Abstract
Grain hardness and starch are two of the most important factors that determine the end-use quality of bread wheat (Triticum aestivum L.) grain. The grain hardness and amylose content are controlled by the puroindolines (Pina-D1 and Pinb-D1) genes, located on chromosomes 5D, and waxy (Wx-A1, -B1 and -D1) genes, located on chromosomes 7A, 4A and 7D. A total of 160 Iranian landraces from the Germplasm Bank of the International Maize and Wheat Improvement Center were evaluated for grain hardness using near infrared spectroscopy to predict the particle size index (PSI). In addition, molecular markers were used to evaluate the states of the corresponding genes. Eight accessions were found to have a hard texture (predicted PSI < 45%); however, only two could be explained by null alleles either in Pina-D1 or Pinb-D1. Additionally, 152 accessions had semi-hard textures (predicted PSI range of 45% to 55%). For the Wx gene, only one accession (CWI 67665) showed the null Wx-D1b allele, while two accessions (CWI 67747 and CWI 57684) were null for Wx-B1b. Single nucleotide polymorphism and sequence tag site marker techniques were used. Our findings further indicate the importance of using these landraces for grain quality improvement in breeding programs.
Introduction
Bread wheat (Triticum aestivum L. ssp. aestivum; 2n = 6x = 42, AABBDD) is an important crop worldwide and is estimated to be the staple food for 2.5 billion people in 89 countries (more than 30% of the world population) [Citation1]. Grain hardness, starch properties and dough (gluten) viscoelastic properties are quality aspects that explain most of the variation in wheat grain quality traits [Citation2–4]. Grain hardness or texture is one of the most important single factors determining the end-use food properties of wheat grain [Citation5–7], and it also determines the marketing of wheat grain [Citation8]. On the basis of this trait, wheat is classified into three main classes: very hard (durum wheat), hard and soft (bread wheat) [Citation9]. Hard and soft wheat mill differently. Compared with soft wheat flours, hard wheat flours have greater levels of damaged starch, which leads to increased water absorption, something desirable for bread making [Citation10, Citation11]. In general, hard wheat is used for making bread and soft wheat is used in the manufacture of cookies, cakes and pastries [Citation7, Citation8].
Grain hardness is genetically controlled by genes which have been reported to be located at the hardness locus (Ha) in the distal end of the short arm of chromosome 5D in bread wheat [Citation8, Citation12–14], but in durum wheat (Triticum turgidum ssp. durum Desf. em. Husn.; 2n = 4x = 28, AABB) and other tetraploid species, these genes are not present, because of the absence of the D genome, and the complete deletion of these genes in the A and B genomes during the evolution of tetraploid wheat [Citation13]. Studies have shown that the hardness (Ha) locus of bread wheat has a complex structure within an 82-kb region [Citation13]. This includes two genes (Pina-D1 and Pinb-D1) that code for two basic grain proteins, the puroindoline a and b (Pina and Pinb) of ∼13 KDa, together with the grain softness protein. The Pins (a and b) are unique among other plant proteins because of their basic cysteine-rich nature and the tryptophan-rich domains [Citation6]. The presence of wild type alleles (Pina-D1a and Pinb-D1a) is correlated with soft grain in bread wheat, whereas nucleotide changes in the coding regions or deletions of whole Pin-D1 genes (null alleles) are correlated with a hard texture [Citation15–18]. The gene polymorphisms lead to differences in the degree of hardness [Citation19]. The literature indicates that genotypes harboring Pina-D1b/Pinb-D1a have harder grains than genotypes that carry Pina-D1a/Pinb-D1b [Citation20–24].
Starch composition is another parameter affecting the processing and end-use quality of wheat grain. This macromolecule is composed of two types of glucose polymers, amylose and amylopectin [Citation25], with a ratio of 20%-30% amylose to 70%-80% amylopectin [Citation26]. The physical and chemical properties of starch (gelatinization, pasting and gelation) depend on the relative amounts of amylose and amylopectin [Citation27, Citation28]. Therefore, starches with different amylose/amylopectin ratios are needed for various industrial applications [Citation29, Citation30]. Environmental factors (such as temperature) and growth conditions can influence this ratio [Citation31]. Granule-bound starch synthase I or waxy (Wx) protein is the key enzyme in amylose synthesis and is encoded in bread wheat by the Wx-A1, -B1 and -D1 genes, which are located on the 7A, 4A and 7D chromosomes [Citation32–34].
