Abstract
The amino acid/auxin permease (AAAP) gene family plays a significant role in the long-distance amino acid transport and is involved in plant stress responses and various developmental processes. The functions of the AAAP family have been extensively discussed in multiple organisms, whereas detailed information of this family in strawberry (Fragaria vesca) has not been reported yet. In this study, we identified 45 AAAP genes in the strawberry genome. Phylogenetic analysis indicated that AAAPs from strawberry and Arabidopsis were clustered into eight groups. Expression analysis of the AAAP family showed that AAAP genes were expressed differentially in different developmental stages of flower and fruit, and were significantly induced after Colletotrichum gloeosporioides infection. Our study provides a comprehensive understanding of the strawberry AAAP family and demonstrates their potential roles in disease resistance, providing more reference for further research.
Introduction
Amino acids are essential organic materials that act as an indispensable source of organic nitrogen for growth and development, playing significant roles in the metabolism, structure and biosynthesis of variant compounds in eukaryotic organisms [Citation1,Citation2]. Amino acid/auxin permease (AAAP) protein is an amino acid transporter which plays a role in long-distance amino acid transportation and regulates the transportation of amino acids going through the cellular membrane [Citation3,Citation4]. The AAAP gene family members have been fully studied in multiple plants, such as Arabidopsis thaliana, Oryza sativa, Brassica rapa [Citation5]. In previous studies, members of some amino acid transporters have been reported to be located in vacuoles, involved in the transport of amino acids between vacuoles and cytoplasm, and in controlling the storage of amino acids in vacuoles [Citation6,Citation7].
The AAAP family is one of the largest families of AATs (amino acid transporters) [Citation8] and every AAAP gene has a specific Aa-trans domain. The genes belonging to the AAAP family are classified into eight subfamilies, which are: amino acid permease (AAP), lysine and histidine transporter (LHT), aminobutyric acid transporter (GAT), auxin transporter (AUX), proline transporter (ProT), aromitic neutral amino acid transporter (ANT) and amino acid transporter-like (ATLa and ATLb) [Citation9]. Previous reports have described the molecular features of many AAAPs in multiple plants, such as Arabidopsis thaliana, Oryza sativa and Glycine max [Citation10]. In Arabidopsis thaliana, as a transporter for lysine and histidine, AtLHT1 plays a vital role in regulating the absorption of amino acids by root and supply of xylem-derived amino acids in leaf mesophyll [Citation11,Citation12]. AtAAP1 is localized in A. thaliana embryos, regulates the absorption of amino acids and plays an important role in protein synthesis storage as well as affects seed yield [Citation13]. A similar gene in O. sativa, OsAAAP6, participates in amino acid absorption in the endosperm and provides amino acids for developing embryos [Citation14]. In G. max, similar genes are GmANT5 and GmANT6; when induced by high concentration of nitrate, they show expression in all organs, with highest abundance in cauline leaves and flowers [Citation15]. In A. thaliana, AAP3 and AAP6 are involved in root-knot nematode parasitism. AtAAP1, AtAAP2 and AtAAP8 contribute to decreasing the number of nematodes [Citation16]. All of the above findings give us an insight into the biological roles of plant AAAPs and have shown that the functions of AAAPs vary significantly across plant species, suggesting that their functions still need to be further investigated.
Strawberry (Frasaria vesca) (2n = 2x = 14) is an important horticultural crop and is grown around the world. It has highly nutritive and economic values, and is a great model plant for studies on rosaceae plants [Citation17–19]. Colletotrichum gloeosporioides is the main pathogen causing anthracnose in plants [Citation20,Citation21]. It can infect many plants such as strawberry, peach, chestnut, loquat, papaya, rubber and so on, causing great economic losses, and seriously threatening the healthy and sustainable development of forestry and agriculture in various countries. At present, domestic scholars mainly focus on the biological characteristics and chemical screening for its control. Very little is known about FvAAAPs and their possible roles in strawberry resistance to Colletotrichum gloeosporioides. To this end, we identified AAAPs at a genomewide level and systematically analyzed the gene structure, conserved motifs. Furthermore, we investigated their expression patterns in different flower and fruit development stages, and the expression characteristics of some genes infected with C.gloeosporioides. The aims of this study are to give a comprehensive understanding of FvAAAPs and to explore their functions during the flower and fruit development and the interaction between strawberry and fungal invasion, thereby provide a theoretical basis for further research.
