Abstract
This study explored whether inflammatory gene including C-reactive protein (CRP) and growth arrest-specific gene 6 (GAS6) are related to the etiology of ischemic stroke (IS), and to verify their potential interaction upon susceptibility to IS in Chinese population. A total of 236 patients with IS and 291 non-IS subjects were enrolled. Five single nucleotide polymorphisms (SNPs) in two candidate genes (CRP and GAS6) for IS were examined and determined by using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and detected by DNA sequencing. We evaluated the association between SNPs and the risk of IS and evaluated their potential gene–gene interactions by using the generalized multifactor dimensionality reduction (GMDR), interaction dendrogram, entropy analysis and Interaction circle graph. A significant gene–gene interaction was then selected. We further tested the best model by logistic regression models. The results showed no significant difference between the IS (or its subtypes) group and the control group in the single-locus of all the analyzed variants. GMDR analysis indicated that the CRP SNPs rs3093059 and the GAS6 SNPs rs7400722 shared strong synergism in LAA analysis (Testing accuracy = 59.66%, CVC = 10, p = 0.0107). Dendrogram and circle graph analysis indicated a more-than-additive effect between the two loci. Furthermore, a recessive model of the two-locus interactions was confirmed by multivariate logistic regression model (OR = 1.940, 95% CI: 1.068–3.525, p = 0.030). The results indicate that CRP and GAS6 genes may fully participate in affecting the susceptibility to IS by gene–gene interaction, and may be conducive to provide a novel area for IS research.
Introduction
Stroke is the second commonest cause of death and leading cause of adult disability worldwide [Citation1,Citation2]. However, it has risen to the leading cause of death since 2010, and has been a concern as a major public health problem in China [Citation3]. Among all subtypes, ischemic stroke (IS), as a polygenic and multifactorial disease, accounting for almost 80% of the total cases of stroke, is considered to be the main type of stroke [Citation4]. In addition to the traditional risk factors, such as hypertension, diabetes mellitus, obesity and smoking, accumulative evidence from animal, clinical and epidemiological studies have repeatedly supported some contribution of unclear genetic risk factors to common IS susceptibility [Citation5,Citation6].
Increasingly, research studies have suggested that inflammation significantly contributes to the risk of IS, progression and outcome [Citation7–9]. A number of studies have indicated that several susceptibility genes for IS will influence not only the risk of IS, but also severity and prognosis of IS [Citation10,Citation11]. Although the precise pathological mechanisms by which these genetic polymorphisms may contribute to IS are unclear, it has been documented that variations in the genes encoding C-reactive protein (CRP) and growth arrest-specific gene 6 (GAS6) could influence the CRP and GAS6 plasma levels [Citation12–18]. While several studies have investigated the role of polymorphisms of the CRP gene in the susceptibility of IS [Citation12,Citation14], there have been few studies on its partner, the GAS6 gene [Citation19]. Historically, most previously published reports have investigated these markers as isolated gene mutations, and several case–control studies of IS candidate genes have produced controversial results. IS leads to a variety of phenotypes, involving genetic and environmental risk factors. Thus, a study regarding investigating the impact of gene–gene risk factors interactions on risk of IS is necessary. Traditionally, most scholars have explored gene–gene and gene–environment interactions using multilocus linkage disequilibrium tests and logistic regression. But there is a certain degree of limitations due to its general application. This drawback is avoided by using the multifactor dimensionality reduction (MDR) method. MDR, known as a nonparametric approach, was first used for selecting estrogen-metabolism genes in sporadic breast cancer [Citation20]. Although MDR cannot analyze the main effect of the risk factors, it can explore higher order interactions, especially explore the gene–gene, gene–environment interactions in the pathogenesis of complex disease, and give a better explanation of the pathogenesis of disease at gene level [Citation21–23].