In recent years, several studies have been conducted to identify new waxy alleles in different wheat-related species, such as einkorn and Aegilops [Citation35, Citation36] and in landraces [Citation37] to increase the genetic resources used to develop wheat varieties with novel starch properties and end-use quality characteristics. Wheat landraces are important sources of genetic diversity that can improve the gene pools of modern cultivars by introducing new alleles [Citation38–42]. Iran is one of the primary habitats of wheat’s ancestors and is, therefore, a reservoir for new alleles. The International Maize and Wheat Improvement Center (CIMMYT) gene bank possesses ∼150,000 wheat and related species accessions, among which 48,600 accessions are of traditional durum and bread wheat landraces. This includes 6,947 accessions from Iran. The main aim of this study was to determine the distribution of Pin-D1 and Wx-1 alleles in a collection of Iranian bread-wheat landraces from the CIMMYT Germplasm Bank.
Materials and methods
Plant materials
A total of 160 Iranian bread-wheat landraces, held as accessions in the germplasm bank of the CIMMYT (Texcoco, Edo. de Mexico, Mexico) (Supplemental Table S1) were used in the study. The accessions were evaluated at the experimental field station ‘CENEB’ located near Ciudad Obregon, Sonora, Northwest Mexico (27_209 N, 109_549 W, 38 m above sea level), during the 2010 − 2011 cropping cycle under optimum growing conditions (full irrigation).
Quality parameters
Quality parameters were determined using the methods established by the American Association of Cereal Chemists [Citation43]. The grain protein content (GPC, 12.5% moisture basis) and hardness particle size index (PSI, %) were determined using a near infrared (NIR) spectroscopic NIR System 6500 (FOSS-Tecator, HillerØd, Denmark), calibrated for protein content using the Kjeldahl method [Citation43] and for hardness using the PSI method [Citation44]. Soft wheat endosperm produces a greater proportion of fine particles that correspond to higher PSI percentages. Based on the predicted PSI data obtained by NIR, samples were classified as hard (30%-44%), semi-hard (45%-55%) and soft (>55%).
Genomic DNA extraction
Genomic DNA was extracted from young leaves of two-week-old seedlings grown in a greenhouse using a modified CTAB method described in Dreisigacker et al. [Citation45]. The quality and quantity of DNA were evaluated in 1% agarose gels and using a Nanodrop 8000 spectrophotometer (Thermo Scientific, USA) and the samples were diluted to a final concentration of 50 ng/mL.
Sequence tag site (STS) markers
Sequences of PCR primers and fragment sizes are shown in . Each 10-µL reaction included 50 ng DNA, 1.5 mmol/L MgCl2, 0.6 µmol/L of each primer, 0.8 µmol/L dNTPs, 1.5 ml 2.0× PCR buffer and 0.05 U Taq polymerase (Go Taq Flexi, Promega Crop., Cat. #M8295). PCR was performed in an ABI Genamp 9700 PCR Thermocycler (Applied Biosystems, USA). The PCR conditions included an initial denaturation step of 2 min at 94 °C, followed by 30 cycles of 1 min at 94 °C, 2 min at 60 °C and 2 min at 72 °C. There was then a final 5-min extension at 72 °C. The amplification products were separated in 2.5% agarose gels.
Table 1. List of STS markers used in this study.
Kompetitive allele-specific PCR (KASP) markers
KASP is single-step genotyping technology that detects, using a fluorescence-based application, pre-identified co-dominant and dominant alleles of both single nucleotide polymorphism (SNP) and insertion/deletion variants [Citation49]. The primer sequences and more detailed allelic information are provided in . For Pina-D1 and Pinb-D1, KASP assays were conducted in a 384-well format and performed in 4.2 µL reactions containing 2.1 mL sterile water, 2 mL 2× KASP Mix, 0.1 µL assay mix and 50 ng of dried DNA. For Wx-B1, KASP assays were performed using optimized buffer that contained MgCl2 (50 nm). PCR amplification was performed in a GenAmp PCR system 9700 Thermal cycler (Applied Biosystems) using the following cycling conditions: 94 °C for 15 min for hot-start Taq DNA polymerase activation, 11 cycles at 94 °C for 30 s, 65 °C for 60 s (-0.8 °C each cycle) and 72 °C for 30 s, followed by 26 cycles at 94 °C for 30 s, 57 °C for 60 s and 72 °C for 30 s. Then, there was a final extension at 72 °C for 5 min. End-point fluorescent images were visualized using PHERAstarplus (BMG LABTECH, Germany), and the data were analyzed using KLuster CallerTM software (LGC Genomics).
Table 2. Kompetitive allele PCR assays for used in this study.
Results and discussion
Grain hardness
A wide range of predicted PSI values was identified for the accessions (), with most of the samples (152) having semi-hard textures (predicted PSIs ranging between 45% and 55%). Eight accessions had hard textures (predicted PSIs < 45%) and none had a soft texture (PSI > 55%).
Table 3. Allelic compositions of puroindoline genes and the PSI values of the Iranian bread-wheat landrace accessions.