Materials and methods
Strawberry growth and Colletotrichum gloeosporioides infection
The experimental plant materials were diploid strawberry (Fragaria vesca), which were maintained in the Institute of Rural Revitalization Science and Technology of Heilongjiang Academy of Agricultural Sciences. They were cultivated in a plastic greenhouse under a photoperiod of 16/8 h (light/dark) and air temperatures of 25/18 °C (day/night). The fungal species used for the test was Colletotrichum gloeosporioides (Penz.) Penz. & Sacc, which was purchased from China's BeNa Culture Collection. The pathogenic fungi used for the experiments were grown in Potato Dextrose (PD) shake-flask cultures for 7-10 days at 25 °C and then were filtered through four layers of gauze into a diluent and adjusted to 106 spore mL−1 using a microbial counting chamber. Then, the conidial suspension was transferred to a watering can, and the plant leaves and stems were sprayed to cover their surfaces with a uniform layer of the suspension. The leaves were harvested for experiments after 0, 1, 6 and 12 d. Plants of 0 d were negative controls with only sprayed diluent. Each treatment had 3 plants. All samples were frozen in liquid nitrogen and stored at −80 °C until use.
Identification of strawberry AAAP genes
The genome sequences were obtained from strawberry genome database (https://www.rosaceae.org/). The Hidden Markov Model (HMM) profiles of the AAAP domain (PF01490) were downloaded from the pfam database (http://pfam.xfam.org/). To find putative AAAP family members, we first searched AAAP domains from the strawberry protein database with an e-value cut-off by using HMMER 3.0 software (http://hmmer.janelia.org/) [Citation22]. Then, we performed BLASTP analysis against to the strawberry database by using Arabidopsis AAAP gene sequences as queries. The integrity of the AAAP domain was verified by using the online program SMART (http://smart.emblheidelberg.de/). The basic information of each gene, including length, molecular weight, isoelectric point and gene structure were predicted by the online ExPasy program (http://www.expasy.org/tools/) and GSDS website (http://gsds1.cbi.pku.edu.cn/index.php).
Phylogenetic tree, gene structure and motif patterns analysis
To investigate the phylogenetic relationship of the AAAP gene family in strawberry, Sequence alignments were done by using ClustalX 1.81. A neighbor-joining (NJ) phylogenetic tree was constructed with the MEGA 7.0 program, setting the bootstrap value at 1000. Gene Structure Display Serve 2.0 software (http://gsds.cbi.pku.edu.cn/) was used to investigate the exon–intron organizations of AAAP genes based on their information given in the phytozome database (http://phytozome.jgi.doe.gov/pz/portal.html). The novel motifs of FvAAAPs were searched using MEME 5.0.3 (http://meme-suite.org/tools/meme).
Transcriptome analysis
Genome-wide expression data of strawberry were used to illustrate the expression patterns of AAAP family in different tissues and developmental stages of strawberry. The transcriptome data of different developmental stages of strawberry flower and fruit were downloaded from Strawberry eFP Browser (http://mb3.towson.edu/efp/cgi-bin/efpWeb.cgi).
