Abstract
Carotenoids belong to a major family of isoprenoids which play vital roles in plant growth and development. Pepper (Capsicum annuum L.) is not only a vegetable and condiment grown worldwide, but also can be used as a model organism in fruit color research. In this study, 61 genes known to be involved in mevalonate (MVA), plastidial 2-C-methyl-d-erythritol-4-phosphate (MEP) and carotenoid metabolism (biosynthesis and catabolism) pathways were identified and characterized based on the genome of pepper plants. In the present study, it was found that 56 of the 61 carotenoid metabolism genes in pepper were unevenly distributed throughout 12 chromosomes. CaGGPPS2, CaGGPPS4a and CaGGPPS4b were generally clustered into one region. This study identified CaHMGR1/CaHMGR3 and CaHMGR1/CaHMGR2 as segregation duplication events. In addition, the pepper carotenoid metabolism genes had high variations in gene structure. The key carotenoid metabolism genes showed different expression patterns during the pepper fruit development and under environmental stress conditions. The results obtained in this research investigation will contribute insight to the evolution and function of carotenoid metabolic pathways in pepper fruit.
Introduction
Carotenoids are a large group of isoprenoid molecules with over 750 members, which are distributed in plants, algae, fungi and bacteria [Citation1, Citation2]. Carotenoids participate in various physiological and developmental processes [Citation3], such as light harvesting, and processes related to protecting species against photo-oxidation and during photosynthesis [Citation1, Citation4–6], photomorphogenesis [Citation7] and non-photochemical quenching [Citation8]. Carotenoids also provide bright colours and flavours which attract insects and animals for seed dispersal [Citation9]. In addition, carotenoids also act as precursors for two types of important phytohormones, abscisic acids (ABA) and strigolactones, which are vital regulators for plant growth and development, as well as biotic/abiotic stress responses [Citation10–12]. Moreover, as part of the composition of various food crops, carotenoids are considered to have essential nutritional benefits for both humans and animals [Citation13, Citation14].
Carotenoid metabolism pathways have been extensively studied in the majority of the species worldwide. A large number of the genes and enzymes associated with carotenoid biosynthesis and degradation have been identified, cloned and studied in various plants species [Citation1, Citation15–19]. Carotenoids are derived from two isoprene isomers, the five-carbon precursors isopentenyl diphosphate (IPP) and its double-bond isomer dimethylallyl diphosphate (DMAPP) [Citation20–22]. Two pathways exist for IPP production in plants: the cytosolic-mevalonic acid pathway (MVA) and the plastidial 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway [Citation23, Citation24], as shown in Supplemental Figure S1. However, carotenoids are generally synthesized from IPP and DMAPP via MEP pathways in plant species.
The first step of carotenoid biosynthesis is the head-to-head condensation of two geranylgeranyldiphosphates to produce carotenoid 15-cis-phytoene, which is catalyzed by phytoene synthase (PSY). This is considered to be the main bottleneck in the carotenoid pathways [Citation1, Citation20, Citation25]. Then, uncoloured phytoene is desaturated and isomerized to red-coloured all-trans-lycopene through the actions of two desaturases (phytoenedesaturase (PDS) and ζ-carotene desaturase (ZDS)) and two isomerases (prolycopeneisomerase (CRTISO) and ζ-carotene isomerase (ZISO)) [Citation2]. Phytoene desaturation by PDS and ZDS requires the transfer of two electrons to plastoquinones re-oxidized by a plastid terminal oxidase (PTOX) using O2 as a terminal acceptor () [Citation26]. PTOX is an oxidoreductase linked to carotenoid biosynthesis and chlororespiration in order to prevent photooxidative damage [Citation27].
Subsequently, the cyclization of the lycopene carbon chain ends is a vital step in carotenoid biosynthesis in terms of generating carotenoid diversity [Citation1]. All-trans-lycopene is converted into the formation of δ-carotene and γ-carotene, which are catalyzed by lycopene ε-cyclase (LCYE) and β-cyclase (LCYB), respectively. Then, the orange carotenes (α- and β-carotene) are synthetized by the enzyme LCYB [Citation1]. Thexanthophylls are produced by hydroxylases and epoxidases. There are two different types of hydroxylases: Cytochrome P450 types and CHYB (BCH) types, which hydroxylate the β-ring of cyclic carotenes [Citation1]. Among the former type, the hydroxylases CYP97A and CYP97C hydroxylate the band ε-rings, respectively. The α-carotene is transformed into lutein, and is catalyzed primarily by CYP97-type hydroxylases. Moreover, the lutein can be also converted into lutein epoxides by epoxidases in some plant species [Citation28]. The β-carotene is converted to zeaxanthin and hydroxylated by CHYB. In addition, zeaxanthin is epoxidized to produce antheraxanthin and violaxanthin by zeaxanthinepoxidase (ZEP), and then further to yield neoxanthin via neoxanthin synthase (NXS). Violaxanthin can be converted back to zeaxanthin via violaxanthin de-epoxidase (VDE). In pepper plants, antheraxanthin and violaxanthin are converted by capsanthin–capsorubin synthase (CCS) into capsanthin and capsorubin [Citation29, Citation30]. Carotenoids are degraded enzymatically by a family of carotenoid cleavage oxygenases (CCOs), which are further divided into carotenoid cleavage dioxygenases (CCDs) and 9-cis-carotenoid dioxygenases (NCEDs) according to the different substrates in the epoxy structure [Citation31–34]. Moreover, these genes have been found to be regulated by WRKY transcription factors in the flower petals of Osmanthus fragrans [Citation35, Citation36].
