Abstract
Overweight and obesity are serious and an ever-growing problem in modern society. It is a major risk factors for a number of chronic diseases, including type 2 diabetes, cardiovascular diseases and cancer. Obesity is a complex condition resulting from the interaction of a range of genetic and environmental factors. The aim of this study was to identify genetic markers predisposing to, and molecular pathways associated with, obesity in Bulgarian healthy individuals. Whole-exome sequencing was performed on two DNA pools: one constructed of 32 Bulgarian centenarians and one of 61 young healthy individuals, both with normal BMI, and allele frequencies of detected variants were estimated for each pool. Centenarians were chosen as their exome could be considered ‘golden standard’ for health and longevity, including being free of genetic variants predisposing to obesity. The young individuals group was chosen so variants predisposing to obesity after adolescence can be evaluated when compared to the centenarians. Of all variants designated to be associated with obesity by the database DisGeNET, only 17% were discovered in the studied pools. Using the platform ToppGene, we identified three over-represented pathways based on genes with variants showing significant prelevance in allele frequency in the young individuals group. These three pathways were all G-protein coupled receptor associated pathways: the GPCR ligand binding pathway, the G alpha (s) signalling events pathway and the Class A/1 (Rhodopsin-like receptors) pathway. Understanding the genetic etiology of obesity in different populations is instrumental in developing pharmacological targets for population-specific obesity therapies.
Introduction
Obesity is a risk factor for а number of socially significant and even life-threatening diseases such as type 2 diabetes, cardiovascular disease and cancer [Citation1]. According to the World Health Organization (WHO), worldwide obesity has nearly tripled since 1975 [2]. It was estimated that in 2016 more than 650 million adults worldwide were obese, and 340 million children and adolescents aged 5–19, along with 41 million under the age of 5, were either overweight or obese [Citation2]. Bulgarian children are at higher risk of being obese or severely obese, around 13%, compared to typically below 10% for Western and Northern European countries [Citation3].
Obesity is a complex condition resulting from the interaction of a range of genetic and environmental factors. Various studies have demonstrated that genetic factors play role in obesity. A twin study performed in the United States estimated heritability for weight and BMI at age 25 years to be 0.81 and 0.84, respectively [Citation4], and obesity risk has been estimated to be two to eight times higher for a person with a family history as opposed to a person with no family history of obesity [Citation5]. Genome-wide association studies have found association of genetic loci with obesity phenotypes, but as these are not easily replicated in different populations much of the heritability of BMI remains unexplained [Citation6].
Identification of genes associated with obesity, and genes interacting with environmental factors that cause obesity, will help clarify the etiology of obesity. Pathway analyses infer the functional significance of a set of genes with over-represented variants. Such approach sets in context the interpretation of multiple comparisons that are inherent to genome-wide approaches. This also facilitates identifying at-risk individuals or groups in terms of their genetic profile and helps develop personalized prevention and treatment strategies. Proper interpretation of the results of the genetic test and providing a personal diet and exercise programs, as well as the inclusion of specialized therapy, are the keys for obesity prevention.
In this study, the allele frequency of variants in obesity-associated genes was compared between Bulgarian centenarians, a cohort devoid of obese individuals and young and healthy Bulgarian subjects, also non-obese, but potentially at risk of developing obesity as adults. The molecular pathways that contain the set of genes with over-represented variants were then examined. The genetic predisposition to obesity in the young, and so far healthy subjects, could, thus, be evaluated.
Subjects and methods
Ethics statement
This study was approved by the Ethics commitee of the Medical University of Sofia in accordance with national and international legislation. Each participant was acquainted with the purpose of the study and signed informed consent forms.
