward numerous microbial genera, and its biosafety has been confirmed for human and animals [Citation18]. The antimicrobial activity of Cts could depend on various factors (including the deacetylation degree, pH, pKa, molecular weight, the interaction with metal cations and the targeted microbial species) [Citation19]. The complexes of Cts with metals are reported to possess augmented microbicidal activities [Citation20].
The biomedical applications of Cts, either individually or combined with further polymers, bioactive compounds or metal NPs, have been extensively examined for antimicrobial therapy and wound dressing [Citation21]. Cts-based nanomaterials have become an attractive research area because these nanocomposites have unique attributes (biodegradability, biocompatibility, non-toxicity, enhanced antimicrobial and antitumor activity, etc). The Cts/metal nanocomposites have been recommended for numerous biomedical applications [Citation8,Citation18,Citation22–24].
The production of Cts from grown fungal mycelia has recently gained increasing attention due to the sustainability, simplicity and efficacy of this technique [Citation25,Citation26]. Fungal chitosan (FC) has comparable bioactivities to standard Cts from crustacean shells for its potential applications as preservative, absorber, anti-cholesterol, anti-diabetic, antimicrobial, antitumor and drug-carrier agent [Citation26–29].
The emergence of infectious pathogens resistant to multiple antibiotics has become a serious health threat worldwide. This microbial resistance involves multifactorial functions such as bacterial cell/antibiotic interaction, antibiotic type and action, environmental factors, host characteristics and antibiotic dosage [Citation30]. The spread of drug resistant (DR) microbial species urged has researchers to discover innovative microbicidal agents/composites from any available origin as alternatives to accustomed antimicrobial/chemotherapeutic compounds [Citation31].
Accordingly, the phycosynthesis of Ag-NPs using marine algae (Codium capitatum) and their combination with FC-NPs to generate innovative antibacterial agent against DR pathogens, were planned and performed in the present investigation.
Materials and methods
Algae extract preparation
The algal samples (Codium capitatum; family: Chlorophyceae) were collected from the Red Sea coastal regions, near Jeddah, KSA. Algae were cleaned and repeatedly washed with deionized water (DIW) and lyophilized. Dried powder of C. capitatum (2.0 g) was immersed in 150 mL of DIW and shaken for 22 min at 62 ± 2 °C, then paper filtered (using Whatman No. 1 paper) and the clear filtrate was held at 4 °C.
Phyco-synthesis of Ag-NPs
Fresh 1.0 mmol/L solution of AgNO3 (Sigma Co. LLC) in DIW was prepared; 85 mL of this solution were incubated with 15 mL of algae extract and were kept statically in a dark container for 36 h without heating. The formed brownish mixture was centrifuged (at 8200 g for 20 min, without cooling) and the sedimented pellet was washed with DIW and lyophilized [Citation13].
Synthesis of loaded nano-fungal chitosan with Ag-NPs
Fungal chitosan extraction
Grown fungal biomass (mycelia) of Aspergillus niger (ATCC-16404), after aerobic agitated propagation in Czapek Dox (Oxoid, UK) broth for 8 days, were harvested by centrifugation (4200 g for 25 min) and oven dried at 46 ± 2 °C.
The FC extraction was done based on Tayel et al. [Citation25]; including treatment of mycelia with 1 mol/L NaOH, washing, treatment with 8% (v/v) acetic acid (overnight, with shaking at 140 rpm and 60 °C), washing, then the immersion and homogenization of dried mycelia with of concentrated NaOH (15 mol/L, 30-fold v/w) and autoclaving for 90 min at 110 °C.
The calculation of FC de-acetylation degree (DD) depended on the polymer infra-red spectrum, and the titration method of Davis and Hayes (1988) [Citation32].