RNA extraction, cDNA synthesis and qRT-PCR
We analyzed the expression patterns of the AAAP family under C. gloeosporioides infection using quantitative real-time polymerase chain reaction (qRT-PCR) analysis. We selected all GAT subfamily genes. The total RNA from strawberry leaves was extracted using Trizol reagent (Tiangen, Beijing, China), and their DNA was digested using RNase-free DNaseI (Tiangen, Beijing, China). First-strand cDNA was synthesized using a PrimeScript First-Strand cDNA Synthesis Kit (Toyobo, Shanghai, China), and qRT-PCR was performed by using SYBR Green and monitored on an ABI 7300 Real-Time PCR System (Applied, Biosystems, CA, USA). Primers were designed according to AAAP CDS with Primer 5 software, the primer pairs are listed in . The PCR conditions were programmed as follows: 95 °C for 2 min; followed by 40 cycles at 95 °C for 30 s, 56 °C for 25 s, and 72 °C for 1 min; and a final dissociation at 95 °C for 15 s. The qRT-PCR reaction system and conditions were prepared following the instruction manual. The reference gene was β-actin. The relative expression levels were calculated with the formula 2−ΔΔCt. Three independent biological replicates were used in the analysis.
Table 1. FvAAAP genes identified in the Fragaria vesca genome and predicted properties of the encoded proteins.
Results and discussion
Identification of AAAP family genes in strawberry
The AAAP family members, which play critical roles in plant growth and response to abiotic stress, have been found and studied in many plants such as Oryza sativa, Arabidopsis thaliana, Populus alba and Medicago truncatula [Citation16, Citation23–25]. But their function in strawberry is less known. In this study, we found 45 members in the AAAP gene family, which were fewer than those reported in other plants, such as Arabidopsis thaliana (46), Zea mays (71), Phyllostachys edulis (55) and Populus alba (71) [Citation26,Citation27]. Their given names are from FvAAAP1 to FvAAAP45, according to their chromosome locations (). The lengths of their CDSs range from 1289 bp to 5891 bp, with a mean size of 3075 bp. The predicted molecular weights of the 45 FvAAAP genes range from 43.54 kDa to 60.45 kDa, with an average value of 52.2 kDa. The predicted pI values range from 5.11 to 10.
Phylogenetic analysis of AAAP genes
To investigate the evolutionary relationships of AAAP proteins in plants, a phylogenetic tree was created with 45 members belonging to strawberry and 55 members belonging to Arabidopsis (). And in the strawberry AAAP gene family, AAP was the largest subfamily, which contained 9 members; subsequently, the GAT, ATLb, AUX, ProT, LHT, ATLb subfamilies contained 8, 7, 6, 5, 4, 4 members, respectively. Besides, ANT was the smallest subfamily with only two members.
Figure 1. Phylogenetic tree of AAAPs. At represented Arabidopsis, Fv represented strawberry. The phylogenetic tree was generated using the amino acid sequences of selected AAAPs via neighbor-joining methods. Bootstrap values by using 1000 replicates are indicated at each node. All strawberry AAAPs, together with Arabidopsis homologues were classified into 8 groups, that are: Amino acid permease (AAP), lysine and histidine transporter (LHT), ?-aminobutyric acid transporter (GAT), auxin transporter (AUX), proline transporter (ProT), aromatic, neutral amino acid transporter (ANT) and amino acid transporter-like (including ATLa and ATLb) subfamilies.
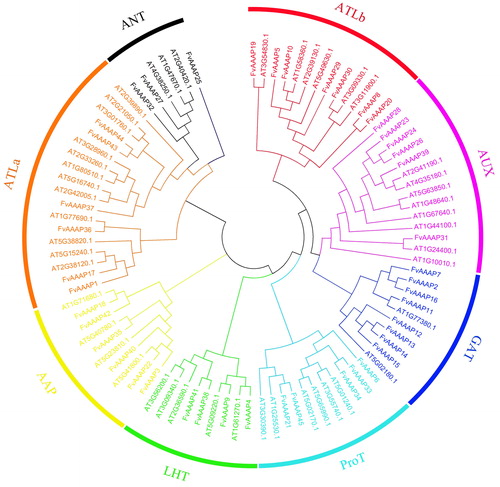
Gene structure and motif analysis of FvAAAPs
The intron–exon distribution could show the evolutionary relationship of gene families. We examined the gene structures of all 45 FvAAAPs and discovered that the number of exons in different subfamilies was uneven (). Most FvAAAPs belong to ATLa, which contained 5-6 exons, and their length of exons was longer than that in other subfamilies members. The number of exons in LHT, ProT, ATLb and AUX subfamilies were similar, ranging from 8 to 11.
