Abstract
Polyploidization events are prevalent in plants and act as an important evolutionary force for speciation, adaptation and diversification. During this process, the genome undergoes reorganization and large changes. However, the evolutionary characteristics of specific gene families are still not well investigated. Cultivated peanut is an allotetraploid that evolved from a diploid. In this study, we focused on the family of mitochondrial transcription termination factors (mTERF), which is increasing in gene number in higher plants and acts in organellar gene expression. The 65 mTERFs were identified in allotetraploid peanut, but only 31 in diploid peanut, which showed that the genome has not doubled simply during the polyploidization event. Some of the mTERFs may have emerged as a result of reverse complements, deletions, insertions, fusions and so on. mTERFs were divided into three groups according to their predicted localization. Six representative proteins showed their different localization. Most of the AhmTERF mRNAs were detected in root and leaf samples and responded to dehydration stress. Our data provide insights into the evolution of the mTERF gene family in peanut and further support the hypothesis that these genes respond to dehydration stress.
Keywords:
Introduction
Peanut is a Fabaceae plant with geocarpy, which can grow on arid and marginal land. The roots of peanut plants harbour colonies of nitrogen-fixing bacteria. With richness in oil and protein contents, peanut has been an important resource of edible oil and protein in many countries. In China, peanut accounts for >46% of total output of all oil crops, and ranks fourth after rice, wheat and corn in market value [Citation1], therefore, peanut is important as a main economic crop. Cultivated peanut (Arachis hypogaea) is an allotetraploid species (AABB genome, 2n = 4x = 40) whose ancestral genomes are probably derived from the A-genome species, A. duranensis (AA genome, 2n = 2x = 20), and the B-genome species, A. ipaensis (BB genome, 2n = 2x = 20) [Citation2–4]. The hybridization event between two diploid species most likely resulted in the allotetraploid A. hypogaea [Citation4–7], which occurred about 11,690 years ago and the domestication of A. hypogaea happened about 4500 years ago [Citation8]. Although agronomic traits differ dramatically between cultivated peanut and its wild progenitors, the A and B subgenomes of cultivated peanut appear to have undergone relatively few changes since polyploidization [Citation1,Citation2,Citation9,Citation10]. The genome size of A. hypogaea (2.7 Gb) is close to the sum of those of A. duranensis (1.25 Gb) and A. ipaensis (1.56 Gb) [Citation1–2], suggesting that there have been no dramatic changes in genome size since polyploidization. However, a polyploidization event is not only the merge of two genomes, but also involves genome reorganization, as manifested by transposable element mobility, insertions, deletions and epigenome restructuring, which results in an altered phenotype and ecology [Citation11].
Mitochondrial transcription termination factor (mTERF) proteins have a modular architecture characterized by the repetition of a 30-amino acid motif named the mTERF motif [Citation12]. Only four mTERF members were found in mammals [Citation13–16]. However, there are many mTERF members in plants, for example, 35 members in Arabidopsis, 46 in rice and 31 in maize. In Arabidopsis thaliana, the 35 mTERF members were divided into five types according to their location and were successively named mTERF1-35 [Citation12]. The mTERFs modulate organellar gene expression in mitochondria and/or chloroplasts. RUG2/BSM is essential for mitochondrion and chloroplast development. The bsm mutant exhibits embryos arrested at the late globular stage of development [Citation17,Citation18]. Loss of function of mTERF5 (mda1 mutant), mTERF6 (PED191 mutant) or mTERF9 (twr-1 mutant) causes abnormal leaf development [Citation19–21]. The mTERF10 and mTERF11 are involved in tolerance to salinity, but knock-down of mTERF10 or mTERF11 has no overt phenotypic effect under normal growth conditions [Citation22]. The mTERF15 regulates the transcription of mitochondrial-DNA-encoded mitochondria complex I components [Citation23]. Disruption of mTERF22 function results in reduced mtRNA accumulation and affects the expression of numerous mitochondrial genes related to organelle biogenesis [Citation24]. Thus, mTERF proteins fulfil diverse roles in the development and stress resistance of plants.