STS marker analysis
Primers from Gautier et al. [Citation6] were used to amplify Pina-D1 and Pinb-D1 genes and detect possible differences in amplicon presence/absence and size. The amplification of Pina-D1 yielded an expected PCR product of ∼349 bp. Most of the accessions produced a PCR product of ∼349 bp (Pina-D1a), except for two accessions (CWI56592 and CWI72061), which produced no amplification products, indicating that they were Pina-D1b(a-null) (). These two accessions were classified as hard and semi-hard based on the predicted PSI values. For the Pinb-D1 gene, most of the accessions produced a PCR product of 250 bp (Pinb-D1a) (), except for five accessions (CWI 73113, CWI 67068, CWI 72525, CWI 71600 and CWI 57139). The lack of product was considered as an indication of the absence of Pinb-D1 (Pinb null). Of these accessions, CWI 73113 was classified as hard and CWI 67068, CWI 72525, CWI 71600 and CWI 57139 were classified as semi-hard based on the predicted PSI values.
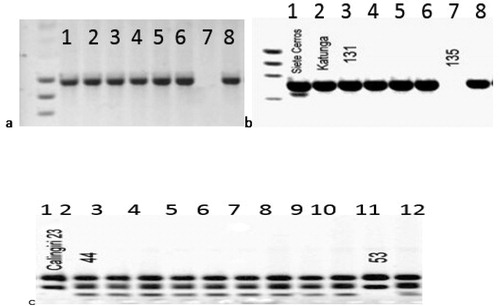
Figure 1. PCR analysis of Iranian bread-wheat landraces using STS primers from Gautier et al. [Citation6].
(a) Pina-D1 amplification in landraces Krichauff (Pina-D1a/Pinb-D1null), Berkut1 (Pina-D1b/Pinb-D1a), number 92 CWI720619 (Pina-D1b/Pinb-D1a) and number 149 CWI56592 (Pina-D1b/Pinb-D1a). (b) Pinb-D1 amplification in landraces Chinese Spring (Pina-D1a/Pinb-D1a), Krichuff (Pina-D1a/Pinb null), number 3 CWI 73113 (Pina-D1a/Pinb-D1 null), number 10 CWI 67068 (Pina-D1a/Pinb-D1 null), number 39 CWI 72525 (Pina-D1a/Pinb-D1 null), number 88 CWI 71600 (Pina-D1a/Pinb-D1 null) and number 119 CWI 57139 (Pina-D1a/Pinb-D1 null).
![Figure 1. PCR analysis of Iranian bread-wheat landraces using STS primers from Gautier et al. [Citation6].(a) Pina-D1 amplification in landraces Krichauff (Pina-D1a/Pinb-D1null), Berkut1 (Pina-D1b/Pinb-D1a), number 92 CWI720619 (Pina-D1b/Pinb-D1a) and number 149 CWI56592 (Pina-D1b/Pinb-D1a). (b) Pinb-D1 amplification in landraces Chinese Spring (Pina-D1a/Pinb-D1a), Krichuff (Pina-D1a/Pinb null), number 3 CWI 73113 (Pina-D1a/Pinb-D1 null), number 10 CWI 67068 (Pina-D1a/Pinb-D1 null), number 39 CWI 72525 (Pina-D1a/Pinb-D1 null), number 88 CWI 71600 (Pina-D1a/Pinb-D1 null) and number 119 CWI 57139 (Pina-D1a/Pinb-D1 null).](/cms/asset/4b9c2997-9e8e-4435-921c-cb68b379c7c5/tbeq_a_1814866_f0001_c.jpg)
For the Wx loci, all the accessions contained the alleles Wx-A1a and Wx-D1a in experiments using the markers MAG264 and MAG269, respectively, except for one accession (CWI67665) that had no amplification product, indicating Wx-D1b (null mutation) (). At the Wx-B1 locus, tested using marker Wx-B1, all the accessions contained the allele Wx-B1a, except for two accessions (CWI67747 and CWI57684), indicating Wx-B1b (null mutation) ().
Figure 2. PCR analysis of Iranian bread-wheat landraces indicating the Wx-D1b and Wx-B1b (null mutations) (a) 1: Pavon (Wx-D1a); 2, Chinese Spring (Wx-D1a); 3, CWI 73213 (Wx-D1a); 4, CWI 7314 (Wx-D1a); 5, CWI 73215 (Wx-D1a); 6, CWI 73216 (Wx-D1a); 7, CWI 67665 (Wx-D1b); 8, CWI 67674 (Wx-D1a); (b) 1, Chinese Spring (Wx-B1a); 2, Katunga (Wx-B1a); 3, CWI 71829 (Wx-B1a); 4, CWI 71830 (Wx-B1a); 5, CWI 57655(Wx-B1a); 6, CWI57662 (Wx-B1a); 7, CWI 57684 (Wx-B1b); 8, CWI 57692 (Wx-B1a); (c) 1, Calingiri 23 (Wx-B1b); 2, CWI 73213 (Wx-B1a); 3, CWI 7314 (Wx-B1a); 4, CWI 73215 (Wx-B1a); 5, CWI 73215 (Wx-B1a); 6, CWI 67665 (Wx-B1a); 7, CWI67674 (Wx-B1a); 8, CWI 67701 (Wx-B1a); 9, CWI 67703 (Wx-B1a); 10, CWI 67708 (Wx-B1a); 11, CWI 67747 (Wx-B1b); 12, CWI 67749 (Wx-B1a).