Nanocomposite synthesis
A solution of 0.1% (w/v) FC in acetic acid solution (1%, v/v) was prepared, filtered and pH adjusted to 6.1. TPP (Sodium tripolyphosphate, Sigma), dissolved in DIW at a concentration of 0.1% (w/v), was also prepared. The FC-NPs synthesis was conducted via slow fine dropping of TPP solution into FC solution while it was vigorously stirred without heating, until reaching a weight ratio of 5:1 of FC: TPP [Citation26]. The stirring continued for 70 min; then the formed FC-NPs were collected via centrifugation (9100 g for 25 min). For the FC/Ag nanocomposite, the method of their synthesis was modified from Ali et al. [Citation33] protocol. Briefly, Ag-NPs (1 mmol/L) was added to the FC-NPs suspension during TPP dropping and was stirred for further 60 min before centrifugation. The separated NPs by centrifugation (at 10,050 g for 30 min) were extensively washed with DIW and re-centrifuged. The formed NPs were then lyophilized and subjected to analysis.
Nanoparticles characterization
FTIR (fourier-transform infrared) spectrophotometry
The infra-red (Fourier transform) spectra of synthesized Ag-NPs, FC-NPs, and their nano-conjugates, were assayed via FTIR (Perkin Elmer™, V. 10.03.08, Germany) over wavenumbers of 450–4000 cm−1 [Citation33].
Physico-chemical analysis of nanoparticles
The NPs size, distribution and shape of synthesized Ag-NPs, FC-NPs and FC/Ag -NPs were assessed; the Zetasizer spectroscopy (Malvern™, UK) was used for determining the size distribution and charges of NPs, after dissolving in DIW and sonication. Subsequently, transmission electron microscopy (TEM) imaging was employed, using TEM (Leica™, Leo 0430, Cambridge Ltd, UK) to determine the NPs size and morphology of FC/Ag nanoconjugates. A coated TEM grid with carbon was charged with NPs suspension and 1% uranyl acetate was added and air dried before loading for imaging.
Antimicrobial activity
Standard identified drug-resistant bacteria, i.e. Staphylococcus aureus (ATCC 43300, Methicillin and Oxacillin resistant) and Salmonella Typhimurium (Salmonella enterica subsp. enterica serovar Typhimurium - ATCC® BAA-190, multidrug-resistant), were activated and grown aerobically in Nutrient broth (NB) and Nutrient agar (NA) media at 37 °C. The antibacterial competency of synthesized NPs and nanoconjugates were evaluated (with qualitative and quantitative antimicrobial assays) using ZOI assay (inhibition zones surrounding assay disc) and MIC determination (minimal inhibitory concentration of NPs), respectively.
For ZOI assay, cell suspensions of the pathogen strains were homogenously swabbed onto NA plates, then sterilized discs (Watman No 2 filter paper, 6 mm diameter) were positioned onto inoculant surfaces and impregnated with each NPs solution (28 μL, with concentration of 50 μg/mL). After incubation at 37 ± 2 °C for 24–30 h, the ZOI diameters were precisely measured. The NPs antimicrobial bioactivities were compared with standard antibiotic discs, i.e. ceftriaxone, gentamycin and tetracycline (Fluka, Buchs, Switzerland − 30 μg/disc), as positive controls, whereas impregnated discs with DIW with 1% acetic acid were the negative controls.
The MIC of NPs (using microdilution method and confirmed by triphenyl tetrazolium chloride indicator) was determined using pathogenic strains, at NPs concentration range of 0.1–50 μg/mL [Citation34].
Scanning microscopy imaging
The mode of FC/Ag-NPs antibacterial action against DR Salmonella Typhimurium, was studied via electron microscope (scanning) imaging (SEM, JEOL JSM-6301F, Japan), after exposure to NPs (at a concentration of 5 μg/mL) for 4 h and 8 h. The micrographs captured the alterations in the morphology and shape of the exposed cells.
Results and discussion
In this study, Ag-NPs, FC-NPs and their nanocomposite were were successfully synthesized. The characteristics of the synthesized NPs were biochemically, physically and biologically evaluated.