However, the function of mTERF family in peanut is still unknown, especially due to the complexity of polyploidization. In this study, we have identified 65 mTERFs coding genes in the allotetraploid peanut and analyzed the sequence changes from wild diploid to allotetraploid. Protoplasts transient transformation assays confirmed the subcellular localization of six representative mitochondrial cluster AhmTERFs. Tissue-specific and dehydration stress-responsive expression patterns for AhmTERFs were examined by transcriptome analyses and six genes were further determined using quantitative real-time polymerase chain reaction (RT-PCR). Taken together, our results gave insight into the mTERFs evolution from wild diploid to allotetraploid and shed light on the involvement of AhmTERF genes in plant growth and development, including the response to dehydration stress.
Materials and methods
Plant material and treatment
The South China peanut cultivar Yueyou7 was supplied by the Crop Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou. Peanut plants were grown under normal conditions as described previously [Citation25]. Seedlings were grown in a greenhouse at 25–28 °C. Plants were removed from soil and any residual soil was washed from their roots to dehydrate for 2 h and then they were rehydrated for 24 h.
Identification of AhmTERFs in peanut and phylogenetic tree construction
To identify potential mTERFs in peanut, we used the gene sequences of AtmTERFs as queries to run the BLAST program against the peanut database (https://www.peanutbase.org/). The phylogenetic tree was generated by NCBI program (https://www.ncbi.nlm.nih.gov/tools/cobalt/re_cobalt.cgi).
Chromosomal localization and gene evolution analyses
The gene location and chromosome number for each peanut mTERF gene was obtained from the peanut database (https://www.peanutbase.org/). The sequence alignment of mTERF genes between diploid and allotetraploid peanut was done by using BLAST (https://www.peanutbase.org/blast).
Subcellular localization assays
The full-length coding sequence of AhmTERFs was introduced into p35S-eGFP to generate p35S-eGFP-AhmTERFs fusion construct. The fusion construct was transfected into protoplast cells for in vivo protein targeting. The protoplast isolation and transient expression were conducted as described previously [Citation26]. After transfection, the protoplasts were incubated at 22 °C for 12 h in the dark, and the distribution of the fusion protein was determined using a confocal laser scanning microscope (LSM800, Carl Zeiss, Germany). The protoplasts were stained by mitochondria-specific dye MitoTracker for 5 min.
RNA isolation and quantitative real-time RT-PCR
RNA was extracted as described previously [Citation27]. For first-strand cDNA synthesis, 1 µg high-quality total RNA was reverse transcribed (RT) using a Prime Script TM RT Reagent Kit (Perfect Real Time, TaKaRa, Dalian, China). The cDNA sample was used as the template. Quantitative real-time RT-PCR assays were performed on three biological replicates on CFX96 Touch system (Bio-Rad, USA). The peanut 18 s gene was used as the internal control, and the relative expression levels of genes were calculated using the 2-ΔΔCT method [Citation28]. Primer sequences are listed in Supplemental Table S1.
Transcriptome analyses
For transcriptome preparation, total RNA was extracted using the TRIzol extraction method [Citation27]. RNA concentration was measured initially on a NanoDrop 2000 and more precise measurements were made on an Agilent 2100 Bioanalyzer. Next, mRNA was enriched using an oligo (dT) resin and then fragmented and reverse-transcribed into cDNA with random primers. Second-strand cDNA was synthesized by DNA polymerase I, RNase H, dNTP and buffer and then purified with a QIAquik PCR Purification Kit (Qiagen, Germany), end repaired and poly(A) added. Purified cDNA was ligated to Illumina sequencing adapters. Sequencing was carried out using an Illumina HiSeq 4000 system by Gene Denovo Biotechnology Co (Guangzhou, China). All raw data have been uploaded to the NCBI Sequence Read Archive (SRA) (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA503795). Analysis of differences in gene expression was carried out as previously described [Citation25].
Results and discussion
Identification and phylogenetic analysis of allotetraploid peanut mTERF family
The protein sequences of mTERFs can be found on peanut database (https://www.peanutbase.org/). In total, 65 mTERF proteins of allotetraploid peanut were identified (). The mRNA of AhmTERF genes ranged from 195 to 4318 bp, whereas the proteins ranged from 64 to 1336 amino acids with calculated molecular weights from 6.86 to 152.26 kDa and isoelectric points from 5.41 to 10.31 ().