Previously, there was no reported study on the association between interactive genetic variants in CRP or GAS6 and IS. We hypothesized that the interaction of single nucleotide polymorphisms (SNPs) in CRP and GAS6 gene might confer higher IS risk than a single susceptibility gene. On the basis of these observations, we therefore focused on a case–control study to examine the independent gene effects of the polymorphisms of two inflammatory genes and also tested the hypothesis whether there were potential gene–gene interactions in the etiology of IS by using MDR and generalized multifactor dimensionality reduction (GMDR). In addition, since obviously there are different genetic risk factors for IS subtypes, we evaluated these genetic marker interactions in the whole database, as well as in IS subtypes.
Subjects and methods
Ethics statement
The study protocol was reviewed and approved by the Local Ethics Committee. All participants gave their informed consent.
Study population
This hospital-based case–control study included 236 first-ever patients with IS and 291 non-IS subjects. The patients were of Han Chinese origin and consecutively recruited from the Department of Neurology, Aerospace Central Hospital, China from October 2010 to December 2012. All cases were newly diagnosed by strict neurological examination, CT and/or MRI examination to confirm the presence of acute IS within 48 h after the admission. The diagnosis of IS was made in accordance with World Health Organization criteria [Citation24]. To determine the subtype of IS, the original TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria were used [Citation25]. Only two main subtypes of IS – large-artery atherosclerotic stroke (LAA) and small-artery occlusive stroke (SAO) – were included, because the candidate genes we selected were related to atherosclerosis and may have little association with other subtypes. Patients with brain tumour, vascular malformations, metabolic disorders (except diabetes mellitus), infections, auto-immune diseases, blood diseases, cancer and severe chronic diseases (e.g. liver and kidney dysfunction) were excluded from this study. Control subjects without history of stroke were randomly enrolled from the Medical Examination Center. The same exclusion criteria as those for patients with IS were also applied to the control group.
Questionnaire investigation was used to collect demographic information among all subjects including age, sex, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), blood glucose, total plasma cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and the history of hypertension, type 2 diabetes mellitus (T2DM), coronary heart disease (CHD), smoking and drinking status. Hypertension was defined as an average of three independent measures of systolic blood pressure ≥140 and (or) diastolic blood pressure ≥90 mmHg, or the use of antihypertensive drugs. Diabetes mellitus was diagnosed when the subject had a fasting glucose level of more than 7.8 mmol/L or was receiving antidiabetic drugs. Coronary heart disease was defined as having a clear history of angina pectoris and myocardial infarction. Cigarette smokers were categorized as more than 10 cigarettes per day for 5 years. Subjects were classified as drinkers if they drank alcohol at least once a week, and more than 50 mL each time, for more than half a year preceding the study. Family history of stroke was defined as at least one first-degree relative having suffered from IS. BMI of more than 25 kg/m2 was considered as obesity or overweight.
SNP selection and genotyping
The CRP gene is located between 1q21 and 1q23 and the gene is 2.3 kb in length, including 2 exons and 1 intron. GAS6 is located on chromosome 13q34, and is 43.8 kb in length, including 15 exons encoding 678 amino acids. SNPs within the CRP gene and GAS6 gene were obtained from the NCBI SNP database (http://www.ncbi.nlm.nih.gov/SNP) and the International HapMap project data for the Han Chinese population (http://hapmap.ncbi.nlm.nih.gov/). Tagging SNPs were selected using the Haploview programme with a minor allele frequency greater than 0.05. We also selected SNPs on the basis of published data previously reporting to have a relationship with IS.
Five variants in these two candidate genes of IS were examined, including rs1130864 (1444C > T), rs1800947 (1059G > C) and rs3093059 (–757A > G) in the CRP gene, and rs8191974 (c.834 + 7G > A) and rs7400722 (c.1478-1315C > T) in GAS6 gene. They were genotyped using polymerase chain reaction-restrictive fragment length polymorphism (PCR-RFLP) approach and 5% of samples were detected by DNA sequencing.