In recent years, with the development of sequencing technology, many plant genomes have been successfully sequenced, which has provided opportunities to identify members of gene families at whole-genome levels [Citation37–42]. Peppers (Capsicum annuum L.) are currently one of the most economically important vegetable crops in the world. The fruit colour is rich and colourful, ranging from green to ivory and yellow at the green stage, while being brown, orange, red, violet or yellow at the mature stages. Due to the wide ranges of Capsicum fruit colours, peppers have become model organisms of fruit colour research. It has been found that the identification of carotenoid metabolic pathway genes in pepper plants is conducive to improving the understanding of the formation mechanisms of fruit colour.
In pepper plants, carotenoids are not only responsible for the visual appeal of the pepper fruit, but also enhance the nutritional value and health benefits for humans [Citation1, Citation43, Citation44]. The aim of this study was to identify and characterize the genes known to be involved in mevalonate (MVA), MEP and carotenoid metabolism (biosynthesis and catabolism) pathways based on the genome of pepper plants, and to explore available data about their differential expression during the pepper fruit development and under biotic and abiotic stress.
Materials and methods
Identification of carotenoid metabolism genes in pepper plants
The identifications of the carotenoid metabolism-related genes were performed via a BLAST-P search in the PGP (pepper genome platform) database (http://passport.pepper.snu.ac.kr/?t=PGENOME) using the amino acid sequences of Arabidopsis thaliana, Vitis vinifera (grape) and Solanum lycopersicum (tomato) as the queries. The gene sequences related to the carotenoid metabolism pathways from Arabidopsis, grape and tomato were acquired from the TAIR database (www.arabidopsis.org); grape genome database (http://genomes.cribi.unipd.it/grape/); and the SNG database (https://solgenomics.net/), respectively. Subsequently, the search processes of the candidate genes in the pepper genome were repeated using BLAST-P. The e-value used was 1e−5. Then, all the candidate genes were evaluated for further verification using the Pfam database (http://pfam.janelia.org/) in order to accurately classify the genes.
Sequence feature analysis of the pepper carotenoid metabolism genes
The genomic and CDS sequences for each of the carotenoid metabolism genes were extracted from the SNG database. The structures of the genes were predicted using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/chinese.php). Then, the molecular weights and theoretical isoelectric points were predicted using the online tool (http://cn.expasy.org/tools/protparam.html).
Chromosomal locations, duplications and phylogenetic analysis of the pepper carotenoid metabolism genes
The information regarding the chromosomal mapping of each carotenoid metabolism-related gene was obtained from the PGP database, and the maps were drafted using MapDraw3.0 software. The duplication events of the pepper carotenoid metabolism genes across the entire pepper genome were identified by conducting BLASTP searches of the pepper carotenoid metabolism gene sequences against the annotated pepper (CM334) protein sequences. The obtained hits of the carotenoid metabolism genes were filtered using the following threshold: Expectation (E) value ≤10−40; cumulative identity percentage (CIP) ≥60%; and cumulative alignment length percentage (CALP) ≥70% [Citation45]. The hits which survived were considered as the duplicated events of the pepper carotenoid metabolism genes in the pepper genome. In addition, gene clusters were defined as regions where two neighbouring homologous genes were less than 200 kb apart with fewer than eight non-carotenoid metabolism-encoding genes between the carotenoid metabolism-encoding genes [Citation46, Citation47]. A tandemly duplicated gene was determined based on the definition introduced by Huang et al. [Citation48], in which tandem-duplicated candidate gene pairs were defined as regions which included two or more adjacent homologous genes within 100 kb.
Then, MEGA X software was used to construct the phylogenetic trees with a neighbour-joining (NJ) method [Citation49]. The pairwise deletion gaps, p-distance model and 1,000 bootstrap replications were selected as the parameters for the NJ analysis. The missing sequence data were treated using pairwise deletions of the gaps. The branch lengths were assigned by utilizing the pairwise calculations of the genetic distances. PhyML software (Version 3.0) was adopted to construct the maximum likelihood (ML) trees. In the present study, a Whelan and Goldman amino acid substitution model, 100 nonparametric bootstrap replicates and γ-distribution were selected for the analysis [Citation50]. In addition, the ProtTest software (Version 2.4) was used for the model selection [Citation51].
Expression analysis of the pepper carotenoid metabolism genes
The transcriptome data of the fruit development, along with the multiple pathogen and abiotic responses, were acquired from the scientific data [Citation52, Citation53]. Then, the expression heatmaps of the pepper carotenoid metabolism genes were built and visualized using TBtools software [Citation54].
Results and discussion
Identification and characterization of 61 genes involved in the pepper carotenoid metabolism pathways
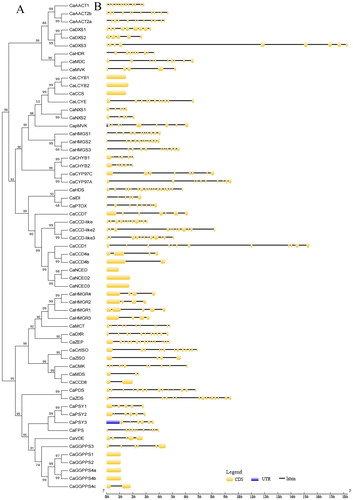
The genes involved in carotenoid metabolism pathways in pepper were identified using a bioinformatics method. The results indicated that at least 61 carotenoid metabolism-related genes could be identified in the PGP database using the amino acid sequences of Arabidopsis, grapevine and tomato as the queries. Each of the carotenoid metabolism genes of the pepper were named based on the enzymatic reactions, similar to those given in the Arabidopsis, grapevine and tomato carotenoid metabolism pathways. Details about each of the carotenoid metabolism (biosynthetic and catabolic) genes are shown in , including the gene ID, chromosome location, gene length, number of exons, protein length, isoelectric point (pI), molecular weight and the subcellular localization ().
Table 1. Carotenoid metabolic genes in pepper.
