Abstract
Leucocalocybe mongolica (S. Imai) is a well-known edible medicinal mushroom. Polysaccharides extracted from L. mongolica show several pharmacological properties. Owing to increased predation and desertification, wild L. mongolica populations are declining and becoming increasingly rare. Microbial fermentation is emerging as a suitable alternative to obtain bioactive compounds from this mushroom. In this study, polysaccharides were isolated from the solid-state fermentation of L. mongolica (LMPs) and their anti-tumor activities were investigated. Treatment with LMPs significantly inhibited the growth of hepatoma H22 tumors after cell transplantation in mice. Notably, LMP treatment protected the spleen and the thymus against tumor-induced damage. Furthermore, LMP administration significantly increased the serum levels of the cytokines interferon gamma, tumor necrosis factor-α, interleukin (IL)-6 and IL-2, while simultaneously decreasing the serum level of vascular endothelial growth factor. Hematoxylin-eosin staining, the TUNEL assay, immunohistochemistry and western blotting indicated that the in vivo anti-tumor activity of LMP was achieved by promoting apoptosis and inhibiting angiogenesis. In summary, this study revealed that LMPs markedly suppressed the tumor growth of H22-transplanted tumors in vivo at least partly by enhancing the immune response, inducing apoptosis and inhibiting angiogenesis. These findings highlight LMP as a potential low-cost anti-tumor drug.
Introduction
Leucocalocybe mongolica (S. Imai) X.D. Yu & Y.J. Yao is a well-known edible and medicinal mushroom belonging to the Tricholomataceae family [Citation1, Citation2]. Its former Latin name is Tricholoma mongolicum S. Imai [Citation3] and its Chinese name is ‘Kou-mo’ or ‘Bai-mo’. Recently, L. mongolica has attracted increased attention owing to the recognition of its bioactive compounds. To date, diverse types of secondary metabolites have been isolated from L. mongolica, such as polysaccharides [Citation4–6], peptides [Citation7], triterpenoids [Citation8] and unsaturated fatty acids, alkaloids and lectins [Citation9, Citation10].
As one of the main bioactive components, polysaccharides are a key research topic [Citation11, Citation12]. Polysaccharides are carbohydrate polymers composed of monosaccharide units bound together by glycosidic linkages, and they are involved in a range of biological processes [Citation13]. Polysaccharides isolated from L. mongolica have multiple pharmacological properties, including hypoglycemic activity [Citation14], and anti-proliferative [Citation5], antitumor [Citation15, Citation16], antioxidative [Citation4] and immunomodulatory properties [Citation15].
However, wild L. mongolica is becoming increasingly rare as a consequence of predation and grassland desertification. Therefore, it is necessary to search for an alternative approach to obtain similar bioactive polysaccharides. Microbial fermentation (such as solid-state fermentation or submerged fermentation) is an effective way to produce bioactive compounds and has therefore gained increasing attention as a method to obtain polysaccharides [Citation17, Citation18]. The use of microbial fermentation to obtain polysaccharides provides numerous potential advantages such as higher productivity, lower water and energy requirements, and easier downstream processing [Citation19]. Several studies have reported the microbial fermentation of L. mongolica and polysaccharide extraction from the microbial fermentation products [Citation20, Citation21]. However, there is limited research on the biological activity of these polysaccharides.
Therefore, in the present study, we extracted polysaccharides from the solid-state fermentation of L. mongolica, and investigated their anti-tumor properties and underlying mechanisms.
Materials and methods
Strain and reagents
The L. mongolica strain (accession number MCCJLAU2015C1) used in this study was maintained at the Strains Laboratory of Fungi at the Engineering Research Center of the Chinese Ministry of Education for Edible and Medicinal Fungi of Jilin Agricultural University. The voucher specimen was preserved at the Herbarium Mycology of Jilin Agriculture University (HMJAU).