A 4-mL peripheral blood sample was obtained from each participant for DNA analysis after overnight fasting and was added into an ethylene diamine tetraacetic acid (EDTA) anticoagulation tube. Genomic DNA from all participants was extracted from peripheral white blood cells using an AxyPrep genomic DNA extraction kit according to the manufacturer’s instructions. The primers and restriction enzymes used for detection of the five SNPs are listed in . Primers were designed using Premier 5.0 software and synthesized by Shanghai Invitrogen Biotechnology Co., Ltd. (Shanghai, China). The total volume of the PCR mixture was 20 μL including 10 ng of template DNA, 10 μL of 2 × PCR buffer, 1.5 U of Taq enzyme, 0.2 mmol/L of dNTP and 1 μL of each 5′ and 3′ primers, following 25 cycles of 30 s at 95 and 60 °C and 90 s at 72 °C. Then, PCR-RFLP products were separated in 3% agarose gels and analyzed using a gel imaging analysis system. PCR products were further verified by digestion with 2 U restriction endonuclease (New England Biolabs, Beijing, China) at 65 °C for 1 h and analyzed by 3% agarose gel electrophoresis.
Table 1. Description and PCR primers, restriction enzymes and PCR product size for 5 SNPs.
Statistical analysis
Database management and statistical analyses were conducted by SPSS 13.0 software package. The differences between the IS group and the control group were calculated by Student’s unpaired t-test for quantitative variables, and Pearson’s χ2 test or Fisher’s exact test for categorical variables. The χ2 test was also used to assess the deviation from the Hardy–Weinberg equilibrium of all the selected SNPs among the controls and investigate the association between SNPs and the risk of IS. To analyze the gene–gene interactions, GMDR was used to determine the best genetic model from several SNPs that could most probably predict the risk of IS [Citation22]. We constructed all possible combinations of genotypes of five SNPs. This method included a combined 10-fold cross-validation and 1000-fold permutation-testing procedure that minimizes false-positive results by multiple examinations of the data. The testing balanced accuracy, cross-validation consistency and the sign test were implemented in the GMDR software (v.0.9). The model with the highest accuracy and maximal CV was considered to be the best. Then, the genetic data were collapsed into two categories with high and low risk in each cell across the 9 two-locus genotype cells. The cumulative score value was calculated within each multifactor cell, which would be labelled either as low-risk (light grey) if the score was less than 0 or as high-risk (dark grey) if the average score met or exceeded 0. Finally, we visualized the nature of the dependencies using an Interactive dendrogram and interaction circle graph by MDR (v.2.0) [Citation21]. Multiple logistic regression analysis was performed to evaluate these genotypes and their combinations results from MDR analyses. Odds ratios and 95% confidence intervals were calculated by logistic regression analysis. A two-tailed p < 0.05 was considered to indicate statistical significance.
Results and discussion
Demographic and medical characteristics
The aim of our study was to establish the role of these inflammatory genes in IS susceptibility, and to detect their interaction upon IS. In our study, there was no significant difference in terms of age and BMI between the IS group and the control group. However, the IS group overall were predominantly male and had a higher levels of smoking and drinking habits, DBP, SBP, diabetes mellitus, history of coronary heart disease and family history of stroke than the control group (p < 0.05). In addition, we analyzed 174 cases of LAA and 62 cases of SAA according to the original TOAST criteria ().
Table 2. Demographic and medical characteristics of the IS and control group.
Single polymorphism analysis
This is the first study to report the interactive association between polymorphisms of CRP and GAS6 gene and IS in Chinese patients. Based on previous studies and Hapmap database, we chose CRP (rs1130864, rs1800947 and rs3093059) and GAS6 (rs8191974 and rs7400722) as the candidate genes. All genotype distributions of the five polymorphisms in the control group were consistent with HWE. However, no significant differences were apparent between the patients with IS and control subjects in the genotype distributions of all variants. The outcome was the same for subgroup analysis ().
Table 3. Association measures between SNPs and IS or its subtypes.