Table 1. Overview of mTERF genes identified in allotetraploid peanut.
To evaluate the evolutionary relationship of allotetraploid peanut mTERFs, a phylogenetic analysis was performed using the protein sequences of mTERFs from allotetraploid peanut and A. thaliana. Arabidopsis mTERF proteins were classified as chloroplast and chloroplast-associated cluster, mitochondria and mitochondrial-associated cluster, and the other cluster [Citation12]. according to the TargetP (http://www.cbs.dtu.dk/services/TargetP/) scores, mTERFs of allotetraploid peanut were also divided into three clusters as those of A. thaliana (). The phylogenetic tree indicated these three clusters were not distributed individually. Evolution and chromosomal localization of mTERFs in peanut.
Figure 1. Phylogenetic tree of mTERFs in allotetraploid peanut and Arabidopsis. The phylogenetic tree was generated by NCBI program (https://www.ncbi.nlm.nih.gov/tools/cobalt/re_cobalt.cgi). Violet lines represent the mitochondria localization or mitochondrial-associated cluster. Green lines represent the chloroplast localization or chloroplast-associated cluster. Black lines represent other localizations or associated clusters.
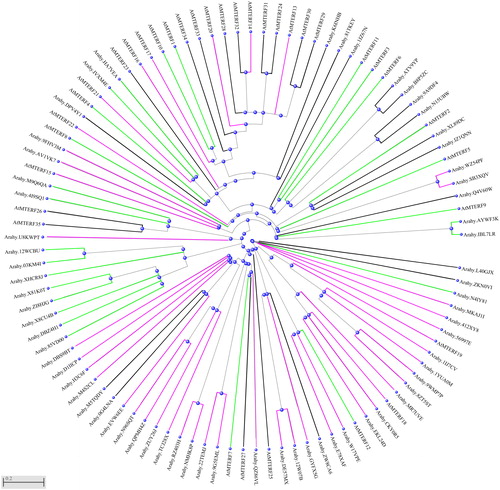
Polyploidy entails far more than simply the merger of two ancestral genomes; rather it involves a whole spectrum of molecular and physiological adjustments [Citation29]. Polyploidy simultaneously duplicates tens of thousands of genes by adding one extra set of genomes [Citation30]. Generally, the merger of A and B diploid genome formed the genome of allotetraploid peanut. Therefore, we compared the mTERF genes in A. duranensis and A. ipaensis with those of allotetraploid peanut to understand the evolution of mTERF genes. The sequences of mTERFs of A. duranensis and A. ipaensis also can be found in the peanut database (https://www.peanutbase.org/). Totally, 31 mTERFs were found in the A and B diploid genome, respectively. However, 65 mTERFs were found in allotetraploid peanut and successively named AhmTERF1-65. They were divided into three subgroups. The I ∼ XI (AhmTERF1∼AhmTERF34) were predicted as mitochondria localization or mitochondria associated. The XII ∼ XVII (AhmTERF35∼AhmTERF49) were predicted as chloroplast localization or chloroplast associated, whereas XVIII ∼ XXV (AhmTERF50∼AhmTERF65) were classified as others ().
Figure 2. Evolution of mTERFs in peanut. The sequences of mTERFs in diploid and allotetraploid peanut were obtained from Peanutbase (https://www.peanutbase.org/). The percentage shows the sequence identity between diploid and allotetraploid peanut.