Rabbit polyclonal antibodies against Bcl-2 (3498S), Bax (2772S) and Cyt-C (4272 T) were purchased from Cell Signaling Technology, Inc. (Shanghai, China). Mouse monoclonal vascular endothelial growth factor (VEGF) antibody was purchased from Salvage Pharmaceutical Co., Ltd. (Guizhou, China). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (HA1001) was purchased from Hangzhou HuaAn Biotechnology Co., Ltd. (Hangzhou, China). DAB detection kit (SP-9000) was purchased from Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China). Reagent kits for quantifying tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-2, IL-6 and VEGF were acquired from AndyGene Biotechnology Co., Ltd. (Beijing, China). All other chemicals and reagents were of analytical grade.
Extraction of polysaccharides from L. mongolica (LMPs)
Solid-state fermentation of L. mongolica was conducted according to a previously described method [Citation22] from mycelia dried at 60 °C and then ground into a powder. The polysaccharides were extracted using a method described by Wu et al. [Citation20] with some modifications. In brief, extraction from the dried mycelia was performed three times with 5 L distilled water for 4 h each at 100 °C. The protein was removed from the crude polysaccharides using the Sevag method, consisting of eight cycles of deproteinization with CHCl3:n-BuOH at a 5:1 (v/v) ratio. The obtained extract was collected and freeze-dried.
Animals
Male Kunming mice (20 g ± 2 g) were obtained from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China) with certificate of quality [SCXK(Liao)-0001] and maintained in a controlled environment at 24 °C ± 2 °C with humidity of 55% ± 10%, a 12-h light/12-h dark cycle, and food and water ad libitum.
Ethics statement
All experiments were conducted in accordance with the guidelines for the care and use of laboratory animals approved by the Animal Ethics Committee of Jilin Agricultural University.
Experimental design
After an acclimatization period of 1 week, murine hepatoma H22 cells (maintained in the ascitic form by sequential passages into the peritoneal cavities of male Kunming mice) were transplanted in mice to induce solid tumor growth according to a previously described method [Citation23]. In brief, 0.2-mL H22 cell suspension (1 × 107 cells/mL) was inoculated subcutaneously in the right forelimb armpit of the recipient mice. The tumor-bearing mice were randomly divided into five groups, with 10 mice per group. A sixth group comprising non-tumor-bearing animals was considered as the normal group (NG). The mice in the model group (MG) and the NG received saline intragastrically. The mice in the positive control group (PG) were administered cyclophosphamide (CTX, 25 mg/kg) by an intraperitoneal injection, and the remaining LMP treatment groups were administered the polysaccharide extract from L. mongolica at a low dose (LD, 100 mg/kg daily), medium dose (MD, 200 mg/kg daily) or high dose (HD, 300 mg/kg daily). Twenty-four hours after the last administration of the test agents, on the 10th day of the experiment, blood samples were collected from the eyes of the mice, and serum was obtained by centrifuging the blood samples at 4000 g for 10 min at 4 °C. All of the mice were sacrificed by cervical dislocation under ether anaesthesia, and the whole bodies, segregated tumor, thymus and spleen were weighed immediately. The tumor growth inhibition rate (%) was calculated using the following formula: [(tumor weight of model group – tumor weight of tested group)/tumor weight of model group] × 100%.
Biochemical analysis
The serum levels of TNF-α, IFN-γ, IL-2, IL-6 and VEGF were determined using enzyme-linked immunosorbent assay (ELISA) following the kit manufacturer’s instructions. The absorbance was measured at 450 nm on an ELISA reader (Bio-Rad, Hercules, CA, USA).
Histopathological observation
After removing the tumor tissues, they were fixed in 10% neutral-buffered formalin for 24 h, dehydrated in increasing grades of ethanol, cleared in xylene and embedded in paraffin. Sections were cut at 5-μm thickness and stained with hematoxylin and eosin (H&E) for histopathological observation under an optical microscope (Olympus BX-51, Tokyo, Japan).
TUNEL staining
Apoptosis in tumor cells was assessed using the In Situ Cell Death Detection Kit (Roche Applied Science, Shanghai, China) in accordance with the manufacturer’s instructions. Briefly, the sections were treated with 20 μg/mL of proteinase K in distilled water for 15 min at 37 °C. After rinsing the slides twice with phosphate buffered saline (PBS), they were incubated in TUNEL reaction mixture for 60 min at 37 °C. To block endogenous peroxidase, the slides were immersed in methanol containing 3% hydrogen peroxide for 10 min at room temperature. The slides were then incubated in converter-POD for 30 min at 37 °C. Finally, the diaminobenzidine substrate was added, and the slides were counterstained with hematoxylin.