FTIR analysis
FTIR analysis of NPs was done to identify the potential responsible biomolecules for the activity and stability of the synthesized NPs (). The leading characteristic peaks, for FC-NPs structure (-FC-NPs) were observed at 653 cm−1 (-OH vibrations), 1421 cm−1 (–CH2 vibrations), 1566 cm−1 (N-H bending of primary amine), 1645 cm−1 (C = O stretch of secondary amide). The broad band at 3428 cm−1 (-H intermolecular bonds) indicated the potential overlapping (between the O − H stretching vibration and the N − H stretching vibration of the polysaccharide moieties), while the band at 2924 cm−1 is due to the aliphatic C − H stretching vibrations of the bonds [Citation26]. The detection of chitosan distinctive biochemical and structural bonds in the FC-NPs FTIR spectrum evidenced the minimal chemical alteration and structural variation that possibly emerged during FC-NPs synthesis [Citation29].
Figure 1. FTIR spectra of nanoparticles synthesized from silver (Ag-NPs), fungal chitosan (FC-NPs) and their nanocomposite.
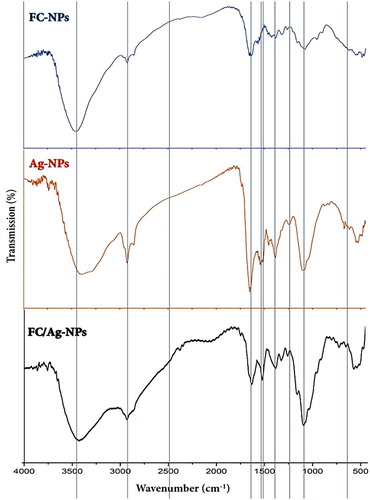
The FTIR spectrum of phycosynthesized Ag-NPs is plotted in . A strong band (corresponding to the bending vibration of secondary amines of proteins) appeared at 1533 cm−1. The observed band at 1632 cm−1 is assumingly assigned to stretched group vibration of (NH) C = O. After Ag-NPs phycosynthesis, the decrease in the band intensity at 1533 cm−1 signified the contribution of algal secondary amines in NPs reduction process. The band at 1632 cm−1 is probably correlated to the (NH) C = O group within the formed NPs; these groups are commonly involved in NPs stabilization [Citation35,Citation36]. Additionally, this band indicates the major involvement of algal peptides in Ag-NPs synthesis. At 1054 cm−1, the detected band could be potentially assigned to the ‒C‒O‒C absorption peaks [Citation7].
The strong band at 1514 cm−1 in Ag-NPs spectrum was assigned to C = C chain stretching vibrations [Citation13]. The distinctive bands at 1054– 1232 cm−1 could indicate the C‒N involvement in the plane vibrations in aliphatic amines; these bonds are frequently detected in proteins, which also indicates the role of algal proteins as ligands for Ag-NPs synthesis and stability [Citation10]. The distinctive peak at 1232 cm−1, in the spectrum of phycosynthesized Ag-NPS, signifies the asymmetric vibration of sulfate groups that commonly exist in seaweeds as components in sulfated polysaccharides [Citation37]. The above findings support those of Venkatpurwar & Pokharkar's (2011) [Citation9], which indicated that marine alga sulfated polysaccharides (from Porphyra vietnamensis) had a powerful capability to synthesize Ag-NPs. This is also in agreement with other previous reports that appointed the presence of sulfated polysaccharides in seaweeds [Citation13,Citation38].
In the IR spectrum of FC/Ag nanocomposite ( FC/Ag-NPs), many distinctive bands were detected, corresponding to relevant bands in plain FC-NPs or Ag-NPs (indicated with the vertical lines in the figure), which indicates the incidence of cross-linkage between the two nanoparticles’ types. The FC/AG-NPs spectrum had some alternations, comparing with the FC-NPs spectrum; the FC characteristic bands, at 1645 cm−1 and 1566 cm−1, shifted to 1631 cm−1 with remarkable transmittance decrement in this region. Moreover, the band intensity at 3428 cm−1 decreased and shifted to wavenumber of 3409 cm−1, which designates the Ag-NPs chelation by both hydroxyl and amino groups of FC-NPs [Citation6].