Fourteen genes, which were determined to encode enzymes involved in six enzymatic steps, were identified in the MVA pathway, which led to the formation of the IPP and DMAPP. These genes consisted of three AATC genes (CaAACT1, CaAACT2a and CaAACT2b); three HMGS genes (CaHMGS1, CaHMGS2 and CaHMGS3); four HMGR genes (CaHMGR1, CaHMGR2, CaHMGR3 and CaHMGR4); one MDC gene (CaMDC); one MVK gene (CaMVK); one PMVK gene (CapMVK); and one FPS gene (CaFPS). Ten genes which were determined to be involved in eight enzymatic steps were identified in the MEP pathway, which were considered as the main synthesis pathway of the IPP and DMAPP. In addition, the DXS enzyme, which catalyzes the first enzymatic step of the MEP pathway and carotenoid biosynthesis, was encoded by the three genes: CaDXS1, CaDXS2 and CaDXS3. In total, 26 genes involved in carotenoid biosynthesis pathways were identified. Six CaGGPPS genes were identified which were determined to encode enzymes that catalyze the condensation of three IPP and one DMAPP units to generate the GGPP that has the role of a universal precursor for all the plastid isoprenoids. Three CaPSY genes (CaPSY1, CaPSY2 and CaPSY3) were associated with the main bottleneck in the carotenoid pathway, which is the catalysis of the condensation of two GGPP molecules in order to produce 15-cisphytoene were also identified. Two different lycopene cyclases were identified as CaLCYB and CaLCYE, respectively. In addition, two CaLCYB genes (CaLCYB1 and CaLCYB2); two CaCHYB genes (CaCHYB1and CaCHYB2); and two CaNXS genes (CaNXS1 and CaNXS2) were identified, respectively. Eleven members of the carotenoid cleavage oxygenase (CCO) gene family involved in the carotenoid catabolism pathway were identified and subsequently divided into two groups.
Chromosomal distribution and evolution of the pepper carotenoid metabolism genes
As shown in , 56 of the 61 identified pepper carotenoid metabolism genes were distributed unevenly throughout the 12 chromosomes, which was similar to the previous research findings regarding rice [Citation55]. Eleven of the genes were located on Chromosome 1, while only one of the carotenoid metabolism genes was mapped in Chromosome 9 (). However, based on the data of the current pepper genome, the remaining five genes (CaDXS2, CaMVK, CaLCYE, CaHMGR4 and CaCCD7) were found to be assembled to any scaffold.
Figure 1. Chromosomal locations and duplications of the paralogous carotenoid metabolic genes on the pepper chromosomes. Note: In the figure, the chromosome numbers are shown at the top of each bar; predicted tandem-duplicated genes are indicated by thick blue lines; and gene clusters are denoted by black lines.
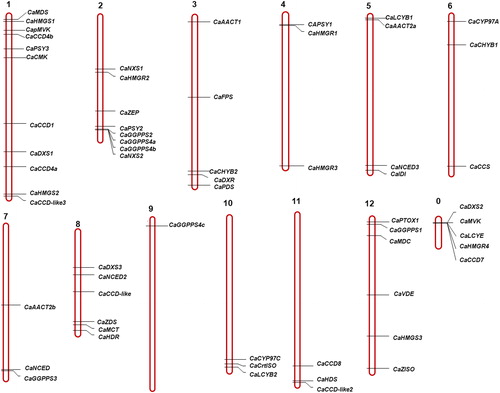
Research has suggested that tandem duplications and segmental duplications are probably the main contributors to the evolutionary momentum of the plants [Citation56]. Currently, several genes known to be related to carotenoid metabolism have been identified through either tandem or segmental duplications [Citation2]. In the present study, in order to understand the mechanism underlying the evolution of carotenoid biosynthesis genes in pepper plants more deeply, tandem and segmental duplications were searched. There were found to be three genes (CaGGPPS2, CaGGPPS4a and CaGGPPS4b) clustered into one tandem duplication region on the pepper Chromosome 2. Two pairs of segment duplication events (CaHMGR1/CaHMGR3 pairing and CaHMGR1/CaHMGR2 pairing) were also identified. They indicated that some of the carotenoid biosynthetic genes in the pepper plants have possibly occurred by gene duplication. Moreover, the segment duplication events we determined also provide a reference for understanding the evolutionary relationships and functional predictions of the carotenoid biosynthetic genes in pepper.
Exon-intron organization of the pepper carotenoid metabolism genes
For the purpose of gaining further insight into the pepper carotenoid metabolism genes, a phylogenetic tree was constructed (Supplemental Figure S2) and the exon–intron structures were also analyzed in this study (). High variations in the numbers of exons and introns among the carotenoid metabolism genes were observed in the pepper plants in this study, as shown in . Ten genes (CaGGPPS1, CaGGPPS2, CaGGPPS4a, CaGGPPS4b, CaNCED, CaNCED2, CaNCED3, CaCCS, CaLCYB1 and CaLCYB2) had no intron in the pepper genome. In addition, among those having introns, the number of introns varied from 1 to 18. However, the CaHDS gene had 19 exons in its coding DNA sequences, which was the highest number of exons contained in this study. The CaDXS3 was the longest carotenoid metabolic gene with 18 kb of genomic sequence. The great variations in the structure of carotenoid metabolism genes indicated that the pepper genome might have undergone significant changes during evolution, as suggested earlier about the genome of grapevine [Citation2].