Study subjects
In the present study two Bulgarian age groups were surveyed: one consisting of 32 centenarians (mean age 102.4) and the other of 61 young healthy individuals (mean age 21.3). Centenarians were interviewed about their lifestyle, medical history, diet, physical activity, tobacco smoking, alcohol consumption, social contacts, mood, memory status, stress episodes, financial problems, family history of long-living individuals, etc. All centenarians had normostenic habitus with BMI < 25 kg/m2. Only 2% stated that they are vegetarians, 84% consume salty foods, 86% sweet foods and 40% food items containing saturated fats. Alcohol and coffee consumption was rare or absent. Seven percent reported to be occasional smokers compared to more than 30% in the country’s population [Citation7]. Thirty-seven percent of the centenarians stated that their daily routine incorporates substantial physical activity and 63%, moderate. All centenarians reported that they have positive attitude and have preserved cognitive functions and memory. Most of them exhibited emotional stability despite difficulties of life. Seventy-six percent had family history of long-living relatives. Similarly to the centenarians in this study, none of the subjects in the young individuals group were obese. The majority reported that they do not follow any special diet, that they engage in some physical activity and report intermediate levels of stress in their daily lives. Some of them however report that they have overweight family members, as well as the presence of common diseases among relatives.
Sampling and whole-genome sequencing (WES)
Buccal swap samples were collected from centenarians and blood samples were collected from young individuals. DNA was extracted with QIAamp DNA Blood Mini Kit (Qiagen) and equimolar amounts were used for the preparation of two pools, a centenarians’ and a young individuals’ pool. Whole-exome sequencing (WES) was performed with BGI v4 chemistry on BGISEQ-500 platform (by BGI Genomics) with 250x coverage. The genetic variants called were annotated using the web-based service wANNOVAR [Citation8]. The variants were filtered as follows: number of individual reads > 30, genotype quality > 99, mapping quality > 60, and number of reads of minor allele frequency (MAF) variant > 2.
Data analysis
Contingency tables were constructed from the number of allele reads for each variant in both pools and Fisher’s exact test was performed to evaluate the significance of the allele frequency differences. False Discovery Rate (FDR) adjustment of Benjamini and Hochberg [Citation9] was used to reduce the number of false positives. All statistical analyses were performed using R scripts [Citation10].
The publicly available DisGeNET database [Citation11] was used to compile a list of variants associated with obesity, and inspect the Bulgarian exomes for their presence. The functional significance of the assemblage of genes that have variants showing significant difference between the two pools was examined using the web-based platform ToppGene [Citation12].
Results and discussion
Variants detected in Bulgarian pools
The aim of this study was to identify genetic variants predisposing to obesity by WES Bulgarian centenarians and young individuals group. The exomes of centenarians, all of whom are with normal BMI, may be expected to be devoid of variants predisposing to obesity. Comparing centenarian exomes to those of young and yet healthty individuals, might aid identifying variants predisposing to obesity after adolescence.
After applying the selected filtering criteria, 89,810 variants were detected in Bulgarian pools, 72,791 in both pools, 8253 in the centenarian pool only and 8766 in the young ingividuals’ (control) pool only. From DisGeNET, 811 variants of genes associated with obesity were selected, of which 138 were detected in our pools.
One hundred and twenty variants were identified in both pools. Of these, 27 variants had significant difference in allele frequencies between the two pools (p-FDR < .05), but 15 were found with greater frequency in the control group ( and ). Six genetic variants were identified only in the group of centenarians and 10 were identified only in the young individuals group (). Variants significantly prevalent, or detected only, in centenarians were not further analized as they could not be considered to be associated with obesity. Thus, of all variants designated to be associated with obesity by DisGeNET, only 17% were discovered in the Bulgarian pools.
Figure 1. WES variants in obesity-associated genes showing significant allele frequency difference between the centenarian and young individuals pools. The filled circles below the identity line represent variants that have significantly higher frequency in the young individual’s pool. The white circles above the identity line represent variants that have significantly higher frequency in the centenarian pool, and these were not further analyzed.
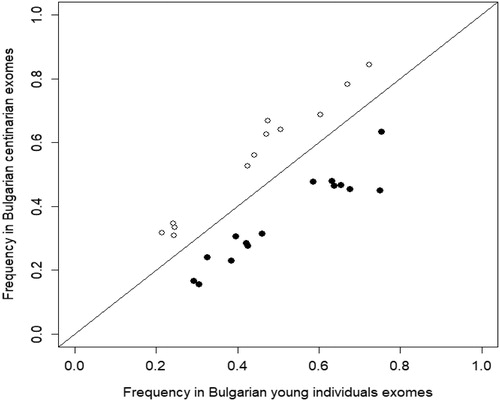
Table 1. Variants showing significant allele frequency difference between centenarian and young individuals pools.