Immunohistochemistry
Immunohistochemical analysis was performed according to a previously described method [Citation12]. After restoration in citric acid buffer (0.01 mol/L, pH 6.0), 1% H2O2 solution was added to the slides to block peroxidase activity. The sections were then incubated with the primary antibodies against Bax, Bcl-2 and VEGF overnight at 4 °C. After washing with PBS, the sections were incubated with biotin-conjugated IgG secondary antibody for 30 min at 37 °C. The substrate was added to the sections for 30 min, followed by DAB staining and hematoxylin counter-staining. The immunostaining intensity was assessed using a light microscope (Olympus BX-51, Tokyo, Japan).
Western blot analysis
Western blot analysis was performed as described previously [Citation24] with minor modifications. In brief, proteins from tumor tissues were extracted and measured using a bicinchoninic acid assay kit (Pierce, Rockford, IL, USA). All proteins were resolved on a 15% sodium dodecyl sulfate-denaturing polyacrylamide gel and then transferred onto a polyvinylidene fluoride membrane. The membranes were incubated with antibodies against Bax (1:1000), Cyt-c (1:1000), Bcl-2 (1:1000) and VEGF (1:1000) overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated sheep anti-rabbit (1:3000) and horse anti-mouse (1:3000) at room temperature for 1 h. Finally, the enhanced chemiluminescence (ECL) reagent was added and protein bands were visualized with an ECL detection system (Amersham Pharmacia Biotechnology, Tokyo, Japan).
Statistical analysis
All data are presented as mean values with standard deviation (± SD). Statistical analysis was performed using SPSS 16.0 software (Chicago, IL, USA), and differences between experimental groups were compared by analysis of variance (ANOVA) followed by a t-test. Differences were considered statistically significant at p < 0.05.
Results and discussion
Polysaccharide is a natural macromolecular compound formed by the connection of aldoses or ketoses through glucoside bonds. Polysaccharides are present in the cell membrane and have a variety of physiological functions, including anti-tumor, antioxidant and anti-inflammatory effects, along with immune regulation [Citation25]. Many studies have demonstrated the potent anti-tumor properties of polysaccharides from various species such as the herb genus Astragalus [Citation26] and the mushroom Ganoderma lucidum [Citation27]. Polysaccharides inhibit tumor growth mainly by suppressing proliferation [Citation28], promoting tumor cell apoptosis [Citation29] and enhancing the immune system [Citation30, Citation31]. In this study, solid-state fermentation was used to extract the bioactive compounds from L. mongolica, and the anti-tumor mechanism of the polysaccharides isolated was investigated.
LMPs inhibited H22 tumor growth in a dose-dependent manner
First, we analyzed the effect of the different treatments on tumor growth in vivo (). In the MG, the average tumor weight was 1.44 ± 0.49 g, which significantly decreased in the PG and LMP-treated groups (LD, MD and HD) (all p < 0.01). CTX as the positive control exhibited the highest tumor inhibitory rate (85.42%). Tumors had significantly lower weight in the LMP-treated groups than those in the MG. Moreover, this decrease was dose-dependent; the tumor inhibitory rates of the LMP-treated groups were 64.58%, 72.92% and 77.78% for LD, MD and HD, respectively.
Table 1. Effects of Leucocalocybe mongolica polysaccharides on tumor weight and relative organ indices in H22 tumor-bearing mice.
LMPs protected the spleen and thymus from tumor-induced damage
Both the spleen and the thymus are crucial for proper functioning of the immune system. The spleen is involved in both specific and non-specific immune responses of the body. The thymus is the central organ of the immune system and participates in cellular immunity. Therefore, the thymus and spleen indices were determined to evaluate the effect of LMP administration on the immune system. As shown in , the spleen and thymus indices in the CTX-treated group (PG) were significantly lower than those in the MG (p < 0.05), which was due to the immunosuppressive effect of CTX. Compared to the NG, the MG showed a significantly enlarged spleen (p < 0.01) and a severely atrophic thymus (p < 0.05). These data suggested that the malignant proliferation of tumor cells induced severe damage to the immune system of mice. However, the organ indices were improved in the LMP-treated groups, suggesting that LMPs protect the spleen and thymus from tumor-induced immune system damage.