KASP marker analysis
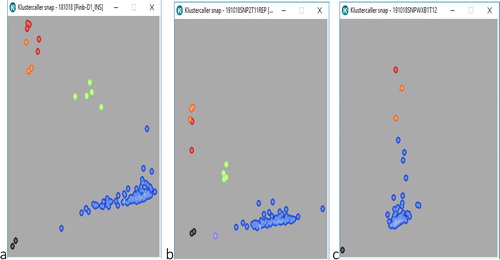
KASP primers were used in this study to identify the pina-D1/pinb-D1 and Wx-B1 genes based on sequences upstream and downstream of the detected SNPs (with the 3′ end of the forward primer positioned at the mutant SNP site). For Pina-D1, two accessions (CWI 56592 and CWI 72061) shown in red (VIC) with null type alleles pina-D1b (A/G SNP) produced a hard texture in bread wheat. For Pinb-D1, five accessions (CWI 73113, CWI 57816, CWI 72525, CWI 71600 and CWI 57139) shown in red (VIC) with Pinb-D1b alleles (C/T SNP) produced hard textures. This identification is due to the codon change Gly-46 to Ser-46 and other accessions shown in blue (FAM) with wild-type alleles (pina-D1a and pinb-D1a) produced soft textures in bread wheat. No heterozygosity was detected (). For Wx-B1a, only one accession (CWI 67747) shown in red (VIC) had the null allele (Wx-B1b), and all the accessions shown in blue (FAM) had the wild-type allele (Wx-B1a) ().
Figure 3. Codominant KASP markers developed in Iranian bead-wheat landraces for identifying (a) and (b) the Pin-D alleles. Black dots represent the non-template controls, blue dots represent the FAM-type or wild-type (soft) pina-D1a/pinb-D1a, green dots represent heterozygous alleles, red dots represent VIC or mutant-type (hard) pina-D1b/pinb-D1b and purple dots represent non amplification. (c) For Wx-B alleles with a dominant marker. Black dots represent the non-template controls; blue dots represent the FAM-type or wild-type Wx-B1a and red dots represent VIC or mutant-type Wx-B1b.
Bread-making quality is a key target of breeding programs. It is controlled by wheat genetics and the environment. Grain hardness influences wheat milling and flour viscoelastic properties [Citation51–54], and it is used for marketing classifications [Citation8]. In the present study, a collection of Iranian bread-wheat landraces held in the CIMMYT’s wheat germplasm bank was analyzed for grain hardness using NIR calibrated by PSI and characterized at the molecular level by Pin compositions and Wx genes. The accessions were classified into hard and semi-hard wheat. In eight of the accessions showing a hard grain texture, two could be explained by a null mutation in either Pina-D1 or Pinb-D1, which corroborated earlier findings [Citation24, Citation55, Citation56]. Accessions harboring Pina-D1 null alleles may be harder than those with other Pin alleles, such as Pinb-D1b [Citation23, Citation57], and have low milling yields [Citation19, Citation22, Citation23]. This mutation has been reported in numerous studies worldwide [Citation16, Citation24, Citation49, Citation57–62]. However, our result did not show differences in grain hardness between Pina-D1b and Pinb-D1null [Citation63]. In this study, the findings may have been influenced by the following: 1) environmental conditions and grain size [Citation14, Citation64–67]; however, Pomeranz et al. [Citation68] showed that hardness-related measurements were more affected by genotype than by environment; 2) other minor genes [Citation23, Citation69–71]; 3) the limited accuracy of NIR to predict PSI; 4) the limited number of accessions with different Pin-D1 alleles and the different genetic backgrounds of the accessions, which led to unidentifiably small differences owing to the presence of different Pin-D1 alleles [Citation53]. Grain protein content is directly related to grain hardness [Citation14, Citation58, Citation70, Citation72–74]. In the present study, the grain protein content was 15.7% on average. Thus, it was not surprising that few of the accessions showing hard grain phenotypes had protein contents of ∼15.43%. (data not shown).
However, Symes [Citation75] reported that the correlation between protein content and hardness was positive in some cultivars and negative in other cultivars, which was corroborated by our results.