Expression patterns of the carotenoid metabolism genes during the development of the pepper fruit
In the present study, in order to gain insight into the functions of the pepper carotenogenic genes during fruit development, the expression levels of 61 genes in two pepper organs during fruit development were analyzed based on published transcriptome data [Citation52] and heatmaps were built from the results (). These pepper carotenogenic genes exhibited significant differential expression patterns in the placenta (PL) and pericarp (PR) tissue during the development stage of the pepper fruit. In the MVA pathway, 10 genes were found to be up regulated in the placenta tissue during pepper fruit development (). It was also observed that the CaAATC1 expression exhibited large changes throughout the pepper developmental stages. CaHMGR2 was found to be similar in expression to CaAATC1, with the exception of the mature green stage. In addition, CaHMGS4 also displayed an expression similar to CaAATC1, with the exception of the 16 DPA and the mature green stage. This study also revealed that CaAATC2a was expressed higher step-by-step following the completion of the fruit development stage. Furthermore, it was determined that CaAATC2b, CaHMGR1, CaHMGS2, CaMDC and CapMVK displayed the highest expression levels at 16 DPA, 25 DPA, 43 DPA, 16 DPA and 48 DPA, respectively. CaMVK was expressed at higher levels at 25 DPA and during the mature green stage. Three of the genes were expressed at higher levels during the fruit development stage in the pericarp tissue. CaAATC1 and CaAATC2a displayed similar expressions at higher levels, with the exception of the 16 DPA and 25 DPA stages. CaHMGR1 was found to be expressed at the highest level at 25 DPA. The remainder of the genes in both the placenta and pericarp tissue during the pepper developmental stages exhibited no or only slight changes. In the MEP pathway, the pepper carotenogenic genes (with the exception of CaDXS3) in the placenta and pericarp tissue were found to be expressed at low to medium level changes during all of the pepper developmental stages. However, CaDXS3 was observed to be expressed at higher levels at the 43 DPA and 48 DPA stages in placenta and pericarp tissue, respectively. These findings indicated that CaDXS3 may play a predominant role in the carotenoid biosynthesis of pepper fruit.
Figure 3. Expression heatmaps of the pepper carotenoid metabolic genes in placenta tissue (A) and pericarp tissue (B) during the fruit development stage based on the RNA-Seq data [Citation52]. Sample names were assigned as follows: placenta (PL); pericarp (PR); Stage 1, 6 DPA (1); Stage 2, 16 DPA (2); Stage 3, 25 DPA (3); mature green, 36 DPA (MG); breaker, 38 DPA (B); breaker plus 5, 43 DPA (B + 5); and breaker plus 10, 48 DPA (B + 10). Note: In the figure, the colour scale represents the relative expression levels based on log2-scaledfold changes of the RPKM during the later stages compared with those in Stage 1; Red indicates high level fold changes and green represents low level fold changes.
![Figure 3. Expression heatmaps of the pepper carotenoid metabolic genes in placenta tissue (A) and pericarp tissue (B) during the fruit development stage based on the RNA-Seq data [Citation52]. Sample names were assigned as follows: placenta (PL); pericarp (PR); Stage 1, 6 DPA (1); Stage 2, 16 DPA (2); Stage 3, 25 DPA (3); mature green, 36 DPA (MG); breaker, 38 DPA (B); breaker plus 5, 43 DPA (B + 5); and breaker plus 10, 48 DPA (B + 10). Note: In the figure, the colour scale represents the relative expression levels based on log2-scaledfold changes of the RPKM during the later stages compared with those in Stage 1; Red indicates high level fold changes and green represents low level fold changes.](/cms/asset/aa7aa9fa-502e-4fdf-9609-8749b4aa4443/tbeq_a_1824618_f0003_c.jpg)
In the carotenoids biosynthesis pathways, the majority of the carotenoid biosynthesis genes will be expressed at high levels during the different fruit development stages. In this study, it was observed that CaGGPS4b was expressed at higher levels at the 38 DPA, 43 DPA and 48 DPA stages in placenta tissue, but had either no or only slight changes in expression in the pericarp tissue throughout the pepper developmental stages. In addition, the transcriptome data showed that CaGGPS3 displayed no or only slight expression changes in the pericarp tissue throughout the pepper developmental stages, with the exception of the 48 DPA. Three CaPSY genes were found to exhibit different expression patterns during the pepper fruit development. For example, CaPSY1 was highly expressed at 38 DPA, as well as during the later development stages both in the placenta and pericarp tissue. CaPSY2 and CaPSY3 displayed only slight changes () in their expression levels during all of the fruit development stages, which suggested that the CaPSY2 and CaPSY3 may not be majorly associated with pepper fruit carotenogenesis. Previous research findings have reported that PSY2 and PSY3 are mainly expressed in the leaves and roots [Citation2]. Higher expressions of CaPTOX were found in the pericarp tissue throughout the pepper developmental stages, and CaCHYB2 was found to be up-regulated earlier in the pericarp than the placenta tissue. CaCCS is known to catalyze the synthesis of capsanthin and capsorubin, which are the main pigments and pungent substances. It was also determined that CaCCS expressed higher levels in the placenta and pericarp during the mature green fruit stage, as well as during the later development stages. It is speculated that the high expression levels of CaCCS may also help to synthesize capsanthin and capsorubin during the fruit development stages.
In the carotenoid catabolism pathway, four genes in the placenta tissue and three genes in the pericarp tissue displayed higher expression levels during the fruit developmental stages. CaCCD1 was highly expressed at 25 DPA, as well as during the later stages of fruit development in the placenta tissue. However, there were only slight changes in the expression levels observed in the pericarp tissue. CaCCD4b was highly expressed at 16 DPA, 25 DPA and during the mature green stage in the pericarp, with no changes observed in the placenta tissue. In addition, CaCCD8 was highly expressed at 16 DPA, 25 DPA and during the mature green stage in the placenta tissue, with no changes observed in the pericarp tissue. There were higher expression levels of CaNCED during the mature green stage and the later stages in the placenta, as well as higher expression levels at 16 DPA, 38 DPA, 43 DPA and 48 DPA, respectively, in the pericarp tissue. CaNCED2 displayed higher expression levels during the mature green stage and the later stages in the placenta, with higher expression levels observed at 16 DPA and the later stages in the pericarp tissue.