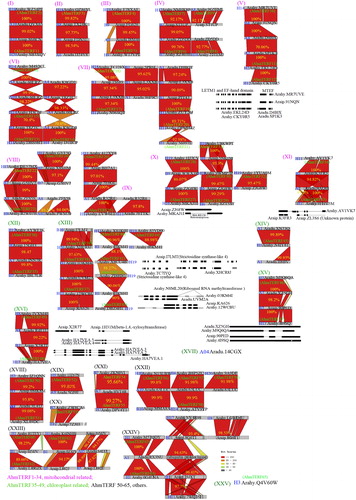
Extensive genomic rearrangements, including exchanges between genomes and gene loss, often arise with the onset of polyploidization [Citation29]. Hence, polyploids often exhibit more genome structural variation than their diploid progenitors [Citation11]. Genome structural variations represent large rearrangements of genomic types including deletion, insertion, inversions, duplications and copy number variations [Citation31]. It was reported that at least one pair of chromosomes of IpaDur1, an induced allotetraploid (A. ipaensis × A. duranensis)4x, has a clear mosaic hybridization pattern indicating recombination between the subgenomes [Citation7]. According to the homology of sequences, most of mTERFs in allotetraploid peanut were obtained from wild diploid, such as AhmTERF1-7, AhmTERF11, AhmTERF13-15, AhmTERF35-36 and AhmTERF50-51. Some mTERFs obtained new segments through reverse complementation (e.g. AhmTERF15-16), deletion (e.g. AhmTERF18, AhmTERF25-26 and AhmTERF48-49), insertion (e.g. AhmTERF27), or fusion (e.g. AhmTERF48). Strikingly, AhmTERF1 (from Aradu.86BCN) and AhmTERF2 (from Araip.QV65X) were highly conserved, with 100% homology. Aradu.14CGX and AhmTERF65 (Arahy.Q4V60W) were changed between diploid and allotetraploid genome. The transcript of Aradu.14CGX was not annotated in A. ipaensis or A. hypogaea peanut, which predicted it expressed in leaves and located in chloroplasts (https://www.peanutbase.org/). It has attracted great attention of us for its function, although whether it does not exist in the A. ipaensis or A. hypogaea peanut still remains to be determined. And AhmTERF65 has no gene annotation in diploid peanut, but only exists in allotetraploid peanut. It can be speculated that AhmTERF65 has developed form another gene or its ancestor gene was lost. As predicted on the Peanutbase, AhmTERF65 is mainly expressed in the nodule, perianth and stamen. Hence, it could be speculated that it plays a significant role for plant growth. These results showed that gene polymorphisms of the mTERF family were found in allotetraploid peanut when evolved from diploid.
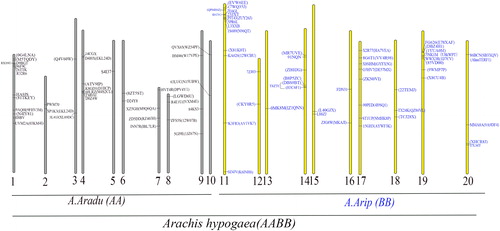
The chromosomal localization of the mTERF genes was further investigated by their genomic distributions on chromosomes. The genes from A. hypogaea are distributed over 19 chromosomes, except chromosomes 5 (). Chromosome 11 contains the largest number of AhmTERF genes with nine, followed by chromosomes 17 and 18 with eight genes each. The mTERF genes of A. duranensis and A. ipaensis are also shown in . The mTERF genes of A. duranensis are distributed over 10 chromosomes. Chromosome 1 has the largest number of mTERF genes with nine. Only one mTERF gene was found on chromosomes 3, 5 and 6. The mTERF genes of A. ipaensis are also distributed over 10 chromosomes. Chromosome 1 has the largest number of mTERF genes with 10. Only one mTERF gene was found on chromosomes 3, 5 and 8. It is noteworthy that the chromosomal localization of several mTERF genes of the A subgenome and B subgenome in A. hypogaea overlapped with those in the A genome and B genome in A. duranensis and A. ipaensis, respectively, suggesting that the chromosomal localization of several mTERFs did not change since the hybridization event, although the sequences of mTERF genes had changed.
Figure 3. Chromosomal localization of mTERF genes in diploid and allotetraploid peanut. The grey pillar indicate chromosomes of diploid, A. duranensis, and the yellow pillar indicate chromosomes of diploid, A. ipaensis. A total of 20 chromosomes indicated chromosomes of allotetraploid peanut. The genes in brackets indicate mTERF genes in allotetraploid peanut.