The 23 genes of the 25 variants showing significantly higher frequency, or detected only in the young individuals’ pool (), were input in the ToppGene platform, which identified three over-represented molecular pathways. These three pathways were all G-protein coupled receptor associated pathways: the GPCR (G protein coupled receptor) ligand binding pathway, the G alpha (s) signalling events pathway and the Class A/1 (Rhodopsin-like receptors) pathway. GPCR ligand binding pathway was significant according to all statistical criteria, G alpha (s) signalling events and Class A/1 (Rhodopsin-like receptors) pathways were significant for all but the FDR B&Y criteria ( and ).
Figure 2. Over-represented pathways generated by ToppGene from the set of genes with variants showing significantly higher allele frequency, or detected only, in the young individuals pool.
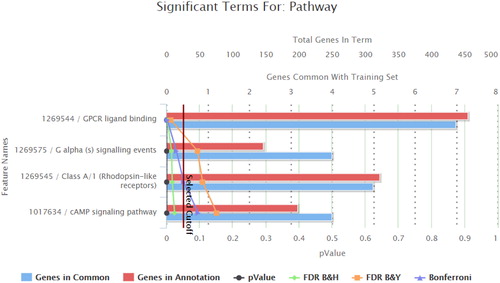
Table 2. Over-represented pathways indicated by genes with variants showing significantly higher allele frequency, or detected only, in the young individuals pool.
A cluster of 7 genes constitute the GPCR ligand binding pathway, and a subset of this cluster is involved in G alpha (s) signalling events and Class A/1 pathways ().
Table 3. Genes from ToppGene involved in over-represented pathways.
In G protein-coupled receptor associated pathways functions a large and diverse family of proteins playing roles in the transduction of extracellular stimuli into intracellular signals. The wide variety of GPCR proteins bind to a diverse set of ligands, including peptide hormones, protease peptides as well as neurotransmitters, ions, lipid-derived mediators and odor molecules. The general function of the G alpha (s) subunits is to trigger intracellular signaling as response to surface GPCR activation… The third significantly over-represented pathway is the largest group of GPCRs, Rhodopsin-like receptors (class A/1). As much as 90% of these receptors are found in the rhodopsin-like family. These include hormones, light and neurotransmitter receptors that take part in, e.g. autocrine, paracrine and endocrine processes [Citation13].
GPCRs are involved in the pathogenesis of obesity-induced type 2 diabetes mellitus (T2DM), and are important targets for the treatment of this disorder. Many GPCRs are associated with inflammation that can lead to obesity-induced T2DM as they are directly involved in the development of insulin resistance and β-cell dysfunction [Citation14]. In fact a key role in the pathogenesis of obesity-induced insulin resistance is played by the immune cell infiltration into adipose tissue causing a chronic low-grade inflammation [Citation15–18]. This is facilitated by the aging processes, sedentary lifestyle as well as high-fat diet that affects functioning of adipocytes and macrophages [Citation19]. Although there are more than 30 GPCRs implicated in the development and progression of insulin resistance and hence T2DM and obesity, so far only the Glucagon-like peptide-1 receptor (GLP-1R) has been successfully therapeutically targeted [Citation20]. Some GPCRs are also involved in nutritional regulation at central nervous system level [Citation21]. They could provide new targets for personalized treatment of obesity. One of the goals of modern pharmaceutical industry is to establish the function and therapeutic relevance of orphan GPCRs, some of which may become novel targets for treatment of obesity and related conditions [Citation22]. In fact, 34% of all FDA approved drugs target members of this receptor family [Citation23].
Gene variants and their potential role in obesity
The three GPCR pathways are constituted by a set or a subset of the seven genes with over-represented variants in the young individuals group (cf. ). Here, we consider these variants and the genes and their potential role in obesity.