CRP is thought to be a strong acute reactant of inflammatory phase and a novel plasma marker of systemic inflammation; many studies have supported its role in the development of IS. Previous studies have shown that CRP can induce the production of adhesion molecules in endothelial cells [Citation26,Citation27], promote the conversion of low density lipoproteins into foam cells [Citation28,Citation29], recruit monocytes through receptor-mediated chemotaxis and induce the release of tissue factor [Citation30,Citation31], which in turn promote the development of atherosclerosis and lead to the occurrence of IS. Elevated CRP level is influenced by genetic factors in many studies [Citation14,Citation17,Citation32] and the potential association between CRP SNPs and IS has led to great interest in the IS genetic research. For example, Kuhlenbaeumer et al. [Citation33] in a German population demonstrated that rs1130864 and rs1800947 of the CRP gene were significantly associated with microangiopathic stroke. A prospective American population-based study also reported a similar association of the T allele within rs1130864 with risk of further IS in patients with symptomatic intracranial atherosclerotic disease [Citation12]. A Japanese study by Morita et al. [Citation34] indicated that the recessive model of rs1800947 was significantly different between patients with IS and control subjects, but there was a negative outcome for rs1130864. A Chinese case–control study including 548 patients with acute IS and 993 age-matched control subjects indicated that rs3093059 was statistically significantly associated with IS [Citation14]. However, controversial views still remain [Citation18,Citation35–37] and that was in accordance with our results.
GAS6 belongs to the family of plasma vitamin K-dependent growth factors, and is expressed in many cell types, including endothelial cells, vascular smooth muscle cells, bone marrow stromal cells and fibroblasts, as well as in human plasma and platelets [Citation38]. Previously, several studies have shown that GAS6 primarily binds to Tyro3, Axl and Mertk (TAM) receptors involved in the inflammatory response, platelet activation, thrombus formation and atherosclerosis [Citation39,Citation40], which suggests that GAS6 may have a relevant role in a common mechanism in the etiology of IS. It has been proved that inhibition of the GAS6/TAM system protects mice against arterial and venous thrombosis and decreases platelet activation responses without increasing bleeding, regarding it as a reasonable clinical trial candidate for novel anti-platelet effect [Citation41]. A study by Munoz et al. [Citation19] reported that GAS6 was associated with cerebrovascular disease and demonstrated that the AA genotype of rs8191974 and the CACA haplotype defined by the intron 3, intron 7 and intron 8, might have a protective role for stroke. However, no association was observed between rs8191974 and carotid atherosclerosis by Hurtado et al.’s study [Citation42]. These conflicting results may be due to allelic or genetic heterogeneity, or limitations in study designs, or differences in stroke etiology between populations. We are the first to choose rs7400722 as a candidate SNP from Chinese population Hapmap database by using the Haploview software; because its minor allele frequency is higher than 5%, we hypothesized that it may be related to stroke. However, no significant correlation was found in this study.
Gene–gene interaction models in IS susceptibility
Although none of the SNPs alone provided substantial evidence for an association with IS, a potential interaction existed. Given that the pathogenesis of IS involves complex multiple gene–gene interactions, the role of a single gene mutation may not be enough to cause disease or only a weak effect. We speculated that the effect that a single SNP contributes to IS might be too small to be observed, and may not be sufficient to to explain complex IS disorders, which do not follow a simple Mendelian pattern of inheritance.
In this study, we used a GMDR model to detect potential synergistic effects among several SNPs, because this model could strengthen the validity of our results to some extent and statistically overcome the small sample size, as well as because it had no limitation on dimension in the interaction analysis. A total of five SNPs of CRP and GAS6 gene were included in the analysis of gene–gene interactions for overall stroke, LAA and SAA by the GMDR method. list the results obtained from GMDR analysis for different combinations of five loci models for gene–gene interaction. In the one-locus model, rs3093059 was the best attribute for predicting LAA (testing accuracy = 46.08%; CVC = 7; p = 0.8281). However, the 2-locus model involving rs3093059 of the CRP gene and rs7400722 of the GAS6 gene scored 10 for cross-validation consistency and 9 for Sign Test (p = 0.0107 on 1000 permutations) and showed the highest level of testing accuracy (59.66%) in LAA analysis (). shows the best model for LAA identified by GMDR, suggesting that their contribution to the risk was due to the joint action of the two genes.
Figure 1. Interaction information for two SNPs by GMDR method. In each cell, the left bar represents a positive score, and the right bar represents a negative score. High-risk cells are indicated by dark shading, low-risk cells by light shading.