LMPs increased the serum cytokine levels and decreased the serum VEGF levels in tumor-bearing mice
Cytokines and VEGF play an important role in inhibiting tumor growth and promoting apoptosis, respectively. IL-2 can promote the synthesis and secretion of IFN-γ and other cytokines and enhance the natural killer cell and cytotoxic T lymphocyte cytotoxic activities against tumor cells [Citation32, Citation33]. IL-6 is involved in the processes of tumor cell proliferation, differentiation, invasion, metastasis and angiogenesis through multiple signaling pathways [Citation34]. TNF-α induces cell lysis by reducing the lysosomal activity of target cells, and it promotes the death of target cells by affecting their glucose metabolism. TNF- α can also promote the apoptosis of tumor cells by increasing the activity of T cells and other killer cells [Citation35]. IFN-γ indirectly inhibits the proliferation of tumor cells and promotes tumor cell apoptosis by inhibiting the expression of c-Myc and c-Fos proto-oncogenes. In addition, IFN-γ can inhibit tumor angiogenesis by inhibiting VEGF-C expression [Citation36].
Therefore, we investigated the effects of LMP treatment on the serum levels of cytokines and VEGF. As shown in , the serum levels of IL-2, IL-6, IFN-γ and TNF-α in all three LMP-treated groups were higher than those in the MG. However, these differences were significant only in the mice treated with LMPs at the MD and HD (p < 0.05). Moreover, treatment with LMPs at all doses markedly and significantly decreased the serum level of VEGF (p < 0.01).
Table 2. Effect of treatment with Leucocalocybe mongolica polysaccharides on serum cytokine and vascular endothelial growth factor levels in H22 tumor-bearing mice.
These data suggested that the tumor-inhibitory effect of LMP could be achieved through the upregulation of cytokines and downregulation of VEGF.
LMPs induced morphological changes and increased the apoptotic rate in tumor tissues
Histopathological characteristics of tumor tissues were evaluated using H&E staining. Tumor cells in the model group were arranged tightly and had a large nucleus and a clear nucleolus (). However, the tumor cells in the CTX- and LMP-treated groups displayed a loose arrangement and a large necrotic region.
Figure 1. Histological examination of the morphological changes in tumors derived from H22-bearing mice. MG, tumor-bearing mice that received saline solution; PG, positive control, received 25 mg/kg body weight of CTX; LD, low dose, received 100 mg/kg body weight of polysaccharide extract from Leucocalocybe mongolica (LMPs); MD, medium dose, received 200 mg/kg body weight of LMPs; HD, high dose, received 300 mg/kg body weight of LMPs. Tumor tissues were stained with hematoxylin and eosin (magnification, 400×) (A) and subjected to the TUNEL assay (magnification, 400×) (B, C). All data are presented as means ± SD (n = 10). *p < 0.05, **p < 0.01 compared with the model group. Scale bar =50 μm.
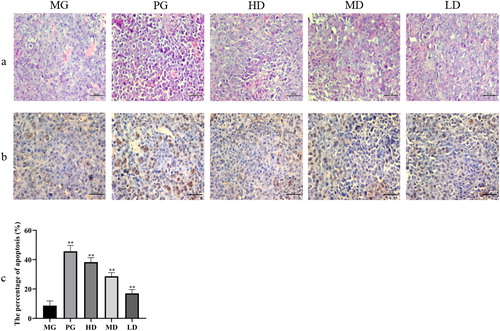
We further explored whether the inhibitory effect of LMP treatment on tumor growth was due to apoptosis of the tumor cells. Representative positive staining and pathologic images of the tumor tissues are shown in . The results suggested that apoptosis was the least frequent in the MG, with only 8% of the cells positively stained. By contrast, the PG exhibited the highest percentage of apoptotic cells. Notably, the percentage of apoptotic cells increased in the LMP-treated groups in a dose-dependent manner. These results support the hypothesis that LMPs inhibit tumor growth via inducing tumor cell death/apoptosis.