Physiognomic analysis of nanoparticles
The physiochemical properties of synthesized Ag-NPs, FC-NPs and their nanocomposite are presented in . The NPs and nanocomposite had median and mean PS diameters of ˂ 85 nm. The average diameter (37.2 nm) of the biosynthesized Ag-NPs indicated the ability of the C. capitatum algal extract to reduce Ag-PS to the nano-scale, without any further treatment. The efficacious phycosynthesis of Ag-NPs in the present work, with minute average diameter was in agreement with previous reports employing different seaweed extracts to stimulate NPs biosynthesis [Citation13–15,Citation39]. The mean diameters of these phycosynthesized Ag-NPs ranged from 5 to 83 nm, according to the seaweed extracts and NPs synthesis conditions. Numerous functional enzymes/groups are found in algal cells, which could be reducing agents for NPs fabrication from metals and their oxides [Citation40].
Table 1. Characteristic attributes of nanoparticles synthesized from silver (Ag-NPs), fungal chitosan (FC-NPs) and their nanocomposite.
The FC-NPs were efficiently synthesized via TPP ionic-gelation technique, with high positive charges (+ 26.3 mV) and minute PS average. The phycosynthesized Ag-NPs, using C. capitatum extract, had spherical shapes and diverse particle sizes, which are possibly due to the algal polysaccharides reducing power. The spherical and potentially irregular shapes of Ag-NPs, with different morphology and dimensions were reported to vary depending on the algal species used for biosynthesis [Citation16]. Ag/FC nanocomposite exhibited outstanding characteristics regarding their combined PS mean (79.6 nm) and particle charges (21.9 mV), which indicated the cross-linkage and combination between the two NP types. The Ag/FC nanocomposite had PS diameter and NP charge of lesser values than the sum of their individual particles. The estimated Ag-NPs size in the FC/Ag-nanocomposite, which was smaller than the size of plain phycosynthesized Ag/NPs, could be a result of their extra reduction throughout FC-NPs synthesis and interaction.
In the regular FC/Ag-NPs synthesis, the polyanion TPP could agglutinate FC units as templates, which attract Ag+ particles inside them. TPP could enhance the Ag+ diffusion process and could regulate the formed Ag-NPs size. Also, the FC glucosamine units act as reducing agents for modulating and decreasing Ag-NPs size [Citation22].
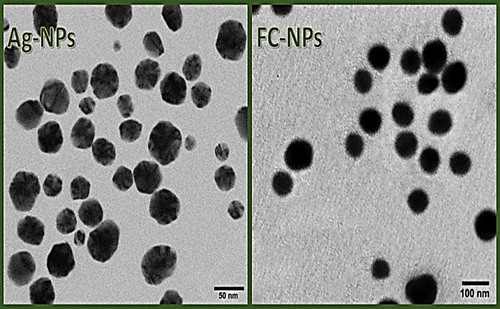
The TEM micrographs of synthesized NPs indicated their structural features and distributions (). The phyco-synthesized Ag-NPs appeared with spherical shapes, good distribution and PS range of 13.8‒68.9 nm (), whereas the FC-NPs had more uniformed PS (with a range of 55.2‒118.9 nm) and uniform shapes (); they also had good distribution within the dissolving solution, with no apparent agglomerations. The diminutive particle size (Ps) mean and relatively high zeta potential positivity, of fabricated FC/Ag-NPs, contributed in the augmented stability and bioactivity of combined NPs during the interaction with bacterial cells. The Ps and surface charges of NPs were reported to critically influence the NPs delivery rate. The NPs size could significantly govern their distribution inside cells, while the NPs charges enable their stability in the structure [Citation1].