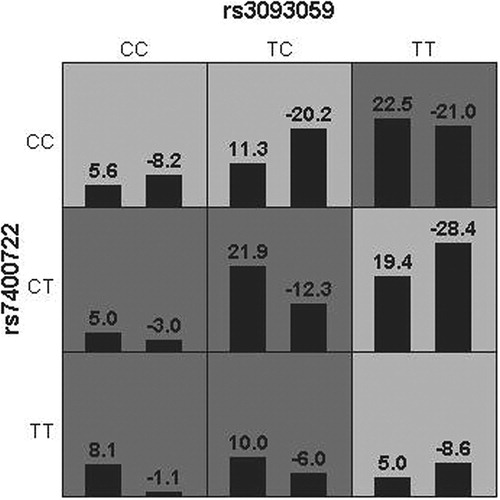
Table 4. Multifactor dimensionality reduction results in total IS patients and control subjects.
Table 5. Multifactor dimensionality reduction results in LAA patients and control subjects.
Table 6. Multifactor dimensionality reduction results in SAA patients and control subjects.
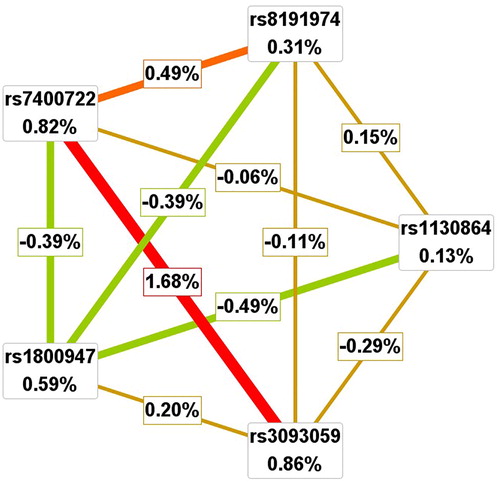
We further applied the interaction dendrogram graph for LAA to determine whether there was a synergistic relationship using the MDR method (). The hierarchical cluster analysis placed rs3093059 of the CRP gene and rs7400722 of the GAS6 gene on the same branch, connected with a red line, and their distinguishingly closer position in the diagram clearly showed that the two-locus model might have a strong synergistic effect on modulating the risk of LAA. Consistent with the results from GMDR analysis and the interaction dendrogram, the interaction circle graph also supported the two-locus model that had an interaction effect. We found that small percentages of the entropy in the case–control status were explained by CRP rs3093059 (0.86%), GAS6 rs7400722 (0.82%) when considered independently, but a large percentage of the circle graph was explained by the pairwise interactions between the SNPs of the two locus model (1.68%), which indicated a synergistic interaction ().
Figure 2. Interaction dendrogram for LAA by MDR method. In the upper panel, a red or orange line connecting two SNPs suggests a positive information gain that can be interpreted as a synergistic or non-additive relationship, whereas a green or blue line suggests a loss of information that can be interpreted as redundancy or correlation. A yellow line indicates independence or additivity. It is clear that the CRP rs3093059 and GAS6 7400722 have the strongest synergistic interaction.

Our GMDR analysis revealed a strong 2-locus interaction on risk of IS between rs3093059 of the CRP gene and rs7400722 of the GAS6 gene. This positive association was strengthened by logistic regression analysis, which suggested participants with rs3093059-TC or CC and rs7400722-CT or TT genotype had a higher risk of IS, compared to participants with rs3093059-TT and rs7400722-CC genotype (OR = 1.867, 95% CI: 1.087–3.206, p = 0.024; ). And this association did not vary by multiple logistic regression analysis after the adjustment for traditional risk factors (OR = 1.940, 95% CI: 1.068–3.525, p = 0.030). The results support the suggestion that the interaction of CRP and GAS6 gene conferred higher risk of LAA than a single susceptibility gene. We believe that there is a synergistic interaction between the two loci. Although their specific combination pairs could give rise to IS as susceptibility factors, the accurate mechanism of the interaction was not known. One possible explanation for the two SNPs interaction was that these two genes all have some association with atherosclerosis, one of the principal pathogenesis factors of IS.