LMPs upregulated pro-apoptotic proteins and downregulated anti-apoptotic and angiogenic factors
Apoptosis is fundamental to maintain the physiological balance of an organism [Citation37]. Accumulating evidence has demonstrated that some chemotherapeutic agents exert their anticancer effects via induction of apoptosis [Citation38]. Here, we found that LMPs could increase the percentage of the apoptotic tumor cells. The mitochondria-dependent pathway is one of the classical apoptosis pathways. The Bcl-2 family plays a pivotal role in mitochondria-dependent apoptosis [Citation39] and includes the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2. When Bcl-2 is highly expressed, it can heterodimerize with Bax to inhibit apoptosis. Conversely, when Bax is highly expressed, Bax homodimerizes to promote apoptosis [Citation40]. Immunohistochemistry () and western blot analysis () showed that the expression level of Bcl-2 was significantly reduced, whereas the expression level of Bax was increased upon treatment with LMP. Moreover, downregulation of Bcl-2 could promote the expression of Cyt-c, which activates caspase 3 to induce apoptosis [Citation41]. Taken together, our data suggest that the anti-tumor effect of polysaccharides may be mediated by regulating the ratio of Bcl-2 and Bax.
Figure 2. Immunohistochemical staining of tumor tissues and the stained area of the tumor tissues. Data are presented as means ± SD (n = 10). *p < 0.05 and **p < 0.01 compared to the model group. Scale bar =50 μm.
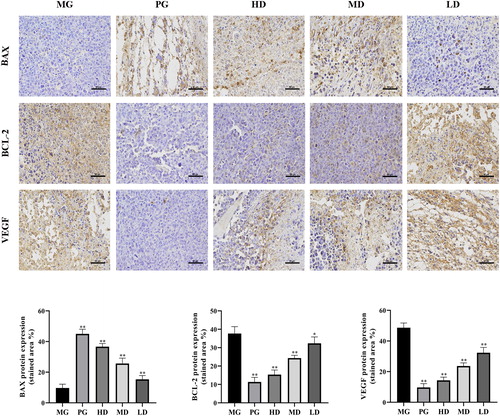
Figure 3. Relative protein expression of Bcl-2-associated X protein (Bax), B-cell lymphoma-2 (Bcl-2), cytochrome C (Cyt-c), caspase-3, cleaved caspase-3 and vascular endothelial growth factor (VEGF) in tumor tissues. β-actin was used as a control. Data are presented as means ± SD (n = 10). * p < 0.05, ** p < 0.01 compared with the model group.
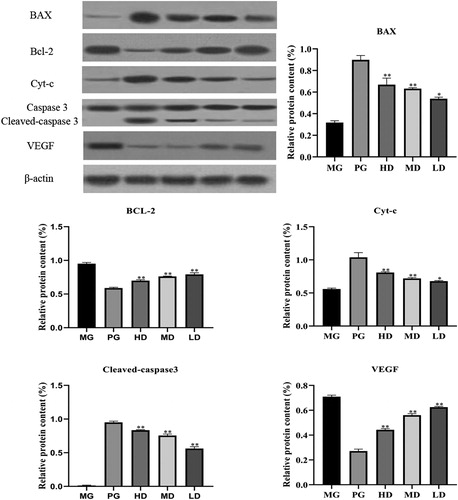
Angiogenesis is important to tumor growth and metastasis. Various growth factors are involved in tumor angiogenesis. Among them, VEGF exerts effects on the whole process of angiogenesis through multiple signal pathways [Citation42]. Hepatocellular carcinoma (HCC) is a highly vascular tumor and VEGF is highly expressed in the hepatocarcinoma tissue. Numerous studies have shown associations between VEGF expression and HCC proliferation, invasion, and migration [Citation43]. In this study, treatment with LMPs significantly decreased the serum levels of VEGF (p < 0.05). Furthermore, immunohistochemistry () and western blotting () confirmed that LMPs significantly inhibited the expression of VEGF in the tumor tissue (p < 0.05).
