?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Bacillus subtilis HLZ-68 can produce l-amino acid oxidase (l-AAO), and dl-arginine can be degraded asymmetrically by suspending the wet bacterial biomass in the degradation liquid. By adding oxygen-vectors to the fermentation medium, the collected amount of wet bacterial biomass can be increased. Taking n-dodecane, n-hexadecane, oleic acid, paraffin and n-hexane as oxygen-vectors, the optimal oxygen-vector was 1.2% (v/v) oleic acid. The wet weight biomass increased by 66.83% and the activity of l-AAO in the fermentation broth increased by 38.88% compared with those before the addition of oxygen-vector. The standard sample dl-arginine was derivatized by phenyl isothiocyanate, and then subjected to high-performance liquid chromatography (HPLC), and the obtained peak area and arginine content were used as standard curves to measure the dl-arginine. The content of d-arginine and l-arginine in the initial degradation solution was 50% each, and the bacterial cells were added to the initial degradation solution of dl-arginine. After 21 h of reaction, l-arginine was completely degraded, leaving 47% of d-arginine. d-alanine was easily extracted from the reaction solution using cation-exchange resin, after centrifugation, decolourization, concentration and vacuum drying, and the chemical and optical purity of the extracted d-arginine were 92.68% and 97.46%, respectively.
Introduction
Each essential amino acid had its optimal intake and minimum intake [Citation1]. Because d-arginine is an unnatural amino acid, its content in nature is very small, so it needs to be obtained through industrial production. d-arginine plays an important role in improving the response of glucocorticoids to normal life pressure [Citation2]. d-arginine can play an analgesic role during acute pain [Citation3]. d-arginine has protective effect against oxygen free radical induced cardiac injury [Citation4,Citation5]. d-arginine also has a wide range of applications in the pharmaceutical and food industries. Adding d-arginine to food can effectively improve its water solubility and extend its shelf life [Citation6]. A study in mice has also suggested that d-arginine may be added to milk to enhance infant brain development [Citation7]. With the widespread use of d-arginine in the food and pharmaceutical industries, the market demands for d-arginine have also increased. In general, arginine can be classified into d-arginine and l-arginine according to its chiral molecular structure [Citation8] and optical rotation [9]. The existing production methods of d-arginine include microbial fermentation, biochemical conversion, asymmetric resolution and asymmetric degradation. Asymmetric degradation is a method that directly utilizes the specificity and selectivity of enzymes in microbial cells to catalyze the oxidation of substrates. For example, l-AAO directly degrades l-amino acids in dl amino acids to produce the process of separation and purification of d-amino acids [Citation10,Citation11]. Microbial cells are a tool for the separation of l-amino acids and d-amino acids in asymmetric degradation [Citation12].
Oxygen is a significant factor affecting the growth, metabolism and morphology of microorganisms. Increasing the amount of dissolved oxygen is conducive to the collection of Bacillus subtilis. The main methods to improve the dissolved oxygen in fermentation include increasing stirring speed, increasing aeration rate, adding oxygen carrier into fermentation medium, etc. The faster the stirring speed, the greater the shear force, which may directly lead to the death of some bacteria. Increasing the volume of ventilation will produce a lot of bubbles, which will not benefit the propagation of the bacteria for a long time. Therefore, adding an oxygen carrier as part of the technology can solve these problems well. Compounds added to the fermentation media that improve the oxygen transfer to microorganisms were defined as oxygen-vectors [Citation13]. The oxygen-vector is an organic solvent which has no toxicity to microbial cells, can combine with oxygen in the system, is insoluble in water, and can transfer oxygen to the fermentation system [Citation14]. The oxygen-vector addition technology was developed in the 1980s. It is found that oxygen-vectors can carry oxygen from the gas phase to the liquid phase, providing more dissolved oxygen and higher oxygen solubility than the water phase, especially in aerobic fermentation system [Citation15]. The yield of 4-acetylantroquinonol B and antroquinonol increased by 15.8-fold and 61.6-fold, respectively, when 5% n-hexadecane was added to the medium, the dissolved oxygen was 56.1% after 28 days of fermentation, which was about 2.2-fold of the control group [Citation16]. When the concentration of n-hexane was 3.48%, the amount of pyocyanin increased twofold [Citation17]. It is important that an oxygen-vector can solve the high cost problem of oxygen transfer in fermentation tanks. Adding an oxygen-vector is economical, simple and practical. In the present study, oleic acid was added as an oxygen-vector to the fermentation system to increase the cell counts of B. subtilis HLZ-68 and the activity of l-amino acid oxidase, thereby increasing the efficiency of dl-arginine degradation and d-arginine production.