Table 7. Combined effects of rs3093059 and rs7400722 on the risk of LAA.
Conclusions
This study demonstrated evidence for the role of CRP and GAS6 gene in IS susceptibility by gene–gene interactions. A combination of the recessive model of rs3093059 of CRP gene and rs7400722 of the GAS6 gene may cause a significantly higher risk of stroke, which may open up a novel strategy in the search for genetic risk of IS. Due to the small sample size, further prospective studies with a larger-scale are necessary. Although this was a basic medical research, our findings may possibly highlight the importance of taking into account such interactions in the etiology of IS.
Authors contribution
Xu Yang and Xinyi Li conceived the project. Xiaofeng Li wrote the paper. Xiaofeng Li, Zhongyun Chen and Yongjun Gao performed the experiments and analyzed the data. Xiaoli Zhao prepared the experimental samples.
Acknowledgements
We thank Director Jin-ying Gao, Department of Medical Examination Center, Aerospace Central Hospital, for providing us with the data of the control group. We thank all the staff and patients who participated in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139(10):56–528.
- Thrift A, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke. 2017;12(1):13–32.
- Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381(9882):1987–2015.
- Della-Morte D, Guadagni F, Palmirotta R, et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics. 2012;13(5):595–613.
- Mallolas J, Hurtado O, Castellanos M, et al. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J Exp Med. 2006;203(3):711–717.
- Jerrard-Dunne P, Cloud G, Hassan A, et al. Evaluating the genetic component of ischemic stroke subtypes: a family history study. Stroke. 2003;34(6):1364–1369.
- Rodriguez-Yanez M, Castillo J. Role of inflammatory markers in brain ischemia. Curr Opin Neurol. 2008;21(3):353–357.
- Shi K, Tian D, Li Z, et al. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–4422.
- Eldahshan W, Fagan S, Ergul A. Inflammation within the neurovascular unit: focus on microglia for stroke injury and recovery. Pharmacol Res. 2019;147:104349. Epub 2019 Jul 14.
- Zhao N, Liu X, Wang Y, et al. Association of inflammatory gene polymorphisms with ischemic stroke in a Chinese Han population. J Neuroinflamm. 2012;9:162.
- Tuttolomondo A, Di Raimondo D, Forte GI, et al. Single nucleotide polymorphisms (SNPs) of pro-inflammatory/anti-inflammatory and thrombotic/fibrinolytic genes in patients with acute ischemic stroke in relation to TOAST subtype. Cytokine. 2012;58(3):398–405.
- Arenillas JF, Massot A, Alvarez-Sabin J, et al. C-reactive protein gene C1444T polymorphism and risk of recurrent ischemic events in patients with symptomatic intracranial atherostenoses. Cerebrovasc Dis. 2009;28(1):95–102.
- Chang SN, Lai LP, Chiang FT, et al. C-reactive protein gene polymorphism predicts the risk of thromboembolic stroke in patients with atrial fibrillation: a more than 10-year prospective follow-up study. J Thromb Haemost. 2017;15(8):1541–1546.
- Shen C, Sun X, Wang H, et al. Association study of CRP gene and ischemic stroke in a Chinese Han population. J Mol Neurosci. 2013;49(3):559–566.
- Lee CH, Chu NF, Shieh YS, et al. The growth arrest-specific 6 (Gas6) gene polymorphism c.834 + 7G > A is associated with type 2 diabetes. Diabetes Res Clin Pract. 2012;95(2):201–206.
- Jiang L, Liu CY, Yang QF, et al. Plasma level of growth arrest-specific 6 (GAS6) protein and genetic variations in the GAS6 gene in patients with acute coronary syndrome. Am J Clin Pathol. 2009;131(5):738–743.
- Ben Assayag E, Shenhar-Tsarfaty S, Bova I, et al. Association of the -757T > C polymorphism in the CRP gene with circulating C-reactive protein levels and carotid atherosclerosis. Thromb Res. 2009;124(4):458–462.