Conclusions
In this study, polysaccharides produced by the solid-state fermentation of L. mongolica were isolated successfully. These LMPs showed a strong anti-tumor effect in H22 tumor-bearing mice. Notably, the anti-tumor effect of LMPs was achieved by enhancing the immune response, increasing the ratio of Bax/Bcl-2, and inhibiting the expression of VEGF. Therefore, LMPs may be used as a low-cost anti-tumor drug and as a supplement in functional and health-care food owing to their strong tumor-inhibitory effect.
Authors' contributions
Xueliang Zhao and Haiying Bao participated in the design and conducted the majority of the experiments of this study and drafted the manuscript. Tolgor Bau contributed to the interpretation of the findings and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (0207-202022934).
Disclosure statement
The authors declare no competing interests.
Additional information
Funding
References
- Yu XD, Deng H, Yao YJ. Leucocalocybe, a new genus for Tricholoma mongolicum (Agaricales, Basidiomycota). Afr J Microbiol Res. 2011;5(31):5750–5756.
- Dong D, Bau T. A study on the taxonomic position of Tricholoma mongolicum. J Fungal Res. 2013;11(3):172–5175.
- Imai S. On an edible Mongolian fungus “Pai-mo-ku. Proc Imp Acad. 1937;13(7):280–282.
- Zhao YM, Song JH, Wang J, et al. Optimization of cellulase-assisted extraction process and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai . J Sci Food Agric. 2016; 96(13):4484–4491.
- Wang J, Zhao Y, Li W, et al. Optimization of polysaccharides extraction from Tricholoma mongolicum Imai and their antioxidant and antiproliferative activities. Carbohydr Polym. 2015;131:322–330.
- You QH, Yin XL, Zhang SN, et al. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydr Polym. 2014;99:1–10.
- Wang DW, Wu EQ, Bau T. Research of preparation technology of Tricholoma mongolicum polypeptide. Food Science. 2007; 27(09):245–249.
- Yao QZ, Zhang ZY, Yan W. Optimization of extraction technology of the Tricholoma mongolicum triterpenoid by the method of response surface analysis. International Conference of National Product and Traditional Medicine. 2009. 327–330. Oct 16-18; Xian, China.
- Wang HX, Ng TB, Liu WK, et al. Isolation and characterization of two distinct lectins with antiproliferative activity from the cultured mycelium of the edible mushroom Tricholoma mongolicum. Int J Pept Protein Res. 1995;46(6):508–513.
- Wang HX, Ooi VEC, Ng TB, et al. Hypotensive and vasorelaxing activities of a lectin from the edible mushroom Tricholoma mongolicum. Pharmacol Toxicol. 1996;79(6):318–323.
- Dae YL, Chan WP, Sue JL, et al. Immunostimulating and antimetastatic effects of polysaccharides purified from ginseng berry. Am J Chin Med. 2019;47(4):1–17.
- Han C, Wei YY, Wang X, et al. Salvia miltiorrhiza polysaccharides protect against lipopolysaccharide-induced liver injury by regulating NF-κb and Nrf2 pathway in mice. Food Agricul Immunol. 2019;30(1):979–994.
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3(2):97–130.
- Zhang GQ, Huang YD, Bian Y, et al. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats . Appl Microbiol Biotechnol. 2006;72(6):1152–1156.
- Wang HX, Ooi VEC, Chang ST, et al. A polysaccharide-peptide complex from cultured mycelia of the mushroom Tricholoma mongolicum with immunoenhancing and antitumor activities. Biochem Cell Biol. 1996;74(1):95–100.
- Bau SRGG, Bao HY, Bau T, et al. Anti-tumor activity of Tricholoma mongolicum fruit bodies. Food Sci. 2012; 33(21):280–284.
- José LAAF, Lucas OS, André GAF, et al. Optimization of the solid-state fermentation conditions and characterization of xylanase produced by Penicillium roqueforti ATCC 10110 using yellow mombin residue (Spondias mombin L.). Chem Eng Commun. 2019;207(1):1–12.
- Zeng YJ, Yang HR, Wu XL, et al. Structure and immunomodulatory activity of polysaccharides from Fusarium solani DO7 by solid-state fermentation. Int J Biol Macromol. 2019; 137:568–575.