Starch composition is another parameter that affects the processing quality and is controlled by Wx genes (Wx-A1, -B1 and -D1). All the accessions contained the allele Wx-A1a with the marker MAG264, which is similar to the results obtained by Liang et al. [Citation76]. In our study, all the accessions contained the allele Wx-D1a with the marker MAG269, and only one accession (CWI 67665) indicated the presence of Wx-D1b (null mutation). However, the lack of Wx-A1 or Wx-D1 protein does not always lead to a significant decrease in the amylose content [Citation34, Citation77], while the absence of Wx-D1 has a greater effect compared with that of Wx-A1 [Citation78]. At the Wx-B1 locus, using primers designed by Saito et al. [Citation48] and Mclauchlan et al. [Citation47], complete deletions of the gene existed and most accessions harbored the Wx-B1a allele, except two accessions (CWI67747 and CWI57684). Yamamori and Quynh [Citation78] reported that Wx-B1b induced a lower amylose content, which was in agreement with an earlier finding [Citation79]. They also analyzed the effects of Wx proteins and ranked the single null genotypes as Wx-B1b > Wx-D1b > Wx-A1b. Molecular characterizations of wheat waxy proteins and their effects on starch properties, as well as the detection of Wx null mutations, have been widely studied [Citation47,Citation48, Citation80–83], which is important for the identification waxy wheats in breeding programs [Citation84]. The majority of KASP markers found in the CIMMYT used in this study were derived from the Pin-D analysis and were in agreement with the STS markers. KASP can be used for genotyping a wide range of species for various purposes that can help the development of grain quality.
Molecular markers for Pin-D1 and Wx genes are imperative to wheat molecular breeding programs, because their different allelic compositions lead to different hardness levels [Citation85] and amylose contents, which affect the end-use food quality of wheat grain [Citation21]. Codominant and dominant markers are preferable for introducing null alleles into grain hardness and Wx proteins. The use of traditional gel-based PCR markers is time-consuming and results in a relatively low throughput, compared with more recently developed methods. The results of this study provide a deeper insight into the molecular and KASP markers that can be used to characterize grain hardness and Wx genes in bread wheat. Moreover, landraces offer an important genetic resource that can be used to improve modern varieties of wheat by means of introducing new alleles or a combination of genes.
Conclusions
Waxy genes, including three null alleles, were found in the Iranian landrace collection described in this study. These alleles may be used to modify starch properties by adjusting of the standard amylose/amylopectin ratio, thereby enhancing the functionality of wheat flour doughs to improve traditional or novel wheat-based foods. In addition grain hardness is of paramount importance to wheat processors, end- users and those involved in wheat breeding and improvement. To this end, null alleles of puroindoline genes were determined in this collection of Iranian landraces. The results, therefore, provide evidence that these genetic materials are important sources of genetic diversity for developing wheat cultivars with improved grain textures.
tbeq_a_1814866_sm7462.xlsx
Download MS Excel (16.3 KB)Acknowledgements
We acknowledge Dr. Thomas Payne for providing landraces for this study, Dr. M.R Jala Kamali for the constructive suggestions on this work and Bacilisa Luna Garrido for her expertise in the molecular markers preparation.
Additional information
Funding
References
- Food and Agriculture Organization (FAO). 2017. FAOSTAT data-base. [cited 2017 Jan 14]. Available from: http://faostat.fao.org/beta/en/.
- Ram S, Mishra B. Biochemical basis and molecular genetics of processing and nutritional quality traits of wheat. J Plant Biochem Biotechnol. 2008;17(2):111–126.
- Alvarez JB, Guzman C. Interspecific and intergeneric hybridization as a sourece of variation for wheat grain quality improvement. Theor Appl Genet. 2018;131(2):225–251.
- Johansson E, Branlard G, Cuniberti M, et al. Genotypic and environment effects on wheat technological and nutritional quality. In: Igrejas G, Ikeda TM, Guzman C, editors. Wheat quality for improving processing and human health. Springer Cham; 2020:171–204.
- Pomeranz Y, Williams PC. Wheat hardness: its genetic, structural, and biochemical background, measurement, and significance. In: Pomeranz Y, editor. Advances in cereal science and technology. St Paul (MN): American Association of Cereal Chemists; 1990; p. 471–544.
- Gautier MF, Aleman ME, Guirao A, et al. Triticum aestivum puroindolines, two basic cystine-rich seed proteins: cDNA sequence analysis and developmental gene expression. Plant Mol Biol. 1994;25(1):43–57.
- Morris CF, Rose SP. Wheat. In: Henry RJ, Kettlewell PS, editors. Cereal grain quality. New York (NY): Chapman & Hall; 1996; p. 3–54.
- Morris CF. Puroindolines: the molecular genetic basis of wheat grain hardness. Plant Mol Biol. 2002;48(5-6):633–647.
- Gautier M-F, Cosson P, Guirao A, et al. Puroindoline genes are highly conserved in diploid ancestor wheats and related species but absent in tetraploid Triticum species. Plant Sci. 2000;153(1):81–91.
- Souza EJ, Graybosch RA, Guttieri M. Breeding wheat for improved milling and baking quality. J Crop Prod. 2002;5(1-2):39–74.