Expression patterns of the pepper carotenogenic-related genes following pathogen infection
This study investigated the functions of pepper carotenogenic genes in response to pathogens by analyzing available data [Citation52]. The results can be seen in the heatmaps displayed in . A greater number of up-regulated genes following the infection with the P. infestans and TMV P0 strains were involved in the MVA pathways. In addition, the CaAACT2b gene was observed to display higher expression levels at 6 h, 12 h and 2 days following the onset of P. infestans infection. CaHMGR4 and CaHMGS1 displayed similar expression patterns of higher expression levels at 6 h, 12 h, 1 day, 2 days and 3.5 days after the onset of P. infestans infection. CaHMGR2 and CaHMGS2 had identical expression patterns, in which higher expression levels were evident following the onset of the P. infestans infection. CaMDC and CaFPS were expressed at higher levels at 12 h, 1 day, 2 days and 3.5 days after the onset of the P. infestans infection. In addition, CaMVK was expressed at higher levels at 6 h and 12 h after the onset of the P. infestans infection. Higher expression levels of CaAACT2b, CaHMGR2, CaHMGS1, CaMDC, CaMVK and CaFPS were observed 2 and 3 days after the onset of the TMV P0 strain infection. Also, CaHMGR4 was more highly expressed at 2 days after the onset of the TMV P0 strain infection. Higher expression levels of CaHMGS2 were observed at 1 day, 2 days and 3 days following the onset of the TMV P0 strain infection.
Figure 4. Expression heatmaps of the pepper carotenoid metabolic genes following pathogen infections based on RNA-Sequence data [Citation52]. Infection by P. infestans (A), pepper mottle virus (PepMov) (B), or tobacco mosaic virus (TMV) P0 strain (C). Note: In the figure, the colour scale represents the relative expression levels based on the log2-scaled fold changes of the RPKM injections at each time point compared with the CK; Red indicates high level fold changes and green represents low level fold changes.
![Figure 4. Expression heatmaps of the pepper carotenoid metabolic genes following pathogen infections based on RNA-Sequence data [Citation52]. Infection by P. infestans (A), pepper mottle virus (PepMov) (B), or tobacco mosaic virus (TMV) P0 strain (C). Note: In the figure, the colour scale represents the relative expression levels based on the log2-scaled fold changes of the RPKM injections at each time point compared with the CK; Red indicates high level fold changes and green represents low level fold changes.](/cms/asset/4dad9eda-2d59-455f-bdbe-4dfcb13353cf/tbeq_a_1824618_f0004_c.jpg)
Only one gene (CaIDI) which had been involved in the MEP pathways displayed higher levels of expression following both the P. infestans and TMV P0 strain infections. In addition, two genes (CaGGPS1 and CaCCS) and three genes (CaGGPS1, CaNXS1 and CaCCS) involved in the carotenoid biosynthetic pathways were determined to display higher expression levels following the P. infestans and TMV P0 strain infections, respectively. Furthermore, one gene (CaCCD-like) and two genes (CaCCD8 and CaCCD-like) involved in the carotenoid catabolism pathways were respectively expressed at higher levels following the P. infestans and TMV P0 strain infection. However, only a few carotenogenic-related genes had displayed strong responses following the onset of pepper mottle virus (PepMov) infection. It was observed that genes CaHMGS2, CaGGPS1, CaCCS, CaCCD-like and CaNCED were all up-regulated following the onset of pepper mottle virus (PepMov) infection in the current study.
Expression patterns of the carotenoid metabolic genes of pepper subjected to temperature stress
It is well known that abiotic stress (low or high temperature levels and/or a deficient or excessive amount of water, heavy metals and hormone molecules, etc.) will affect plant growth and development [Citation54–69]. In order to investigate the functions of pepper carotenogenic gene responses to temperature stress, the available transcriptome data of pepper plants subjected to cold (10 °C) and heat treatments (40 °C) were acquired [Citation53]. The results are illustrated in the heatmaps displayed in . CaAACT1, CaHMGS2 and CaPTOX displayed higher expression levels at 24 and 72 h after the cold treatments (). CaAACT2b, CaHMGR2, CaHMGS1, CaFPS, CaIDI and CaCCD8 had higher expression levels at 72 h after the cold treatments. In addition, the expression levels of CaHMGR4, CaGGPS3 and CaLCYB1 were higher at 6 h after the cold treatments. CaDXS1, CaZEP, CaCHYB1 and CaCCS were more highly expressed at 12 h after the cold treatments. CaCCD7 displayed a higher expression level at 24 h after the cold treatments. Meanwhile, CaCHYB2 and CaNCED2 had higher expression levels at 6, 12 and 24 h after the cold treatments. Higher expression levels were observed for CaNCED at 3, 6, 12 and 24 h after the cold treatments. As can be seen in , according to the abiotic stress experiment results, only CaGGPS4a, CaGGPS4b, CaCHYB1, CaCCD7, CaNCED and CaNCED2 displayed higher expression levels following the heat treatments.
Figure 5. Expression heatmaps of the pepper carotenoid metabolic genes under temperature stress based on the RNA-Sequence data [Citation52]. Cold stress at 10 °C (A) and heat stress at 40 °C (B). Note: In the figure, the colour scale represents the relative expression levels based on the log2-scaledfold changes in the RPKM of the injections at each time point compared with the CK; Red indicates high level fold changes and green represents low level fold changes.