Subcellular localization of AhmTERFs
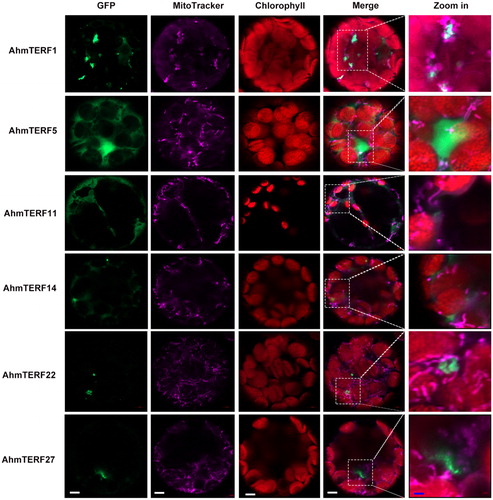
Prediction analysis indicated that AhmTERFs exhibit various patterns of subcellular localization, such as the mitochondria, chloroplast and cytoplasm. Members of the chloroplast and chloroplast-associated clusters are principally involved in chloroplast gene expression, embryogenesis and protein catabolism, whereas representatives of the mitochondria and mitochondria-associated clusters seem to participate in DNA and RNA metabolism in the mitochondria, the nucleus and the cytoplasm. And the mTERF proteins in ‘the other’ cluster are weakly expressed, but may act in the nucleus [Citation12]. As shown in , the mTERFs of allotetraploid peanut were divided into three subgroups. Here, we selected several mTERFs to further determine the subcellular localization of AhmTERF proteins. Full length cDNAs were fused to the eGFP driven by the CaMV 35S promoter and then transiently expressed in isolated protoplasts. These genes are associated with mitochondria, as suggested by protein sequence analysis. Therefore, we confirmed this possibility by showing that the mitochondria-specific dye MitoTracker. As shown in , AhmTERF proteins were not entirely located in mitochondria. AhmTERF1 and AhmTERF22 were surrounded by mitochondria, were AhmTERF5 and AhmTERF11 were widespread. AhmTERF14 and AhmTERF27 were located close to mitochondria. It is noteworthy that the mitochondria exhibited different forms in protoplasts with different AhmTERF proteins, implying that AhmTERFs differently impact the mitochondria.
Figure 4. Subcellular localization of AhmTERFs. AhmTERFs-eGFP fusion constructs were used to determine the subcellular localization of AhmTERFs. MitoTracker was used as mitochondria marker. Fluorescence images were captured with a confocal laser scanning microscope. Scale bars in white, 5 µm, scale bars in blue, 1 µm.
Tissue and organ specific expression of mTERFs in allotetraploid peanut
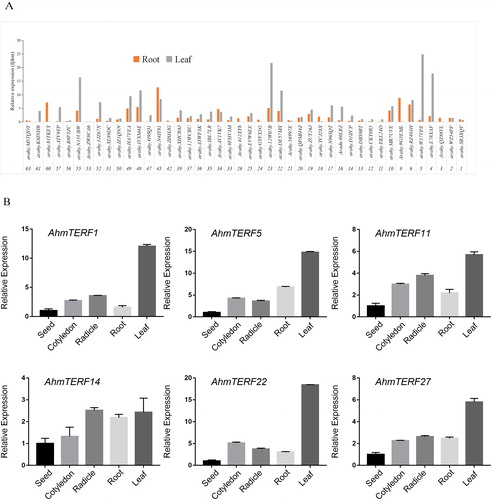
To investigate the tissue-specific expression profiles of AhmTERF genes in allotetraploid peanut, we analyzed the AhmTERF genes expression levels in transcriptomes of leaf and root at four-leaf stage. Here, we found that a total of 44 AhmTERFs can be detected (). Next, we conducted Quantitative real-time RT-PCR to determine the expression of AhmTERF1, 5, 11, 14, 22 and 27 in seed, cotyledon, radicle, root and leaf. However, unlike the results of transcriptome, AhmTERF1 mRNAs were highly expressed in leaves, illustrating that there are differential expression levels in different stages. AhmTERF5, 11, 22 and 27 mRNAs were also accumulated in leaves. The AhmTERF14 transcript level was increased after seed germination ().
Figure 5. Tissue- and organ-specific expression patterns of AhmTERF genes in allotetraploid peanut. (A) Expression patterns of AhmTERF genes in transcriptomes of root and leaf at the four-leaf stage. The numbers below the x-axis represent the name of AhmTERF. (B) The x-axis represents different tissues or organs. The y-axis shows the gene expression levels after normalization to reference gene 18 s. Note: Seeds, cotyledons and radicle of 5-day-old peanut and roots and leaves of two-leaf stage were collected for total RNA isolation. Quantitative real-time RT-PCR was performed using gene-specific primers. Data are the mean values with standard deviation (±SD) of three independent experiments.