- Martins S, Mussatto SI, Martínez-Avila G, et al. Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review. Biotechnol Adv. 2011;29(3):365–373.
- Wu XT, Xu RH, Ren QW, et al. Factors affecting extracellular and intracellular polysaccharide production in submerged cultivation of Tricholoma mongolicum. Afr J Microbiol Res. 2012; 6(5):909–916.
- Lu T, Bau T, Ohga S. Physiological study of the wild edible mushroom Leucocalocybe mongolica. J Fac Agricul Kyushu Univ. 2017; 62(1):1–8.
- Wang XY, Bao HY, Bau T. Nutritional value and volatiles of the edible mushroom Leucocalocybe mongolica. Quality Assur Safety Crops Foods. 2019; 11(8):679–685.
- Lin M, Li L, Zhao Y, et al. 2-Naphthoic acid ergosterol ester, an ergosterol derivative, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis. Steroids. 2017; 122:9–15.
- Zhang H, Li H. miR-137 inhibits renal cell carcinoma growth in vitro and in vivo. Oncol Lett. 2016; 12(1):715–720.
- Zhang L, Li X, Xu X, et al. Correlation between antitumor,activity molecular weight, and conformation of lentinan. Carbohydr Res. 2005; 340(8):1515–1521.
- Tian QE, De Li H, Yan M, et al. Effects of astragalus polysaccharides on p-glycoprotein efflux pump function and protein expression in H22 hepatoma cells in vitro. BMC Complem Altern Med. 2012;12(1):94.
- Lu J, Sun L-X, Lin Z-B, et al. Antagonism by Ganoderma lucidum polysaccharides against the suppression by culture supernatants of B16F10 melanoma cells on macrophage. Phytother Res. 2014; 28(2):200–206.
- Synytsya A, Kim WJ, Kim SM, et al. Structure and antitumor activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr Polym. 2010;81(1):41–48. [cited Feb 10, 2020];
- Zhao XK, Ma S, Liu N, et al. A polysaccharide from Trametes robiniophila inhibits human osteosarcoma xenograft tumor growth in vivo. Carbohydr Polym. 2015; 124(124):157–163.
- Cui H, Li T, Wang LP, et al. Dioscoreabulbifera polysaccharide and cyclophosphamide combination enhances anti-cervical cancer effect and attenuates immunosuppression and oxidative stress in mice. Sci Rep. 2016;5:19185.
- Han S, Ma C, Hu M, et al. A polysaccharide from Dictyophora indusiata inhibits the immunosuppressive function of cancer-associated fibroblasts. Cell Biochem Funct. 2017;35(7):414–419.
- Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6(11):1071–1072.
- Li XC, Demirci G, Ferrari-Lacraz S, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7(1):114–118.
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):218–228.
- Lundblad LK, Thompson-Figueroa J, Leclair T, et al. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171(12):1363–1370.
- Muller-Hermelink N, Braumuller H, Pichler B, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13(6):507–518.
- Hsu YL, Kuo PL, Liu CF, et al. Acacetin-induced cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Cancer Lett. 2004;212(1):53–60.
- Ji HK, Gi SN, Sung HK, et al. Orostachys japonicus exerts antipancreatic cancer activity through induction of apoptosis and cell cycle arrest in PANCcells. Food Sci Nutr. 2019;7(16):1–11.
- Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256(1):50–57.
- Mohana-Kumaran N, Hill DS, Allen JD, et al. Targeting the intrinsic apoptosis pathway as a strategy for melanoma therapy. Pigment Cell Melanoma Res. 2014;27(4):525–539.
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–418.
- Wei F, Hu Q, Huang J, et al. Screening active compounds from Corydalis yanhusuo by combining high expression VEGF receptor HEK293 cell membrane chromatography with HPLC - ESI - IT - TOF - MSn method . J Pharm Biomed Anal. 2017;136:134–139.
- Amaoka N, Saio M, Nonaka K, et al. Expression of vascular endothelial growth factor receptors is closely related to the histological grade of hepatocellular carcinoma. Oncol Rep. 2006;16(1):3–10.