Antibacterial activity of synthesized nanoparticles
The antibacterial activities of the synthesized NPs against DR strains of S. Typhimurium and S. aureus were demonstrated via quantitative and qualitative antimicrobial assays (). Where the control treatments (using 1% acetic acid solution) did not exhibit any biocidal activities against bacteria, the individual and composited NPs were able to inhibit the DR bacterial strains, but with varying capabilities. Generally, Ag-NPs were significantly more forceful than FC-NPs in their antibacterial activity, the combined nanocomposite from them (Ag/FC-NPs) exhibited significantly more powerful biocidal activity than each individual type of NPs. The two DR bacterial strains were sensitive to all NPs, but with no significance between the gram-positive (S. aureus) and gram-negative (S. Typhimurium) strains toward each NPs type (). The positive controls in the antibacterial assay (using ceftriaxone-, gentamycin- and tetracycline-loaded discs) confirmed the DR attributes of the examined strains. S. aureus was resistant to ceftriaxone and S. Typhimurium was resistant tetracycline. The Ag/FC-NPs had significantly stronger antibacterial activity than ceftriaxone and equivalent activity with gentamycin toward S. Typhimurium, whereas no significant difference was recorded between the antibacterial activity of Ag/FC-NPs and tetracycline toward S. aureus.
Table 2. Antimicrobial potentialities of nanoparticles synthesized from silver (Ag-NPs), fungal chitosan (FC-NPs) and their nanocomposite (Ag/FC-NPs) against drug resistant bacterial pathogens.
The bactericidal effects of Ag-NPs possibly depend on their self-organizing and spontaneous properties, which allow the NPs to transform into Ag ions, and accordingly cause generation of ROS (reactive oxygen species) that increase their biocidal action [Citation5]. Moreover, Ag-NPs could possibly disrupt the bacterial cell signaling pathways, which are associated with the tyrosine phosphorylation of cell wall proteins, leading to cytoplasmic membrane delamination and cell wall degradation, then finally cell death [Citation40]. Furthermore, Ag ions were suggested to cause DNA condensation, owing to DNA replication failure inside bacterial cells [Citation11,Citation41].
The high positively charged FC-NPs surfaces can favor their docking onto biological surfaces (negatively charged) and facilitate the penetration of other NPs from them. The Ag-NPs could be easily diffused outside containing FC-NPs and could interact with cell membranes. These were previously described regarding the interaction between Ag/CS-NPs and adenocarcinoma cells [Citation23].
The combined antibacterial activity of FC/Ag-NPs strongly depended on the small Ps of the entire nanoparticles, with positively charged surfaces, which enable their interaction with exact areas in microbial cell surfaces [Citation22].
The FC/Ag-NPs antibacterial activities did not significantly differ toward the examined gram-positive and gram-negative strains, indicating the capability of the synthesized nanoconjugates to interact with bacterial cells via diverse mechanisms, rather than specific interaction with cell membranes. Many previous investigations have reported such insignificant differences regarding Ag-NPs and FC/Ag-NPs antibacterial actions and growth inhibition toward different bacterial pathogens [Citation42–44]. The positive charges of FC/Ag-NPs are assumed to strengthen their interaction with bacterial cells via a governed electrostatical docking process, which is a natural dynamic mechanism, as most cellular components have negatively charged surfaces [Citation42].
The fabricated nanocomposites (FC/Ag-NPs) could be suggested as ideal candidates for topical applications, e.g. for skin disinfection formulations, hygienic textiles finishing, wound healing and disinfection, tissue engineering, etc., due to their favorable and applicable attributes for these issues.
The hybrid composites from natural polymers and nanometals are a promising class of bioactive materials; the intrinsic features of Cts could be employed for preventing the potential negative risks from metal NPs and augmenting their applications in numerous biomedical areas [Citation45]. The Cts incorporation with nanometals considerably affects their colloid steric stabilization, generates further functional groups for conjugating biomolecules, protects nanometals from extra oxidation/reduction, increases their biocompatibility and reduces their cytotoxicity [Citation46]. The antibacterial action of in situ prepared Cts/Ag-NPs against DR S. aureus was stated to provide a prospective progress for personalized medicine [Citation47].