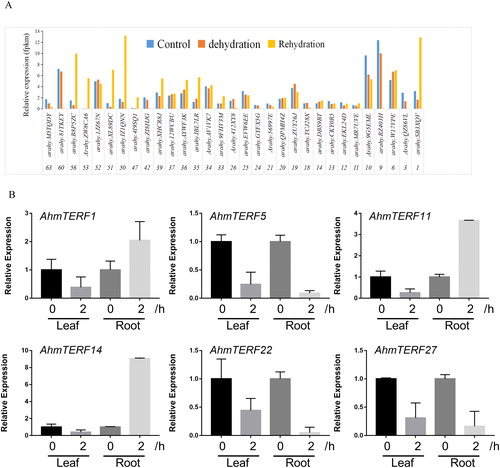
The expression levels of AhmTERFs in response to dehydration and rehydration
The mTERF family of genes mediate acclimation of plants to adversity conditions through a complex network, which is further supported by recent finding [Citation32]. Dehydration stress negatively affects plant growth and development. However, the role of mTERFs in allotetraploid peanut under dehydration stress remains unknown. We uncovered the AhmTERF genes expression patterns through transcriptome analysis of leaf samples under dehydration stress and rehydration. Although several genes were not detected in the transcriptome, 31 genes showed differential expression patterns in dehydration stress and rehydration (). To further examine the expression levels of AhmTERFs under dehydration stress in different tissues, we conducted Quantitative real-time RT-PCR to detect the expression levels of six genes in root and leaf samples under dehydration stress and found that there were different expression patterns. AhmTERF1, 11 and 14 mRNAs were accumulated in roots under dehydration stress for 2 h, whereas AhmTERF5, 22 and 27 mRNA levels were decreased. But all six genes were down-regulated in leaf samples under dehydration stress for 2 h (). These results hinted that the AhmTERF genes have different functions in roots and leaves when plants are exposed to dehydration stress, probably because there are different sensitivities to dehydration stress.
Figure 6. Expression profiles of AhmTERF genes under dehydration stress in allotetraploid peanut. (A) Expression patterns of AhmTERF genes in the transcriptome of leaf at the four-leaf stage under dehydration for 2 h and rehydration for 24 h. The numbers below the x-axis represented the name of AhmTERF. (B) Expression patterns of 6 genes in leaf and root under dehydration stress for 2 h. Quantitative real-time RT-PCR was performed using gene-specific primers. Data are the mean values ± SD of three independent experiments.
Polyploidization events are prevalent in plants, and have been recognized as an important evolutionary force for speciation, adaptation and diversification [Citation29,Citation33,Citation34]. Novel phenotypes are known to emerge from this polyploidization, including some with high visibility to natural selection, such as organ size and flowering time [Citation29]. In general, polyploids exhibit larger organs compared to their ancestors. It is well acknowledged that A. duranensis (AA genome) and A. ipaensis (BB genome) have formed the allotetraploid A. hypogaea (AABB, 2n = 4x = 40) [Citation1]. The diploid and allotetraploid peanut have great differences in agronomic traits. Pod development directly affects peanut yield, and a large pod is a major domestication trait; cultivated peanuts have the pod size four to ten times higher than that of its progenitor [Citation8]. The global environmental selection on the polyploidy has played significant roles. Twenty-five sequenced plant genomes representing major lineages of angiosperms were used to identify the gene families retaining duplicated genes, supporting the idea about the significant contribution of paleopolyploidy to plant adaptation during global environmental changes in the evolutionary history of angiosperms [Citation30]. The majority of mTERFs in allotetraploid peanut were obtained from diploid ancestors, suggesting that the retained mTERFs have critical functions to help plants to adapt to environmental changes.
Indeed, the mTERF family has a pivotal role in plant growth and development [Citation17,Citation18,Citation35,Citation36], and is involved in abiotic stress responses [Citation22,Citation29,Citation37]. Most of the AhmTERF mRNAs in peanut can be detected in the root and leaf transcriptomes. Notably, AhmTERFs respond to dehydration stress, further showing the importance of AhmTERFs which were retained after the polyploidization event with the dramatic environment changes.