![Figure 5. Expression heatmaps of the pepper carotenoid metabolic genes under temperature stress based on the RNA-Sequence data [Citation52]. Cold stress at 10 °C (A) and heat stress at 40 °C (B). Note: In the figure, the colour scale represents the relative expression levels based on the log2-scaledfold changes in the RPKM of the injections at each time point compared with the CK; Red indicates high level fold changes and green represents low level fold changes.](/cms/asset/07f3da61-d202-4885-81a1-3c49a12c22c1/tbeq_a_1824618_f0005_c.jpg)
Expression patterns of the carotenoid metabolic genes of pepper subjected to osmotic stress
Drought and salinity stress conditions are important abiotic environmental factors and significant plant stressors. Such stress conditions will potentially have negative effects plant development and productivity processes, and result in serious agricultural yield losses [Citation70–81]. In order to examine the expression patterns of pepper carotenoid metabolic gene responses to osmotic stresses, data regarding pepper plants treated with mannitol (400 mmol/L) and NaCl (400 mmol/L) were acquired [Citation53]. The acquired data are detailed in the heatmaps shown in , respectively. details the majority of the carotenoid metabolic gene responses to osmotic stress conditions. It can be seen that CaDXS2 was expressed at higher levels at 72 h after the mannitol treatments. CaGGPS1 was expressed at higher levels at 24 and 72 h following the mannitol treatments. The levels of CaCHYB1 and CaCCD-like2 expression were higher at the 12-hour point following the mannitol treatments. CaCCS was expressed at higher levels at 12 and 24 h after the mannitol treatments. CaCCD4a displayed higher levels of expression at 6 h after the mannitol treatments. At the 24-hour point following the mannitol treatments, it could be seen that the CaCCD4b expression levels were also higher. Higher levels of expression were observed for CaCCD7 at 6 and 24 h after the mannitol treatments. However, slight decreases in expression were observed at the 12-hour point following the mannitol treatments. In addition, at the 6-, 24- and 72-hour points following the mannitol treatments, the CaCCD8 expression levels were at higher levels. It was also noted that CaNCED was expressed at higher levels, with the exception of the 72-hour point after the mannitol treatments. CaNCED2 was also generally expressed at higher levels, with the exception of the 3- and 72-hour points following the mannitol treatments. In the present study, it was confirmed that the expression patterns of CaGGPS1, CaCHYB1, CaCCS, CaCCD7 and CaNCED subjected to salinity stress conditions were almost identical to the expression patterns of those subjected to mannitol stress conditions.
Figure 6. Expression heatmaps of the pepper carotenoid metabolic genes under osmotic stress based on the RNA-Seq data [Citation53]. Osmotic stress following 400 mmol/L mannitol treatment (A) and 400 mmol/L NaCl treatment (B). Note: In the figure, the colour scale represents the relative expression levels based on the log2-scaled fold changes in the RPKM of the injections at each time point compared with the CK; Red indicates high level fold changes and green represents low level fold changes.
![Figure 6. Expression heatmaps of the pepper carotenoid metabolic genes under osmotic stress based on the RNA-Seq data [Citation53]. Osmotic stress following 400 mmol/L mannitol treatment (A) and 400 mmol/L NaCl treatment (B). Note: In the figure, the colour scale represents the relative expression levels based on the log2-scaled fold changes in the RPKM of the injections at each time point compared with the CK; Red indicates high level fold changes and green represents low level fold changes.](/cms/asset/dcf98499-8c4b-4e4e-b08f-46111c998f5c/tbeq_a_1824618_f0006_c.jpg)
Conclusions
In this study, we analyzed the carotenoid metabolism genes based on the pepper genome and their expression patterns using available transcriptome data during the pepper fruit development and under biotic and abiotic stress. A total of 61 carotenoid metabolism-related genes were identified and have different expression patterns during the pepper fruit development and under biotic and abiotic stress. The results indicated that the carotenoid metabolism-related genes play important roles in growth and development, and responses to environmental stress. Overall, comparative genome analysis can provide reference for the research of the carotenoid metabolism in pepper and molecular breeding in pepper.
Supplemental Material
Download PDF (277.1 KB)Disclosure statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [HJW], upon reasonable request.
References
- Yuan H, Zhang JX, Nageswaran D, et al. Carotenoid metabolism and regulation in horticultural crops. Hortic Res. 2015;2:15036.
- Leng XP, Wang PP, Wang C, et al. Genome-wide identification and characterization of genes involved in carotenoid metabolic in three stages of grapevine fruit development. Sci Rep. 2017;7(1):4216
- Cazzonelli CI. Carotenoids in nature: insights from plants and beyond. Funct Plant Biol. 2011;38(11):833–847.
- Holt NE, Zigmantas D, Valkunas L, et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307(5708):433–436.
- Dall'Osto L, Fiore A, Cazzaniga S, et al. Different roles of alpha- and beta-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem. 2007;282(48):35056–35068.
- Domonkos I, Kis M, Gombos Z, et al. Carotenoids, versatile components of oxygenic photosynthesis. Prog Lipid Res. 2013;52(4):539–561.
- Howitt CA, Pogson BJ. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006;29(3):435–445.
- Franco AC, Matsubara S, Orthen B. Photoinhibition, carotenoid composition and the co-regulation of photochemical and non-photochemical quenching in neotropical savanna trees. Tree Physiol. 2007;27(5):717–725.
- Nisar N, Li L, Lu S, et al. Carotenoid metabolism in plants. Mol Plant. 2015;8(1):68–82.
- Milborrow BV, Lee HS. Endogenous biosynthetic precursors of (+)-abscisic acid. VI. Carotenoids and ABA are formed by the ‘non-mevalonate’ triose-pyruvate pathway in chloroplasts. Aust J Plant Physiol. 1998;25:507–512.
- Auldridge ME, Block A, Vogel JT, et al. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006;45(6):982–993.
- Havaux M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014;79(4):597–606.
- Cooper DA. Carotenoids in health and disease: recent scientific evaluations, research recommendations and the consumer. J Nutr. 2004;134(1):221S–224S.
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6(2):466–488.