Recent researches illustrated the effectiveness of Cts-based formulations in miscellaneous pharmaceutical and biomedical applications, e.g. gene delivery, drug-delivery, tissue engineering, cell encapsulation, bioimaging, wound healing, antibacterial food packaging, etc. [Citation48]. The ASTM (American Society of Testing Materials, F04 division IV) established standard guidelines for suitable and recommended usage of Cts-based materials in tissue-engineering products, pharmaceutical and biomedical applications [Citation49]. Currently, chitosan applications for wound dressings and cartilage repairing formulations are approved [Citation46].
NPs mode of action observed using scanning microscopy
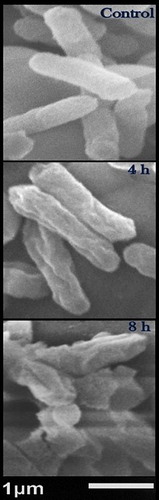
The bactericidal action of Ag/FC nanocomposite was further elucidated against S. Typhimurium, via SEM imaging (). The surface of the control cells appeared with normal topography (smooth, uniform surface and healthy appearance). After 4 h of exposure to Ag/FC-NPs, the bacterial cells were shrunken and distorted, with obvious gaps and chasms formed in their surfaces (). By the end of the exposure (after 8 h), the morphological alterations in the cells’ surface increased and many cells were ruptured or completely lysed.
Figure 3. Scanning micrographs of resistant Salmonella Typhimurium treated with silver/fungal chitosan nanocomposite for 4 h and 8 h, compared with control (non-treated) cells.
Note: The examined Ag/FC-NPs concentration was 5 µg/mL
The cumulative antibacterial action of NPs composed of the cationic chitosan and Ag-NPs was proven via electrostatic interaction between positively charged polymer NPs and the negative charged bacterial wall components, which eventually cause membrane disruption and cell death [Citation8,Citation50]. The inhibitory mechanisms of Ag-NPs toward microorganisms has been proposed (based on electron spin resonance spectroscopy and the antioxidant properties of NPs) to depend on the derived unparalleled free radicals from Ag-NPs surface, which affect lipid membranes of microorganisms and lead to the failure of membranes functionalities [Citation4,Citation51].
Generally, NPs uptake by bacterial cells was stated to have a mechanism with two-steps. Firstly the NPs bind onto cell membranes (majorly governed by the electrostatic interactions), then the internalization step follows. The electrostatic interactions of Cts-coated metal NPs are much stronger and lead to higher adsorption capability and higher internalization [Citation45].
Photocatalytic ROS could be generated from Ag-NPs in the presence of UV light, but some experimental proof demonstrated their large ROS production even in darkness [Citation52]. In accordance with the present results, the Ag-NPs have been reported to stimulate free radical development, which causes destructive damage to microbial cellular membranes [Citation16], which was observed via SEM imaging of exposed bacterial cells to NPs. The free radical generation from NPs and their toxic effect on numerous enzymes in unicellular pathogens was reported [Citation4]; without any harmful consequences on human enzymes.
Conclusions
The phycosynthesis of Ag-NPs, using C. capitatum, could be recommended to generate metal NPs by an eco-friendly procedure. The nanoconjugates form fungal chitosan NPs and Ag-NPs had minute particle size with increasing bioactivity as antimicrobial agents to control drug resistant bacterial pathogens, which recommends their topical applications in biomedical sectors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sahoo SK, Parvee S, Panda J. The present and future of nanotechnology in human health care. Nanomedicine. 2007;3(1):20–31.
- Siddiqi KS, Husen A. Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Res Lett. 2016;11(1):363–373.