Figure 2. a Phylogenetic tree and gene structure of strawberry AAAPs. Phylogenetic tree analysis derived from neighbor-joining methods of FvAAAP proteins. Bootstrap values by using 1000 replicates are indicated at each node. Analysis of exon-intron structures of FvAAAP genes. Their Exon/intron organizations are represented by yellow box and black lines, respectively. UTR are displayed using green box. b Phylogenetic tree and motif patterns of strawberry AAAPs. Phylogenetic tree analysis derived from neighbor-joining methods of FvAAAP proteins. Bootstrap values by using 1000 replicates are indicated at each node. Summary for the distribution of conserved motifs identified from 45 FvAAAP proteins by each group given separately. Every motif is represented by a number in color box.
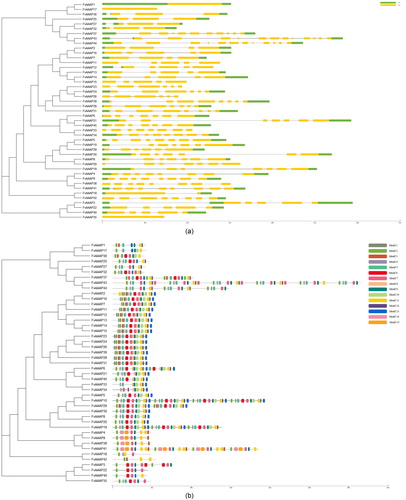
To further investigate the phylogenetic relationships and explore latent features, we analyzed the conserved amino acid sequence domains of FvAAAPs (). A total of 15 various conserved motifs were obtained. As the figure shows, some motifs such as motif 2, motif 6 and motif 11, broadly existed in all subfamilies. Similarly, motif 11 existed in most of the subfamilies except ANT. Conversely, in motif distribution, most subfamilies were highly conserved. For instance, motif 13 was only found in GAT subfamily and motif 14 existed in LHT subfamily.
Research of gene structure showed occurrence of same exon–intron composition of most genes within a class. Distinctions of structures between different FvAAAP genes were revealed through the number of exons-introns. For instance, most FvAAAP genes which belong to ATLa subfamily had 5-6 exons, the ones belonging to LHT contained 8-11 exons. Similar phenomena were reported in Arabidopsis, indicating that FvAAAPs are highly conserved during evolution [Citation16]. In total, we found 15 motifs encoding a characterized domain and some motifs only existing in a certain subfamily, suggesting that these motifs are closely related to the function of this subfamily.
Expression patterns of AAAPs in different developmental stages of strawberry flower and fruit
Strawberry flowers undergo 13 developmental stages, which are divided into three sections: Anther 7-12, early flower development; Carpel 8-12, initiation and development of reproductive organs; and pollen, differentiation of floral organs. We analyzed the transcriptomic data to obtain the expression pattern of FvAAAP genes at different stages of flower development (). In addition, we obtained the expression pattern of FvAAAP genes at different stages of fruit development (), including Wall 1-5, Style 1-2, Ghost (Seed coat and Endosperm) 3-5.
Figure 3. a. Expression patterns of FvAAAP genes involved in different developmental stages of strawberry flower. The Y-axis indicates relative expression levels, and the X-axis indicates the different tissues and stages of flower. b. Expression patterns of FvAAAP genes involved in different developmental stages of strawberry flower. The Y-axis indicates relative expression levels, and the X-axis indicates the different tissues and stages of flower.