- Morris CF, DeMacon VL, Giroux MJ. Wheat grain hardness among chromosome 5D homozygous recombinant substitution lines using different methods of measurement. Cereal Chem. 1999;76(2):249–252.
- Law CN, Young CF, Brown JWS, et al. The study of grain-protein control in wheat using whole-chromosome substitution lines. In: Seed protein improvement by nuclear techniques. Vienna: International Atomic Energy Agency; 1978; p. 483–502.
- Chantret N, Salse J, Sabot F, et al. Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell. 2005;17(4):1033–1045.
- Bhave M, Morris C. Molecular genetics of puroindolines and related genes: allelic diversity in wheat and other grasses. Plant Mol Biol. 2008;66(3):205–e219.
- Giroux MJ, Morris CF. A glycine to serine change in puroindoline-b is associated with wheat grain hardness and low levels of starch-surface friabilin. Theor Appl Genet. 1997;95:857–864.
- Chang C, Zhang H, Xu J, et al. Identification of allelic variations of puroindoline genes controlling grain hardness in wheat using a modified denaturing PAGE. Euphytica. 2006;152(2):225–234.
- Morris CF, Bhave M. Reconciliation of D-genome puroindoline allele designations with current DNA sequence data. J Cereal Sci. 2008;48(2):277–287.
- Morris CF, Lillemo M, Simeone MC, et al. Prevalence of puroindoline grain hardness genotypes among historically significant North American spring and winter wheats. Crop Sci. 2001;41(1):218–228.
- Chen F, Yu Y, Xia X, et al. Prevalence of a novel puroindoline b allele in Yunnan endemic wheats (Triticum aestivum ssp. yunnanense King). Euphytica. 2007;156(1-2):39–46.
- Chen F, Li H, Li X, et al. Alveograph and Mixolab parameters associated with Puroindoline-D1 genes in Chinese winter wheats. J Sci Food Agric. 2013;93(10):2541–2548.
- Eagles HA, Cane K, Eastwood RF, et al. Contributions of glutenins and puroindoline genes to grain quality traits in Southern Australian wheat breeding programs. Aust J Agric Res. 2006;57(2):179–186.
- Giroux MJ, Talbert L, Habernicht DK, et al. Association of puroindoline sequence type and grain hardness in hard red spring wheat. Crop Sci. 2000;40(2):370–374.
- Martin JM, Frohberg RC, Morris CF, et al. Milling and bread baking traits associated with puroindoline sequence type in hard red spring wheat. Crop Sci. 2001;41(1):228–234.
- Lillemo M, Chen F, Xia X, et al. Puroindoline grain hardness alleles in CIMMYT bread wheat germplasm. J Cereal Sci. 2006;44(1):86–92.
- James MG, Denyer K, Myers AM. Starch synthesis in the cereal endosperm. Curr Opin Plant Biol. 2003;6(3):215–222.
- Sestili F, Botticella E, Bedo Z, et al. Production of novel allelic variation for genes involved in starch biosynthesis through mutagenesis. Mol Breeding. 2010;25(1):145–154.
- Fredriksson H, Silverio J, Andersson R, et al. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr Polym. 1998;35(3-4):119–134.
- Zeng M, Morris CF, Batey IL, et al. Sources of variation for starch gelatinization, pasting, and gelation properties in wheat. Cereal Chem. 1997;74(1):63–71.
- Baldwin PM. Starch granule-associated proteins and polypeptides: a review. Starch/Stärke. 2001;53(10):475–503.
- Yu H, Yang Y, Chen XY, et al. Comparison of endosperm amyloplast development and degration in waxy and non-waxy wheat. Cereal Res Commun. 2018; 46(2):333–343.
- Morell MK, Rahman S, Regina A, et al. Wheat starch biosynthesis. Euphytica. 2001;119(1/2):55–58.
- Chao S, Sharp PJ, Worland AJ, et al. RFLP-based genetic maps of wheat homoeologous group 7 chromosomes. Theor Appl Genet. 1989;78(4):495–504.
- Ainsworth C, Clark J, Balsdon J. Expression, organisation and structure of the genes encoding the waxy protein (granule-bound starch synthase) in wheat . Plant Mol Biol. 1993;22(1):67–82.
- Yamamori M, Nakamura T, Endo TR, et al. Waxy protein deficiency and chromosomal location of coding genes in common wheat. Theor Appl Genet. 1994;89(2-3):179–184.
- Guzmán C, Alvarez JB. Molecular characterization of a novel waxy allele (Wx-Au1a) from Triticum urartu Thum. ex Gandil. Genet Resour Crop Evol. 2012;59(6):971–979.
- Ortega R, Alvarez JB, Guzmán C. Characterization of the Wx gene in diploid Aegilops species and its potential use in wheat breeding. Genet Resour Crop Evol. 2014;61(2):369–382.