- Crabtree JH, Burchette RJ, Siddiqi RA, et al. The efficacy of silver-ion implanted catheters in reducing peritoneal dialysis-related infections. Perit Dial Int. 2003;23(4):368–374.
- Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3(1):95–101.·
- Jeyaraj M, Sathishkumar G, Sivanandhan G, et al. Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids Surf B Biointerfaces. 2013;106:86–92.
- Chen Q, Jiang H, Ye H, et al. Preparation, antibacterial, and antioxidant activities of silver/chitosan composites. J Carbohydr Chem. 2014;33(6):298–312.
- Jain R, Jordan N, Tsushima S, et al. Shape change of biogenic elemental selenium nanomaterials from nanospheres to nanorods decreases their colloidal stability. Environ Sci: Nano. 2017;4(5):1054–1063.
- Wei D, Sun W, Qian W, et al. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr Res. 2009;344(17):2375–2382.
- Venkatpurwar V, Pokharkar V. Green synthesis of silver nanoparticles using marine polysaccharide: study of in-vitro antibacterial activity. Mater Lett. 2011;65(6):999–1002.
- Jacob SJ, Finub JS, Narayanan A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf B Biointerfaces. 2012;91:212–214.
- Davoodbasha MA, Kim SC, Lee SY, et al. The facile synthesis of chitosan-based silver nano-biocomposites via a solution plasma process and their potential antimicrobial efficacy. Arch Biochem Biophys. 2016;605:49–58.
- El-Rafie HM, El-Rafie MH, Zahran MK. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr Polym. 2013;96(2):403–410.
- Kannan RR, Stirk WA, Van Staden J. Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S Afr J Bot. 2013;86:1–4.
- Kumar P, Senthamil Selvi S, Govindaraju M. Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl Nanosci. 2013;3(6):495–500.
- Kathiraven T, Sundaramanickam A, Shanmugam N, et al. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl Nanosci. 2015;5(4):499–504.
- Patel V, Berthold D, Puranik P, et al. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep (Amst)). 2015;5:112–119.
- Honarkar H, Barikani M, Honarkar H, et al. Applications of biopolymers: I. Monatsh Chem. 2009;140(12):1403–1420.
- Goy RC, Britto D, Assis OB. A review of the antimicrobial activity of chitosan. Polímeros. 2009;19(3):241–247.
- Fei Liu X, Guan LY, Zhi YD, et al. Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci. 2001;79(7):1324–1335.
- Wang X, Du Y, Fan L, et al. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and structure-activity study. Polym Bull. 2005;55(1-2):105–113.
- Li J, Cai C, Li J, et al. Chitosan-based nanomaterials for drug delivery. Molecules. 2018;23(10):2661.
- Rodríguez-Argüelles MC, Sieiro C, Cao R, et al. Chitosan and silver nanoparticles as pudding with raisins with antimicrobial properties. J Colloid Interface Sci. 2011;364(1):80–84.
- Sanpui P, Chattopadhyay A, Ghosh SS. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl Mater Interfaces. 2011;3(2):218–228.
- Yu B, Liu T, Du Y, et al. X-ray-responsive selenium nanoparticles for enhanced cancer chemo-radiotherapy. Colloids Surf B Biointerfaces. 2016;139:180–189.
- Tayel AA, Ibrahim SA, Al-Saman MA, et al. Production of fungal chitosan from date wastes and its application as a biopreservative for minced meat. Int J Biol Macromol. 2014;69:471–475.
- Alsharari S, Tayel AA, Moussa SH. Soil emendation with nano-fungal chitosan for heavy metals biosorption. Int J Biol Macromol. 2018;118(Pt B):2265–2268.
- Moussa SH, Tayel AA, Al-Turki AI. Evaluation of fungal chitosan as a biocontrol and antibacterial agent using fluorescence-labeling. Int J Biol Macromol. 2013;54:204–208.
- Tayel AA, Gharieb MM, Zaki HR, et al. Bio-clarification of water from heavy metals and microbial effluence using fungal chitosan. Int J Biol Macromol. 2016;83:277–281.