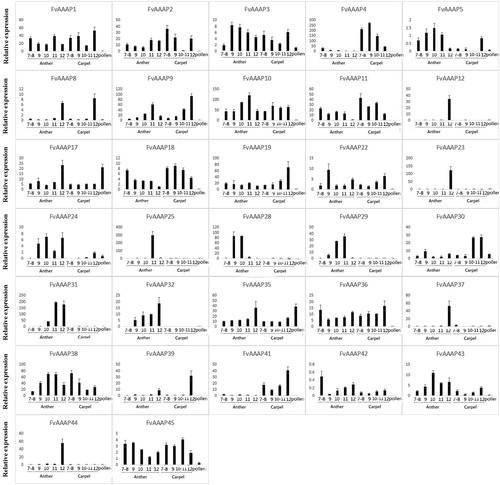
Figure 4. a. Expression patterns of FvAAAP genes involved in different developmental stages of strawberry fruit. The Y-axis indicates relative expression levels, and the X-axis indicates the different tissues and stages of fruit. b. Expression patterns of FvAAAP genes involved in different developmental stages of strawberry fruit. The Y-axis indicates relative expression levels, and the X-axis indicates the different tissues and stages of fruit.
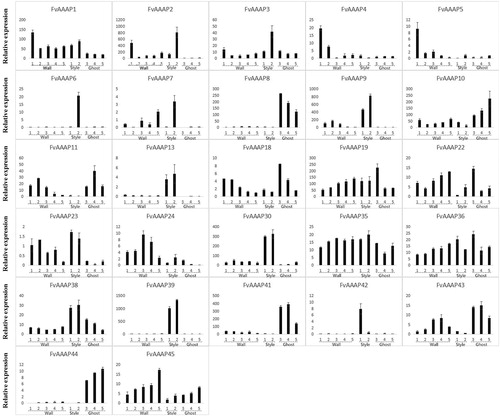
Members of the AAAP gene family show various expression patterns in different tissues of plants and play important roles during plant growth. For instance, in Arabidopsis, AtAUX1 was primarily expressed in the root and promoted lateral root formation; in Oryza sativa, OsAUX1, an ortholog of AUX1, had high expression levels in all organs, especially in young flowers [Citation28,Citation29]. In our study, FvAAAP28, which belongs to the AUX subfamily, was highly expressed in the early flower development stage and it showed a similar function as OsAUX1. In Arabidopsis, AtATLb1 encodes a specific transporter for lysine and histidine and was highly expressed in flower tissues [Citation30]. FvAAAP10, which is an ortholog of AtATLb1, was highly expressed in all flower and fruit stages. As reported, the functions of Anther were to produce and protect the male gametophyte [Citation31]. The expression levels of FvAAAP10 and FvAAAP25 in Anther11 were much higher than at other stages of flower, suggesting that they play key roles in male gametophyte. FvAAAP4 had highest expression in carpel 9 and then declined at later stages of carpel tissue. It might be involved in the early stage of seed production.
Expression patterns of FvAAAP genes after Colletotrichum gloeosporioides infection
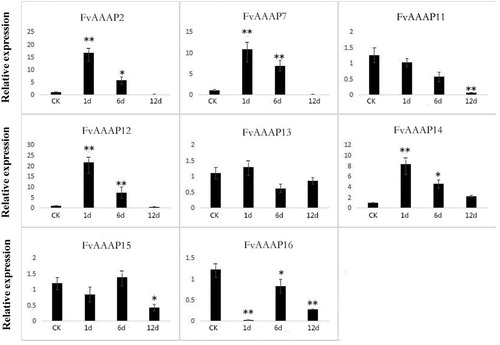
As reported, the expression of GAT subfamily genes which encode the high affinity γ-aminobutyric acid (GABA) transporter was upregulated with the increase in the level of GABA [Citation27]. GABA is produced in plants from glutamate by glutamate decarboxylase and can respond to various conditions of biotic stress [Citation32]. For example, in tomato, GABA levels were increased in response to Cladosporium fulvum infection; and in Oryza sativa, the GABA shunt is activated to upon infection with blast fungus [Citation33]. These reports all suggest that GABA might play a vital role in regulating the communication between plants and microorganisms. Therefore, to explore the functions of FvAAAPs in response to Colletotrichum gloeosporioides infection, we selected all GAT subfamily genes to perform qRT-PCR. Among these genes, some FvAAAPs were down-regulated, such as FvAAAP11 and FvAAAP15 (). But most of the FvAAAPs were up-regulated and then down-regulated in response to Colletotrichum gloeosporioides. For instance, the expression levels of FvAAAP2, FvAAAP7, FvAAAP12 and FvAAAP14 reached a peak at 1 d and were 15, 10, 20, 8 times that of the control (CK, 0 d) respectively. Following the peak, there was a decrease in their expression. The results showed that most GAT subfamily genes were up-regulated and then down-regulated, suggesting that GAT subfamily genes were involved in response to C. gloeosporioides infection. Therefore, we suggest that GAT subfamily genes might be involved in the response to C. gloeosporioides infection by encoding GABA transporters to improve GABA levels. And GAT subfamily might play a crucial role in plants under bacterial infection.