- Ladenvall C, Jood K, Blomstrand C, et al. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke. 2006;37(8):2018–2023.
- Munoz X, Obach V, Hurtado B, et al. Association of specific haplotypes of GAS6 gene with stroke. Thromb Haemost. 2007;98(2):406–412.
- Ritchie MD, Hahn LW, Moore JH. Power of multifactor dimensionality reduction for detecting gene-gene interactions in the presence of genotyping error, missing data, phenocopy, and genetic heterogeneity. Genet Epidemiol. 2003;24(2):150–157.
- Ritchie MD, Hahn LW, Roodi N, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69(1):138–147.
- Xu HM, Xu LF, Hou TT, et al. GMDR: versatile software for detecting gene–gene and gene–environment interactions underlying complex traits. Curr Genomics. 2016;17(5):396–402.
- Moore JH. Computational analysis of gene-gene interactions using multifactor dimensionality reduction. Expert Rev Mol Diagn. 2004;4(6):795–803.
- Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20(10):1407–1431.
- Adams H, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41.
- Chen C, Nan B, Lin P, et al. C-reactive protein increases plasminogen activator inhibitor-1 expression in human endothelial cells. Thromb Res. 2008;122(1):125–133.
- Han C, Liu J, Liu X, et al. Angiotensin II induces C-reactive protein expression through ERK1/2 and JNK signaling in human aortic endothelial cells. Atherosclerosis. 2010;212(1):206–212.
- Bhakdi S, Torzewski M, Klouche M, et al. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999;19(10):2348–2354.
- Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103(9):1194–1197.
- Huang X, Zhang J, Liu J, et al. C-reactive protein promotes adhesion of monocytes to endothelial cells via NADPH oxidase-mediated oxidative stress. J Cell Biochem. 2012;113(3):857–867.
- Cermak J, Key NS, Bach RR, et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82(2):513–520.
- den Hertog HM, van den Herik EG, Dippel DW, et al. Variation in the C-reactive protein gene is associated with serum levels of CRP in patients with acute ischemic stroke. Cerebrovasc Dis. 2010;29(4):372–375.
- Kuhlenbaeumer G, Huge A, Berger K, et al. Genetic variants in the C-reactive protein gene are associated with microangiopathic ischemic stroke. Cerebrovasc Dis. 2010;30(5):476–482.
- Morita A, Nakayama T, Soma M. Association study between C-reactive protein genes and ischemic stroke in Japanese subjects. Am J Hypertens. 2006;19(6):593–600.
- Das S, Roy S, Kaul S, et al. CRP gene (1059G > C) polymorphism and its plasma levels in ischemic stroke and hemorrhagic stroke in a south Indian population. Inflammation. 2014;37(5):1683–1688.
- Andersson J, Johansson L, Ladenvall P, et al. C-reactive protein is a determinant of first-ever stroke: prospective nested case-referent study. Cerebrovasc Dis. 2009;27(6):544–551.
- Flex A, Gaetani E, Papaleo P, et al. Proinflammatory genetic profiles in subjects with history of ischemic stroke. Stroke. 2004;35(10):2270–2275.
- Furukawa M, Wang X, Ohkawara H, et al. A critical role of the Gas6-Mer axis in endothelial dysfunction contributing to TA-TMA associated with GVHD. Blood Adv. 2019;3(14):2128–2143.
- van der Meer JH, van der Poll T, van't Veer C. TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood. 2014;123(16):2460–2469.
- Zhou J, Yang A, Wang Y, et al. Tyro3, Axl, and Mertk receptors differentially participate in platelet activation and thrombus formation. Cell Commun Signal. 2018;16(1):98.
- Law LA, Graham DK, Di Paola J, et al. GAS6/TAM pathway signaling in hemostasis and thrombosis. Front Med (Lausanne). 2018;5:137.
- Hurtado B, Abasolo N, Muñoz X, et al. Association study between polymorphims in GAS6-TAM genes and carotid atherosclerosis. Thromb Haemost. 2010;104(3):592–598.