- El Rabey HA, Almutairi FM, Alalawy AI, et al. Augmented control of drug-resistant Candida spp. via fluconazole loading into fungal chitosan nanoparticles. Int J Biol Macromol. 2019;141:511–516.
- Desselberger U. Emerging and re-emerging infectious diseases. J Infect. 2000;40(1):3–15.
- Cederlund H, Mårdh PA. Antibacterial activities of non-antibiotic drugs. J Antimicrob Chemother. 1993;32(3):355–365.
- Davis HD, Hayes ER. Determination of degree of acetylation of chitin and chitosan. Methods Enzymol. 1988;161:442–446.
- Ali SW, Rajendran S, Joshi M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr Polym. 2011;83(2):438–446.
- Tayel AA, El-Tras WF, Moussa SH, et al. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J Food Saf. 2011;31(2):211–218.
- Huang J, Li Q, Sun D, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnanonum camphora leaf. Nanotechnology. 2007;18(10):105104.
- Philip D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim Acta A Mol Biomol Spectrosc. 2009;73(2):374–381.
- Witvrouw M, De Clercq E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol. 1997;29(4):497–511.
- Matsubara K, Matsubara Y, Bacic A, et al. Anticoagulant properties of a sulfated galacton preparation from a marine alga, Codium cylindricum. Int J Biol Macromol. 2001;28(5):395–399.
- Selvam GG, Sivakumar K. Phycosynthesis of silver nanoparticles and photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Hypnea musciformis (Wulfen) J.V. Lamouroux. Appl Nanosci. 2015;5(5):617–622.
- Jung WK, Koo HC, Kim KW, et al. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74(7):2171–2178.
- Crookes-Goodson WJ, Slocik JM, Naik RR. Bio-directed synthesis and assembly of nanomaterials. Chem Soc Rev. 2008;37(11):2403–2412.
- Feng QL, Wu J, Chen GQ, et al. A mechanistic study of the antibacterial effect of silver ions on Escherchia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–668.
- Roy H, Dare K, Ibba M. Adaptation of the bacterial membrane to changing environments using aminoacylated phospholipids: MicroCommentary. Mol Microbiol. 2009;71(3):547–550.
- Yoksan R, Chirachanchai S. Silver nanoparticle-loaded chitosan–starch based films: Fabrication and evaluation of tensile, barrier and antimicrobial properties. Mater Sci Eng: C. 2010;30(6):891–897.
- Mohammad F, Al-Lohedan HA, Al-Haque HN. Chitosan-mediated fabrication of metal nanocomposites for enhanced biomedical applications. Adv Mater Lett. 2017;8(2):89–100.
- Yaneva Z, Ivanova D, Nikolova N, et al. The 21st century revival of chitosan in service to bio-organic chemistry. Biotechnol Biotechnol Equip. 2020;34(1):221–237.
- Elgorban AM, El-Samawaty AM, Yassin MA, et al. Antifungal silver nanoparticles: synthesis, characterization and biological evaluation. Biotechnol Biotechnol Equip. 2016;30(1):56–62.
- Shariatinia Z. Pharmaceutical applications of chitosan. Adv Colloid Interface Sci. 2019;263:131–194.
- American Society of Testing Materials - ASTM F2103-01 2001. Standard guide for characterization and testing of chitosan salts as starting materials intended for use in biomedical and tissue-engineered medical product applications. West Conshohocken, PA: ASTM International.
- Sambhy V, MacBride MM, Peterson BR, et al. Silver bromide nanoparticle/polymer composites: dual action tunable antimicrobial materials. J Am Chem Soc. 2006;128(30):9798–9808.
- Sarasam AR, Brown P, Khajotia SS, et al. Antibacterial activity of chitosan-based matrices on oral pathogens. J Mater Sci Mater Med. 2008;19(3):1083–1090.
- Choi O, Hu ZQ. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol. 2008;42(12):4583–4588.