Figure 5. Expression patterns of FvAAAP genes after C. gloeosporioides infection. The Y-axis indicates relative expression levels, and the X-axis indicates the C. gloeosporioides infection time. One asterisk indicates significant at 0.05 level differences (p < 0.05, Duncan) between different time stages, two asterisk indicates significant at 0.01 level differences (p < 0.01, Duncan) between different time stages.
Conclusions
In this study, we identified 45 AAAP genes in the strawberry genome. Phylogenetic analysis indicated that AAAPs from strawberry and Arabidopsis were clustered into eight groups. Expression analysis of the AAAP family showed that AAAP genes were expressed differentially in different developmental stages of flower and fruit, and were significantly induced after Colletotrichum gloeosporioides infection. Our study provides a deeper understanding of the strawberry AAAP family members and demonstrates their potential roles in disease resistance, therefore providing more reference for further research.
Supplemental Material
Download MS Excel (27 KB)Disclosure statement
No author has any conflict of interest to disclose.
Additional information
Funding
References
- Young VR, Wayler A, Garza C, et al. A long-term metabolic balance study in young men to assess the nutritional quality of an isolated soy protein and beef proteins. Am J Clin Nutr. 1984;39(1):8–15.
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17.
- Carrillo C, Canepa GE, Giacometti A, et al. Trypanosoma cruzi amino acid transporter TcAAAP411 mediates arginine uptake in yeasts. FEMS Microbiol Lett. 2010;306(2):97–102.
- Conlon NJ, Edwards N, Cmh A, et al. Rheogenic amino acid transport by Drosophila CG4991 of the Amino Acid Auxin Permease (AAAP) transporter family. FASEB J. 2013 March;27:1093.
- Hu L, Yin W, Chen Y, et al. Functional divergence and evolutionary dynamics of the putative AAAP gene family in Brassica rapa. Plant Mol Biol Rep. 2014;32(2):517–530.
- Okumoto S, Koch W, Tegeder M, et al. Root phloem-specific expression of the plasma membrane amino acid proton co-transporter AAP3. J Exp Bot. 2004;55(406):2155–2168.
- Etxeberria E, Gonzalez P, Pozueta-Romero J. Sucrose transport into citrus juice cells: evidence for an endocytic transport system. JASHS. 2005;130(2):269–274.
- Ortiz-Lopez A, Chang H, Bush DR. Amino acid transporters in plants. Biochim Biophys Acta (BBA)-Biomembranes. 2000;1465(1-2):275–280.
- Xia J, Yang Z, Cheng G, et al. Genome-wide identification and expression analysis of amino acid transporters in the Whitefly, Bemisia tabaci (Gennadius). Int J Biol Sci. 2017;13(6):735–747.
- Igarashi Y, Yoshiba Y, Takeshita T, et al. Molecular cloning and characterization of a cDNA encoding proline transporter in rice. Plant Cell Physiol. 2000;41(6):750–756.