Conclusions
In this study, we analyzed the gene family of mTERFs in peanut. A total of 65 mTERFs were identified in allotetraploid peanut, but only 31 in diploid peanut. Some of the mTERFs probably involved reverse complements, deletions, insertions, fusions and so on. mTERFs were divided into three groups according to their predicted localization. Six representative proteins showed their different localization. Most of the AhmTERF mRNAs were detected in roots and leaves and responded to dehydration stress. Overall, this paper provides a new perspective to the evolution of the mTERF gene family in peanut and further supports the hypothesis that these genes responded to dehydration stress.
Author contributions
LXY and LL designed this research. LXY and HB analyzed the gene family and transcriptome data. LLM conducted quantitative real-time RT-PCR assay and wrote the manuscript.
Supplemental Material
Download PDF (86.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zhuang W, Chen H, Yang M, et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat Genet. 2019;51(5):865–876.
- Bertioli DJ, Cannon SB, Froenicke L, et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat Genet. 2016;48(4):438–446.
- Kochert G, Stalker T, Gimenes M, et al. RFLP and cytogenetic evidence on the origin and evolution of allotetraploid domesticated peanut, Arachis hypogaea (Leguminosae). Am J Bot. 1996;83(10):1282–1291.
- Seijo G, Lavia GI, Fernández A, et al. Genomic relationships between the cultivated peanut (Arachis hypogaea, Leguminosae) and its close relatives revealed by double GISH. Am J Bot. 2007;94(12):1963–1971.
- Fávero AP, Simpson CE, Valls Jose FM, et al. Study of evolution of cultivated peanut through crossability studies among Arachis ipaensis, A. duranensis and A. hypogaea. Crop Sci. 2006;46(4):1546–1552.
- Grabiele M, Chalup L, Robledo G, et al. Genetic and geographic origin of domesticated peanut as evidenced by 5S rDNA and chloroplast DNA sequences. Plant Syst Evol. 2012;298(6):1151–1165.
- do Nascimento EFMB, dos Santos BV, Marques LOC, et al. The genome structure of Arachis hypogaea (Linnaeus, 1753) and an induced Arachis allotetraploid revealed by molecular cytogenetics. Comp Cytogenet. 2018;12(1):111–140.
- Yin D, Ji C, Song Q, et al. Comparison of Arachis monticola with diploid and cultivated tetraploid genomes reveals asymmetric subgenome evolution and improvement of peanut. Adv Sci (Weinh)). 2020;7(4):1901672.
- Nielen S, Vidigal BS, Leal-Bertioli SC, et al. Matita, a new retroelement from peanut: characterization and evolutionary context in the light of the Arachis A-B genome divergence. Mol Genet Genom.. 2012;287(1):21–38.
- Samoluk SS, Robledo G, Podio M, et al. First insight into divergence, representation and chromosome distribution of reverse transcriptase fragments from L1 retrotransposons in peanut and wild relative species. Genetica. 2015;143(1):113–125.
- Leitch AR, Leitch IJ. Genomic plasticity and the diversity of polyploid plants. Science. 2008;320(5875):481–483.
- Kleine T. Arabidopsis thaliana mTERF proteins: evolution and functional classification. Front Plant Sci. 2012;3(233):1–15.
- Kruse B, Narasimhan N, Attardi G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989;58(2):391–397.
- Park CB, Asin-Cayuela J, Cámara Y, et al. MTERF3 is a negative regulator of mammalian mtDNA transcription. Cell. 2007;130(2):273–285.
- Cámara Y, Asin-Cayuela J, Park CB, et al. MTERF4 regulates translation by targeting the methyltransferase NSUN4 to the mammalian mitochondrial ribosome. Cell Metab. 2011;13(5):527–539.
- Wenz T, Luca C, Torraco A, et al. mTERF2 regulates oxidative phosphorylation by modulating mtDNA transcription. Cell Metab. 2009;9(6):499–511.