GIPR (rs1800437)
The gene encodes a gastric inhibitory polypeptide receptor (GIP-R), also known as the glucose-dependent insulinotropic polypeptide receptor. A member of the G protein–coupled receptors [Citation24], it is also found on beta-cells in the pancreas [Citation25,Citation26]. G proteins activate adenylyl cyclase, thus, mediating the activity of this receptor. Its function is related not only to inhibition of gastric acid secretion and gastrin release but it also stimulates insulin release in case of elevated glucose levels [Citation27,Citation28]. It is known that obesity leads to insulin resistance and excessive insulin secretion after meal ingestion, and plasma GIP concentrations are elevated in obese and diabetes suffering subjects [Citation28]. The variant rs1800437 was detected in the young individuals group only, thus suggesting a possible role of this variant in predisposition to age-related conditions, including obesity. This is in line with results of a study on German families with at least one obese index patient that find a possible association of this variant with obesity [Citation29]. A study on obese and normal children failed to find association of this variant with obesity, but does find association with glucose homeostasis [Citation30].
SAA1 (rs12218)
This gene encodes a protein called serum amyloid A1, which is produced in the liver and circulates at low levels in the blood. This protein could play various roles in the immune system, e.g. signaling cell migration, acting as an antibacterial agent and helping repair damaged tissues. Several receptors for SAA1 have been identified. These receptors include the G protein-coupled chemoattractant receptor FPR2 (formyl peptide receptor 2) for its role in SAA1-dependent cholesterol metabolism [Citation31]. In our study, the rs12218 variant had significantly higher frequency in the young individuals’ group. The higher frequency of the C allele in the young individuals’ pool in our analysis might indicate higher predisposition to obesity in this group. A study on Chinese children found association of the rs12218 variant in this gene with obesity [Citation32]. More specifically, they found that the CC genotype of this viariant is more common in obese patients than in control subjects.
NPY2R (rs1047214, rs2880415)
The gene product is neuropeptide Y receptor type 2 (Y2R), a member of the neuropeptide Y receptor family of G-protein coupled receptors. It mediates different biological actions including stimulation of food intake and modulation of circadian rhythm, both of which are associated with obesity. In our study, we found two SNPs of NPY2R gene, rs1047214 and rs2880415, which were significantly more prevalent in the young individual group. A study on French Caucasian children with severe obesity has identified rs1047214 to be significantly associated with the waist–hip ratio [Citation33]. A study on Caucasian Utah subjects has established significant association of rs2880415 with BMI but only in males and the effect on BMI was dependent on the genotype of another locus (rs17376826) [Citation34]. Also, a study on Korean children has found that the CT and CC genotypes of this variant showed poorer abdominal and hip flexor strength and endurance, higher risk of obesity and lower physical fitness [Citation35]. Neuropeptide Y plays a key role in controlling food intake and body weight. Some studies suggest the therapeutic potential of some NPY subtypes and these are potential pharmacological targets for personalized therapy of obesity [Citation36].
ADRB1 (rs1801253)
The gene encodes an adrenergic receptor beta 1. Adrenergic receptors are a family of guanine nucleotide binding regulatory protein-coupled receptors that mediate the physiological effects of the hormone epinephrine and the neurotransmitter norepinephrine. They also mediate the catecholamine-induced activation of adenylate cyclase through the action of G proteins. Catecholamines play a role in energy expenditure and lipolysis, and genes involved in their regulation may be important in influence in the epidemiology of obesity. In our study, the alternative allele of the rs1801253 variant is with higher frequency in the young individual pool. A similar result for association with obesity was obtained by a study on 6- to 12-year-old Mexican children [Citation37]. A study on healthy 18- to 49-year-old Caucasians has, however, found that BMI was significantly higher among GG and CG carriers compared to CC in this locus [Citation38], whereas a study on Saudi population aged 18–36 years found no association between the genotype at this locus with BMI [Citation39]. In our study the C allele is with higher frequency in the young individual pool, emphasizing the need for further studies on the significance of this locus in different populations.
GNB3 (rs5443)
This gene encodes a protein subunit that regulates certain signal transduction receptors and effectors. The rs5443 variant in this gene is known to be associated with essential hypertension and obesity. According to meta-analyses involving a large number of studies and subjects, there is a significant association of the TT genotype of rs5443 with overweight or obesity [Citation40,Citation41]. The T allele is significantly more prevalent in the young individuals in our study, suggesting possible predisposition to obesity.