- Farre G, Sanahuja G, Naqvi S, et al. Travel advice on the road to carotenoids in plants. Plant Sci. 2010;179(1–2):28–48.
- Kato M. Mechanism of carotenoid accumulation in citrus fruit. J Japan Soc Hort Sci. 2012;81(3):219–233.
- Rodriguez-Concepcion M, Stange C. Biosynthesis of carotenoids in carrot: an underground story comes to light. Arch Biochem Biophys. 2013;539(2):110–116.
- Ohmiya A. Qualitative and quantitative control of carotenoid accumulation in flower petals. Sci Hortic (Amsterdam). 2013;163:10–19.
- Liu L, Shao Z, Zhang M, et al. Regulation of carotenoid metabolism in tomato. Mol Plant. 2015;8(1):28–39.
- Vranová E, Coman D, Gruissem W. Structure and dynamics of the isoprenoid pathway network. Mol Plant. 2012;5(2):318–333.
- Vranová E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol. 2013;64:665–700.
- Abby JC, Christopher IC, Eleanore TW, et al. Carotenoids. In: Fabrice Rébeillé, Roland Douce, editors. Biosynthesis of vitamins in plants part A. Vitamins A, B1, B2, B3, B5. Vol. 58. Amsterdam (Netherlands): Academic Press; 2011. p. 1–36.
- Rodriguez-Concepcion M, Boronat A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002;130(3):1079–1089.
- Eisenreich W, Bacher A, Arigoni D, et al. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci. 2004;61(12):1401–1426.
- Ruiz-Solaa MA, Rodríguez-Concepción M. Carotenoid biosynthesis in arabidopsis: a colorful pathway. The Arabidopsis Book. 2012;10:e0158.
- Carol P, Kuntz M. A plastid terminal oxidase comes to light: Implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci. 2001;6(1):31–36.
- Rumeau D, Peltier G, Cournac L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007;30(9):1041–1051.
- Forster B, Pogson BJ, Osmond CB. Lutein from deepoxidation of lutein epoxide replaces zeaxanthin to sustain an enhanced capacity for nonphotochemical chlorophyll fluorescence quenching in avocado shade leaves in the dark. Plant Physiol. 2011;156(1):393–403.
- Guzman I, Hamby S, Romero J, et al. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci. 2010;179(1–2):49–59.
- Jeknic Z, Morre JT, Jeknic S, et al. Cloning and functional characterization of a gene for capsanthin-capsorubin synthase from tiger lily (lilium lancifolium thunb.‘splendens’). Plant Cell Physiol. 2012;53:1899–1912.
- Tan BC, Joseph LM, Deng WT, et al. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35(1):44–56.
- Walter MH, Strack D. Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep. 2011;28(4):663–692.
- Auldridge ME, McCarty DR, Klee HJ. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol. 2006b;9(3):315–321.
- Zhang XH, Liu HQ, Guo QW, et al. Genome-wide identification, phylogenetic relationships, and expression analysis of the carotenoid cleavage oxygenase gene family in pepper. Genet Mol Res. 2016;15(4):15048695.
- Han YJ, Wang XH, Chen WC, et al. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in flower petal of Osmanthus fragrans. Tree Genet. Genomes. 2014;10(2):329–338.
- Han Y, Wu M, Cao L, et al. Characterization of OfWRKY3, a transcription factor that positively regulates the carotenoid cleavage dioxygenase gene OfCCD4 in Osmanthus fragrans. Plant Mol Biol. 2016;91(4–5):485–496.
- Lu T, Zhang G, Sun L, et al. Genome-wide identification of CBL family and expression analysis of CBLs in response to potassium deficiency in cotton. Peer J. 2017;5:e3653.
- Chen MY, Li K, Li HP, et al. The glutathione peroxidase gene family in Gossypium hirsutum: genome-wide identification, classification, gene expression and functional analysis. Sci Rep. 2017;7:44743.
- Sun Q, Wang GH, Zhang X, et al. Genome-wide identification of the TIFY gene family in three cultivated Gossypium species and the expression of JAZ genes. Sci Rep. 2017;7:42418.
- Guo YW, Guo HL, Li X, et al. Two type III polyketide synthases from Polygonum cuspidatum: gene structure evolutionary route and metabolites. Plant Biotechnol Rep. 2013;7(3):371–381.
- Gao W, Xu FC, Guo DD, et al. Calcium-dependent protein kinases in cotton: insights into early plant responses to salt stress. BMC Plant Biol. 2018;18(1):15.
- Zhang G, Lu T, Miao W, et al. Genome-wide identification of ABA receptor PYL family and expression analysis of PYLs in response to ABA and osmotic stress in Gossypium. Peer J. 2017;5:e4126.
- Oliveira C, Silva-Ferreira AC, Mendes-Pinto MM, et al. Carotenoid compounds in grapes and their relationship to plant water status. J Agric Food Chem. 2003;51(20):5967–5971.
- Mendes-Pinto MM, Silva-Ferreira AC, Caris-Veyrat C, et al. Carotenoid, chlorophyll, and chlorophyll-derived compounds in grapes and port wines. J Agric Food Chem. 2005;53(26):10034–10041.
- Zhang R, Murat F, Pont C, et al. Paleo-evolutionary plasticity of plant disease resistance genes. BMC Genomics. 2014;15:187.
- Meyers BC, Dickerman AW, Michelmore RW, et.al. Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J. 1999;20(3):317–332.
- Paterson AH, Bowers JE, Bruggmann R, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556.
- Huang S, Gao Y, Liu J, et al. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol Genet Genomics. 2012;287(6):495–513.
- Kumar S, Stecher G, Li M, et al. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549.
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52(5):696–704.
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21(9):2104–2105.