- Guzmán C, Ortega R, Yamamori M, et al. Molecular characterization of two novel null waxy alleles in Mexican bread wheat landraces. J Cereal Sci. 2015; 62:8–14.
- Ciaffi M, Dominici L, Lafiandra D, et al. Seed storage protein of wild wheat progenitors and their relationships with technological properties. Hereditas. 2008;116(3):315–322.
- Feldman M, Sears ER. The wild gene resources of wheat. Sci Am. 1981;244(1):102–112.
- Nevo E, Payne PI. Wheat storage protein diversity of HWM glutenin subunits in wild emmer from Israel. 1. Geographical patterns and ecogeographical predicabality. Theoret Appl Genet. 1987;74(6):827–836.
- Kokten K, Akcura M. Mineral concentrations ofgrain of bread wheat landraces originated from eastern Anatolia of Turky. Prog Nutr. 2018; 20:119–126.
- Li X, Li Y, Zhang M, et al. Diversity of Puroindoline genes and their association with kernel hardness in Chinese wheat cultivars and landraces. Mol Breeding. 2019;39(4). Doi:10.1007/s11032-019-0967-6.
- American Association of Cereal Chemists. Approved methods of the AACC. St. Paul, MN: AACC International; 2010.
- American Association of Cereal. Chemists. Approved Methods of the AACC. St. Paul, MN: Association of Cereal Chemists, 2000.
- Dreisigacker S, Sehgal D, Reyes Jaimez AE, et al. CIMMYT wheat molecular genetics: laboratory protocols and applications to wheat breeding. Mexico: CIMMYT; 2016.
- Liu L, He Z, Yan J, et al. Allelic variation at the Glu-1 and Glu-3 loci, presence of the 1B.1R translocation, and their effects on mixoEgraphic properties in Chinese bread wheats. Euphytica. 2005;142(3):197–204.
- McLauchlan A, Ogbonnaya FC, Hollingsworth B, et al. Development of robust PCR-based DNA markers for each homoeo-allele of granule-bound starch synthase and their application in wheat breeding programs. Aust J Agric Res. 2001;52(12):1409–1416.
- Saito M, Vrinten P, Ishikawa G, et al. A novel codominant marker for selection of the null Wx-B1 allele in wheat breeding programs. Mol Breeding. 2009;23(2):209–217.
- Semagn K, Babu R, Hearne S, et al. Single nucleotide polymorphism genotyping using kompetitive allele specific PCR (KASP):overview of the technology and its application in crop improvement. Mol Breeding. 2014;33(1):1–14.
- Giroux M, Morris CF. Wheat grain hardness results from highly conserved mutations in the friabilin components Puroindoline a and b. Proc Natl Acad Sci USA. 1998; 95(11):6262–6266.
- Boehm JD, Jr, Ibba MI, Kiszonas AM, et al. End-use quality of CIMMYT-derived soft-kernel durum wheat germplasm: I Grain, milling and soft wheat quality. Crop Sci. 2017;57(3):1475–1484.
- Murray JC, Kiszonas AM, Morris CF. Influence of soft kernel texture on the flour, water absorption, rheology and baking quality of durum wheat. Cereal Chem. 2017;94(2):215–222.
- Lullien-Pellerin V, Haraszi R, Anderson RS, et al. Understanding the mechanics of wheat grain fractionation and the impact of puroindolines on milling and product quality. In: Igrejas G, Ikeda TM, Guzman C, editor. Wheat quality for improving processing and human health. 2020.
- Wang D, Zhang K, Dong L, et al. Molecular genetic and genomic analysis of wheat milling and end-use traits in China: progress and perspectives. Crop J. 2018; 6(1):68–81.
- Ayala M, Guzman C, Peña RJ, et al. Genetic diversity and molecular characterization of puroindoline genes (Pina-D1 and Pinb-D1) in bread wheat landraces from Andalusia (Southern Spain). J Cereal Sci. 2016;71:61–65.
- Ma X, Sajjad M, Wang J, et al. Diversity, distribution of puroindoline genes and their effect on kernel hardness in a diverse panel of Chinese wheat germplasm. BMC Plant Biol. 2017;17(1):158.
- Xia LQ, Chen F, He ZH, et al. Occurrence of puroindoline alleles in Chinese winter wheats. Cereal Chem. 2005;82(1):38–43.
- Cane K, Spackman M, Eagles H. Puroindoline genes and their effects on grain quality traits in southern Australian wheat cultivars. Aust J Agric Res. 2004;55(1):89–95.
- Ikeda TM, Ohnishi N, Nagamine T, et al. Identification of new puroindoline genotypes and their relationship to flour texture among wheat cultivars. J Cereal Sci. 2005;41(1):1–6.
- Chen F, He Z, Xia XC, et al. Molecular and biochemical characterization of puroindoline a and b alleles in Chinese landraces and historical cultivars. Theor Appl Genet. 2006;112(3):400–409.