EDN1 (rs5370)
A peptide of the endothelin/sarafotoxin family is the product of the preproprotein encoded by this gene. The protein is a potent vasoconstrictor and its cognate receptors are therapeutic targets in the treatment of pulmonary arterial hypertension. Among its related pathways are Interleukin-1 family signaling pathways and cAMP signaling pathway. We find the variant rs5370 only in young individuals, suggesting its possible role in age-related conditions. Results from a study on Taiwanese subjects suggest that this variant is involved in determination of blood pressure levels in Asian obese subjects [Citation42]. The role of this variant in obesity should be studied in Europeans as different populations are genetically heterogenous.
FSHR (rs6166)
The gene encodes follicle stimulating hormone receptor that belongs to family 1 of G-protein coupled receptors. The variant rs6166 was found in significantly higher frequency in young Bulgarian individuals. The clinical significance of this variant has been established in other studies that show association with Polycystic Ovarian Syndrome where 70% of the patients are either overweight or obese [Citation43]. The rs6166 variant of the FSHR gene is associated with significant elevation of LH, LH/FSH, as well as BMI [Citation43]. A study on Iraqi women finds association of this variant and PCOS, and hence, obesity [Citation41].
Conclusions
Centenarian genomes may be expected to be devoid of genetic variants predisposing to age-related diseases. Disease predisposing variants could be detected when comparing centenarian exomes to those of young individuals. Of all variants designated to be associated with obesity by DisGeNET, only 17% were replicated in Bulgarians. Using the platform ToppGene, we identified three over-represented pathways based on genes with variants showing significant prelevance in allele frequency in the young individuals group. These three pathways were all G-protein coupled receptor associated pathways, in which seven genes had over-represented variants in young Bulgarian individuals. Understanding the genetic etiology of obesity in different populations is essential for developing population-specific obesity therapies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Eng J Med. 2017;377(1):13–27.
- WHO. Obesity and overweight. 2018. Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
- Spinelli A, Buoncristiano M, Kovacs VA, et al. Prevalence of severe obesity among primary school children in 21 European countries. Obes Facts. 2019;12(2):244–258.
- Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA. 1986;256(1):51–54.
- Loos RJ, Bouchard C. Obesity-is it a genetic disorder? J Intern Med. 2003;254(5):401–425.
- Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005;6(3):221–234.
- EUROSTAT. Tobacco consumption statistics, Luxembourg. 2014.
- Yang H, Wang K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat Protoc. 2015;10(10):1556–1566.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57(1):289–300.
- Team RC. R: a language and environment for statistical computing Vienna. 2018. Available from: https://www.R-project.org/
- Piñero J, Bravo À, Queralt-Rosinach N, et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45(D1):D833–D839.
- Chen J, Bardes EE, Aronow BJ, et al. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311.
- Joost P, Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002;3(11):research0063. 1.
- Luo J, Sun P, Siwko S, et al. The role of GPCRs in bone diseases and dysfunctions. Bone Res. 2019;7(1):19.
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246.
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107.
- Shu CJ, Benoist C, Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24(6):436–442.
- Krinninger P, Ensenauer R, Ehlers K, et al. Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. J Clin Endocrinol Metab. 2014;99(7):2500–2509.
- Tanti J-F, Ceppo F, Jager J, et al. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne). 2012;3:181.
- Cantini G, Mannucci E, Luconi M. Perspectives in GLP-1 research: new targets, new receptors. Trends Endocrinol Metab. 2016;27(6):427–438.
- Hazell GGJ, Hindmarch CC, Pope GR, et al. G protein-coupled receptors in the hypothalamic paraventricular and supraoptic nuclei-serpentine gateways to neuroendocrine homeostasis. Front Neuroendocrinol. 2012;33(1):45–66.
- Bjenning C, Al-Shamma H, Thomsen W, et al. G protein-coupled receptors as therapeutic targets for obesity and type 2 diabetes. Curr Opin Invest Dr. 2004;5(10):1051–1062.