- Quesada V, Sarmiento-Mañús R, González-Bayón R, et al. Arabidopsis RUGOSA2 encodes an mTERF family member required for mitochondrion, chloroplast and leaf development. Plant J. 2011;68(4):738–753.
- Babiychuk E, Vandepoele K, Wissing J, et al. Plastid gene expression and plant development require a plastidic protein of the mitochondrial transcription termination factor family. Proc Natl Acad Sci USA. 2011;108(16):6674–6679.
- Robles P, Micol JL, Quesada V. Arabidopsis MDA1, a nuclear-encoded protein, functions in chloroplast development and abiotic stress responses. PLoS One. 2012;7(8):e42924.
- Robles P, Micol JL, Quesada V. Mutations in the plant-conserved MTERF9 alter chloroplast gene expression, development and tolerance to abiotic stress in Arabidopsis thaliana. Physiol Plant. 2015;154(2):297–313.
- Robles P, Núñez-Delegido E, Ferrández-Ayela A, et al. Arabidopsis mTERF6 is required for leaf patterning. Plant Sci. 2018;266:117–129.
- Xu D, Leister D, Kleine T. Arabidopsis thaliana mTERF10 and mTERF11, but not mTERF12, are involved in the response to salt stress. Front Plant Sci. 2017;8:1213.
- Hsu YW, Wang HJ, Hsieh MH, et al. Arabidopsis mTERF15 is required for mitochondrial nad2 intron 3 splicing and functional complex I activity. PLoS One. 2014;9(11):e112360.
- Shevtsov S, Nevo-Dinur K, Faigon L, et al. Control of organelle gene expression by the mitochondrial transcription termination factor mTERF22 in Arabidopsis thaliana plants. PLoS One. 2018;13(7):e0201631.
- Li X, Lu J, Liu S, et al. Identification of rapidly induced genes in the response of peanut (Arachis hypogaea) to water deficit and abscisic acid. BMC Biotechnol. 2014;14:58.
- Liu S, Li M, Su L, et al. Negative feedback regulation of ABA biosynthesis in peanut (Arachis hypogaea): a transcription factor complex inhibits AhNCED1 expression during water stress. Sci Rep. 2016;6:37943.
- Wan XR, Li L. Regulation of ABA level and water-stress tolerance of Arabidopsis by ectopic expression of a peanut 9-cis-epoxycarotenoid dioxygenase gene. Biochem Biophys Res Commun. 2006;347(4):1030–1038.
- Kim JM, To TK, Matsui A, et al. Acetate-mediated novel survival strategy against drought in plants. Nat Plants. 2017; 3:17097.
- Adams KL, Wendel JF. Polyploidy and genome evolution in plants. Curr Opin Plant Biol. 2005;8(2):135–141.
- Wu S, Han B, Jiao Y. Genetic contribution of paleopolyploidy to adaptive evolution in Angiosperms. Mol Plant. 2020;13(1):59–71.
- Tuzun E, Sharp AJ, Bailey JA, et al. Fine-scale structural variation of the human genome. Nat Genet. 2005;37(7):727–732.
- Quesada V. The roles of mitochondrial transcription termination factors (MTERFs) in plants. Physiol Plant. 2016;157(3):389–399.
- Wood TE, Takebayashi N, Barker MS, et al. The frequency of polyploid speciation in vascular plants. Proc Natl Acad Sci USA. 2009;106(33):13875–13879.
- Soltis PS, Soltis DE. Ancient WGD events as drivers of key innovations in angiosperms. Curr Opin Plant Biol. 2016;30:159–165.
- Meskauskiene R, Würsch M, Laloi C, et al. A mutation in the Arabidopsis mTERF-related plastid protein SOLDAT10 activates retrograde signaling and suppresses (1)O(2)-induced cell death. Plant J. 2009;60(3):399–410.
- Romani I, Manavski N, Morosetti A, et al. A Member of the Arabidopsis mitochondrial transcription termination factor family is required for maturation of chloroplast transfer RNAIle(GAU). Plant Physiol. 2015;169(1):627–646.
- Robles P, Navarro-Cartagena S, Ferrández-Ayela A, et al. The Characterization of Arabidopsis mterf6 mutants reveals a new role for mTERF6 in tolerance to abiotic stress. IJMS. 2018;19(8):2388.