- Pickering PA, Bhave M. Comprehensive analysis of Australian hard wheat cultivars shows limited puroindoline allele diversity. Plant Sci. 2007;172(2):371–379.
- Rasheed A, Wen W, Gao F, et al. Development and validation of KASP assays for functional genes underpinning key economic traits in wheat. Theor Appl Genet. 2016;129(10):1843–1860.
- Ayala M, Guzman C, Alvarez JB, et al. Characterization of genetic diversity of puroindoline genes in Mexican wheat landraces. Euphytica. 2013;190(1):53–63.
- Huebner FR, Gaines CS. Relation between wheat kernel hardness, environment, and gliadin composition. Cereal Chem. 1992; 69:148–151.
- Peterson CJ, Graybosch RA, Baenziger PS, et al. Genotype and environment effects on quality characteristics of hard erd winter wheat. Crop Sci. 1992;32(1):98–103.
- Turnbull KM, Rahman S. Endosperm texture in wheat. J Cereal Sci. 2002;36(3):327–337.
- Surma M, Adamski T, Banaszak Z, et al. Effect of genotype, environment and their interaction on quality parameters of wheat breeding lines of diverse grain hardness. Plant Prod Sci. 2012; 15(3):192–203.
- Pomeranz Y, Peterson CJ, Mattern PJ. Hardness of winter wheats grown under widely different climatic conditions. Cereal Chem. 1985; 62:463–467.
- Oury FX, Lasme P, Michelet C, et al. Relationships between wheat grain physical characteristics studied through near-isogenic lines with distinct puroindoline-b allele. Theor Appl Genet. 2015;128(5):913–929.
- Igrejas G, Leroy P, Charmet G, et al. Mapping QTLs for grain hardness and puroindoline content in wheat (Triticum aestivum L.). Theor Appl Genet. 2002;106(1):19–27.
- Tsilo TJ, Simsek S, Ohm JB, et al. Quantitative trait loci influencing endosperm texture, dough-mixing strength, and bread-making properties of the hard red spring wheat breeding lines . Genome. 2011;54(6):460–470.
- Dubreil L, Méliande S, Chiron H, et al. Effect of puroindolines on the bread making properties of wheat flour. Cereal Chem. 1998;75(2):222–229.
- Igrejas G, Gaborit T, Oury FX, et al. Geneticand environmental effects on puroindoline-a and puroindoline-b content and their relationship to technological properties in French bread wheats. J Cereal Sci. 2001;34(1):37–47.
- Mikulikova D. The effect of friabilin on wheat grain hardness: a review. Czech J Genet Plant Breed. 2018;43(2):35–43.
- Symes KJ. The inheritance of grain hardness in wheat as measured by the particle size index. Aust J Agric Res. 1965;16(2):113–123.
- Liang D, Tang JW, Pena RJ, et al. Characterization of CIMMYT bread wheats for high- and low- molecular weight glutenin subunits and other quality-related genes with SDS-PAGE, RP-HPLC and molecular markers. Euphytica. 2010;172(2):235–250.
- Kim W, Johnson JW, Graybosch RA, et al. Physicochemical properties and end-use quality of wheat starch as a function of waxy protein alleles. J Cereal Sci. 2003;37(2):195–204.
- Yamamori M, Quynh NT. Differential effects of Wx-A1,-B1 and-D1 protein deficiencies on apparent amylose content and starch pasting properties in common wheat. Theor Appl Genet. 2000;100(1):32–38.
- Miura H, Sugawara A. Dosage effects of the three Wx genes on amylose synthesis in wheat endosperm. Theor Appl Genet. 1996;93(7):1066–1070.
- Guzman C, Alvarez JB. Wheat waxy proteins: polymorphism, molecular characterization and effects on starch properties. Theor Appl Genet. 2016; 129(1):1–16.
- Shan XY, Clayshulte SR, Haley SD, et al. Variation for glutenin and waxy alleles in the US hard winter wheat germplasm. J Cereal Sci. 2007;45(2):199–208.
- Liu YX, Li W, Chen HP, et al. Variation for glutenin and waxy alleles and their effect on quality properties in Sichuan wheat landraces. J Plant Sci. 2008;3(4):266–276.
- Nakamura T, Vrinten P, Saito M, et al. Rapid classification of partial waxy wheats using PCR-based markers. Genome. 2002;45(6):1150–1156.
- Li S, Zhong X, Zhang X, et al. Production of waxy tetraploid wheat (Triticum turgidum durum L.) by EMS mutagenesis. Genet Resour Crop Evol. 2020;67(2):433–443.
- Takata K, Ikeda TM, Yanaka M, et al. Comparison of five puroindoline alleles on grain hardness and flour properties using near isogenic wheat lines. Breed Sci. 2010;60(3):228–232.