- Myung-Shin K, Seungill K, Jongbum J, et al. Global gene expression profiling for fruit organs and pathogen infections in the pepper, Capsicum annuum L. Sci Data. 2018;5:180103.
- Kang W, Sim YM, Koo N, et al. Transcriptome profiling of abiotic responses to heat, cold, salt, and osmotic stress of Capsicum annuum L. Sci Data. 2020;7(1):17. [
- Chen C, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–1202.
- Neetu C, Aashima N, Jitendra P, et al. Carotenoid biosynthesis genes in rice: structural analysis, genome-wide expression prowling and phylogenetic analysis. Mol Genet Genomics. 2010;283:13–33.
- Chothia C, Gough J, Vogel C, et al. Evolution of the protein repertoire. Science. 2003;300(5626):1701–1703.
- Liang JY, Xia JY, Liu LL, et al. Global patterns of the responses of leaf-level photosynthesis and respiration in terrestrial plants to experimental warming. J. Plant Ecol. 2013;6(6):437–447.
- Zhao Q, Chen W, Bian J, et al. Proteomics and phosphoproteomics of heat stress-responsive mechanisms in spinach. Front Plant Sci. 2018;9:800.
- Hao F, Zhao S, Dong H, et al. Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J Integr Plant Biol. 2010;52(3):298–307.
- Pang Y, Li J, Qi B, et al. Aquaporin AtTIP5;1 as an essential target of gibberellins promotes hypocotyl cell elongation in Arabidopsis thaliana under excess boron stress. Funct Plant Biol. 2018;45(3):305–314.
- Lü D, Wang W, Miao C. ATHK1 acts downstream of hydrogen peroxide to mediate ABA signaling through regulation of calcium channel activity in Arabidopsis guard cells. Chin Sci Bull. 2013;58(3):336–343.
- Ma XN, Zhang XR, Yang L, et al. Hydrogen peroxide plays an important role in PERK4-mediated abscisic acid-regulated root growth in Arabidopsis. Functional Plant Biol. 2019;46(2):165–174.
- Wang K, He JN, Zhao Y, et al. EAR1 negatively regulates ABA signaling by enhancing 2C protein phosphatase activity. Plant Cell. 2018;30(4):815–834.
- Li W, de Ollas C, Dodd IC. Long-distance ABA transport can mediate distal tissue responses by affecting local ABA concentrations. J Integr Plant Biol. 2018;60(1):16–33.
- Sun L, Ma L, He S, et al. AtrbohD functions downstream of ROP2 and positively regulates waterlogging response in Arabidopsis. Plant Signal Behav. 2018;13(9):1–5.
- Li L, Hou M, Cao L, et al. Glutathione S-transferases modulate Cu tolerance in Oryza sativa. Environ Exp Bot. 2018;155:313–320.
- Guo S, Dai S, Singh PK. et al. A membrane-bound NAC-like transcription factor OsNTL5 represses the flowering in Oryza sativa. Front Plant Sci. 2018;9:555.
- Zhao X, Wang YL, Qiao XR, et al. Phototropins function in high-intensity blue light-induced hypocotyl phototropism in Arabidopsis by altering cytosolic calcium. Plant Physiol. 2013;162(3):1539–1551.
- Zhao X, Li YY, Xiao HL, et al. Nitric oxide blocks blue light-induced K + influx by elevating the cytosolic Ca2+ concentration in Vicia faba L. guard cells. J Integr Plant Biol. 2013;55(6):527–536.
- Lv S, Yu D, Sun Q, et al. Activation of gibberellin 20-oxidase 2 undermines auxin-dependent root and root hair growth in NaCl-stressed Arabidopsis seedlings. Plant Growth Regul. 2018;84(2):225–236.
- Zhao X, Wang YJ, Wang YL, et al. Extracellular Ca2+ alleviates NaCl-induced stomatal opening through a pathway involving H2O2-blocked Na+ influx in Vicia guard cells. J Plant Physiol. 2011;168(9):903–910.
- Ma L, Zhang H, Sun L, et al. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress . J Exp Bot. 2012;63(1):305–317.
- Qi J, Song CP, Wang B, et al. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol. 2018;60(9):805–826.
- Wang PT, Liu H, Hua HJ, et al. A vacuole localized beta-glucosidase contributes to drought tolerance in Arabidopsis. Chin Sci Bull. 2011;56(33):3538–3546.
- Xu LH, Wang WY, Guo JJ, et al. Zinc improves salt tolerance by increasing reactive oxygen species scavenging and reducing Na+ accumulation in wheat seedlings. Biologia Plant. 2014;58(4):751–757.
- Li W, Zhao F, Fang W, et al. Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L.) employing iTRAQ-based proteomic technique. Front Plant Sci. 2015;116:732.
- Zhang J, Wang F, Zhang C, et al. A novel VIGS method by agroinoculation of cotton seeds and application for elucidating functions of GhBI-1 in salt-stress response. Plant Cell Rep. 2018;37(8):1091–1100.
- Xu F, Liu H, Xu Y, et al. Heterogeneous expression of the cotton R2R3-MYB transcription factor GbMYB60 increases salt sensitivity in transgenic Arabidopsis. Plant Cell Tiss Organ Cult. 2018;133(1):15–25.
- Li K, Yang FB, Miao YC, et al. Abscisic acid signaling is involved in regulating the mitogen-activated protein kinase cascade module AIK1-MKK5-MPK6. BMC Plant Biol. 2017;12(5):e1321188.
- Liu LY, Li N, Yao CP, et al. Functional analysis of the ABA-responsive protein family in ABA and stress signal transduction in Arabidopsis. Chin Sci Bull. 2013;58(31):3721–3730.
- Song Y, Xiang F, Zhang G, et al. Abscisic acid as an internal integrator of multiple physiological processes modulates leaf senescence onset in Arabidopsis thaliana. Front Plant Sci. 2016;197:181.