- Hauser AS, Chavali S, Masuho I, et al. Pharmacogenomics of GPCR drug targets. Cell. 2018;172(1–2):41–54. e19.
- N’Diaye N, Tremblay J, Hamet P, et al. Adrenocortical overexpression of gastric inhibitory polypeptide receptor underlies food-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83(8):2781–2785.
- Tseng C-C, Zhang X-Y. Role of G protein-coupled receptor kinases in glucose-dependent insulinotropic polypeptide receptor signaling. Endocrinology. 2000;141(3):947–952.
- Bollag RJ, Zhong Q, Phillips P, et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141(3):1228–1235.
- Yamada Y, Hayami T, Nakamura K, et al. Human gastric inhibitory polypeptide receptor: cloning of the gene (GIPR) and cDNA. Genomics. 1995;29(3):773–776.
- Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117(1):24–32.
- Vogel CI, Scherag A, Brönner G, et al. Gastric inhibitory polypeptide receptor: association analyses for obesity of several polymorphisms in large study groups. BMC Med Genet. 2009;10(1):19.
- Sauber J, Grothe J, Behm M, et al. Association of variants in gastric inhibitory polypeptide receptor gene with impaired glucose homeostasis in obese children and adolescents from Berlin. Eur J Endocrinol. 2010;163(2):259–264.
- Su SB, Gong W, Gao J-L, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189(2):395–402.
- Zhang X, Tang Q-Z, Wan A-Y, et al. SAA1 gene variants and childhood obesity in China. Lipids Health Dis. 2013;12(1):161.
- Siddiq A, Gueorguiev M, Samson C, et al. Single nucleotide polymorphisms in the neuropeptide Y2 receptor (NPY2R) gene and association with severe obesity in French white subjects. Diabetologia. 2007;50(3):574–584.
- Hunt SC, Hasstedt SJ, Xin Y, et al. Polymorphisms in the NPY2R gene show significant associations with BMI that are additive to FTO, MC4R, and NPFFR2 gene effects. Obesity (Silver Spring). 2011;19(11):2241–2247.
- Kim HJ, Lee SY, Kim CM. Association between gene polymorphisms and obesity and physical fitness in Korean children. Biol Sport. 2018;35(1):21.
- Yulyaningsih E, Zhang L, Herzog H, et al. NPY receptors as potential targets for anti-obesity drug development. Br J Pharmacol. 2011;163(6):1170–1202.
- Aradillas-García C, Cruz M, Pérez-Luque E, et al. Obesity is associated with the Arg389Gly ADRB1 but not with the Trp64Arg ADRB3 polymorphism in children from San Luis Potosí and León, México. J Biomed Res. 2017;31(1):40–46.
- Lima JJ, Feng H, Duckworth L, et al. Association analyses of adrenergic receptor polymorphisms with obesity and metabolic alterations. Metab Clin Exp. 2007;56(6):757–765.
- Daghestani M, Daghestani M, Daghistani M, et al. ADRB3 polymorphism rs4994 (Trp64Arg) associates significantly with bodyweight elevation and dyslipidaemias in Saudis but not rs1801253 (Arg389Gly) polymorphism in ARDB1. Lipids Health Dis. 2018;17(1):58.
- Li H-L, Zhang Y-J, Chen X-P, et al. Association between GNB3 c. 825C > T polymorphism and the risk of overweight and obesity: a meta-analysis. Meta Gene. 2016;9:18–25.
- Ramadhan RS. Molecular analysis of FSH receptor gene in Iraqi women with PCOS syndrome. Middle East Fertil Soc J. 2018;23(4):404–408.
- Li T-C, Li C-I, Liao L-N, et al. Associations of EDNRA and EDN1 polymorphisms with carotid intima media thickness through interactions with gender, regular exercise, and obesity in subjects in Taiwan: Taichung Community Health Study (TCHS). Biomedicine (Taipei). 2015;5(2):8.
- Sujatha T, Jayashankar E, Addepally U, et al. Association of follicle-stimulating hormone receptor gene ser680 asn (rs6166) polymorphism with polycystic ovarian syndrome. Int J Reprod Contracept Obstet Gynecol. 2016;5(9):3126–3132.