- Svennerstam H, Ganeteg U, Bellini C, et al. Comprehensive screening of Arabidopsis mutants suggests the lysine histidine transporter 1 to be involved in plant uptake of amino acids. Plant Physiol. 2007;143(4):1853–1860.
- Lee CP, Wirtz M, Hell R. Evidence for several cysteine transport mechanisms in the mitochondrial membranes of Arabidopsis thaliana. Plant Physiol. 2014;55(1):64–73.
- Sanders A, Collier R, Trethewy A, et al. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009;59(4):540–552.
- Peng B, Kong H, Li Y, et al. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat Commun. 2014; September 11; 5:4847
- Cheng L, Yuan H, Ren R, et al. Genome-wide identification, classification, and expression analysis of amino acid transporter gene family in Glycine Max. Front Plant Sci. 2016; April 20; 7:515
- Marella H, Nielsen E, Schachtman DP, et al. The amino acid permeases AAP3 and AAP6 are involved in root-knot nematode parasitism of Arabidopsis. Mol Plant Microbe Interact. 2013;26(1):44–54.
- Farinati S, Rasori A, Varotto S, et al. Rosaceae fruit development, ripening and post-harvest: an epigenetic perspective. Front Plant Sci. 2017; July 17; 8:1247
- Tahir HE, Zou X, Shi J, et al. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J Food Biochem. 2018;42(3):e12527. January 21;
- Kim J, Lee SY, Kim D, et al. Genotyping of octoploid strawberry inbred lines by SNP discovery using genotyping-by-sequencing. Hortic Environ Biotechnol. 2019;60(1):69–80.
- Peres NA, Timmer LW, Adaskaveg JE, et al. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005;89(8):784–796.
- McInnes TB, Black LL, Gatti JM. Disease-free plants for management of strawberry anthracnose crown rot. Plant Dis. 1992;76(3):260–264.
- Lim S, Seo J, Choi H, et al. Metagenome analysis of protein domain collocation within cellulase genes of goat rumen microbes. Asian-Australas J Anim Sci. 2013;26(8):1144–1151.
- Zhao H, Ma H, Yu L, et al. Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.). PLoS One. 2012;7(11):e49210November 15;
- Wu M, Wu S, Chen Z, et al. Genome-wide survey and expression analysis of the amino acid transporter gene family in poplar. Tree Genet Genomes. 2015;11(4):83. July 31;
- Qu Y, Ling L, Wang D, et al. Genome-wide identification and expression analysis of the AAAP family in Medicago truncatula. Genetica. 2019;147(2):185–196.
- Sheng L, Deng L, Yan H, et al. A genome-wide analysis of the AAAP gene family in maize. Journal of Proteomics and Bioinformatics. 2014; 7:23–33.
- Liu H, Wu M, Zhu D, et al. Genome-wide analysis of the AAAP gene family in moso bamboo (Phyllostachys edulis). BMC Plant Biol. 2017;17(1):29January 31;
- de Billy F, Grosjean C, May S, et al. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol Plant Microbe Interact. 2001;14(3):267–277.
- Zhao H, Ma T, Wang X, et al. OsAUX1 controls lateral root initiation in rice (Oryza sativa L.). Plant Cell Environ. 2015;38(11):2208–2222.
- Liu X, Bush DR. Expression and transcriptional regulation of amino acid transporters in plants. Amino Acids. 2006;30(2):113–120.
- Pacini E, Dolferus R. The trials and tribulations of the plant male gametophyte—understanding reproductive stage stress tolerance. In: Shanker A, Shanker C, editors. Abiotic and biotic stress in plants - recent advances and future perspectives. London: IntechOpen; 2016. p. 704–754.
- Michaeli S, Fromm H. Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined? Front Plant Sci. 2015 June;6:419.
- Mead O, Thynne E, Winterberg B, et al. Characterising the role of GABA and its metabolism in the wheat pathogen Stagonospora nodorum. PLoS One. 2013 November;8(11):e78368.