Materials and methods
Materials
Source of bacteria
Bacillus subtilis HLZ-68 cells were obtained from Guangxi University, China.
Reagents and solutions
Reagent A: 1.2% (v/v) phenyl isothiocyanate (PITC) acetonitrile solution.
Reagent B: 14% (v/v) triethylamine (tea) acetonitrile solution.
Mobile phase A: 0.2 mol/L acetic acid sodium acetate buffer solution with pH 6.5.
Mobile phase B: acetonitrile: water: 4:1.
Methods
Selection of oxygen-vector and preparation of cell suspension
Bacillus subtilis HLZ-68 was fermented for 24 h in a 5-L fermentor with 80% filling factor, at 30 °C with an aeration of 1.0 vvm and agitation of 300 r/min. The oxygen-vectors selected for fermentation and their added amount were 1.5% n-dodecane, 1.5% n-hexadecane, 1.5% oleic acid, 1.5% paraffin and 0.8% n-hexane (…). The fermentation medium was composed of (w/v) 2% dl-alanine, 0.5% yeast extract, 0.2% K2HPO4, 0.09% MgSO4 · 7H2O and 0.02% CaCl2. We determined the effect of different oxygen-vectors on the wet weight biomass and the enzyme activity of l-AAO, to select the best oxygen-vector. After fermentation, the cells were collected by centrifugation (6500 g for 10 min), washed twice with 0.9% saline and resuspended in 0.9% saline.
Determination of d-arginine and l-arginine in degradation solution
Different optical isomers of arginine were derivatized with phenyl isothiocyanate, and then the arginine isomers were separated and determined by high-performance liquid chromatography (HPLC) using standard curves (Supplemental Figures S1 and S2).
Derivatization of sample. One millilitre of diluted dl-arginine was added into a 10 mL centrifuge tube with a pipette gun; 1 mL of reagent A and 1 mL of reagent B were added into the 10 mL centrifuge tube. The mixture was derivatized at room temperature without light shock for 1 h; then 3 mL of n-hexane were added and extraction was performed for 2 min at room temperature. The lower layer was used as the sample.
Detection conditions. Supelco chiral column (25 cm × 4.6 mm, 5 μm); mobile phase A: mobile phase B: 5:1; total flow rate of mobile phase A and mobile phase B: 1 mL/min; manual injection 20 μL; detection wavelength of UV detector 254 nm; column temperature of 25 °C.
Determination of the ability of bacteria to degrade dl-arginine
First, 4 g dl-arginine was added into 50/250 mL initial degradation solution (KH2PO4 5 g/L, Mg2SO4 · 7H2O 2 g/L, CaCl2 0.1 g/L, pH = 7.0) containing 3 g wet biomass to obtain shake-flask cultures. The degradation temperature was 30 °C, and the rotation speed of the shaking table was 180 r/min. The contents of d-arginine and l-arginine were determined every 3 h, and each group was run in parallel three times. The ability of wet biomass to degrade l-arginine in dl-arginine was studied by HPLC.
Extraction of d-alanine from the degradation mixture
The isoelectric point of d-arginine was 10.76. The pH of the degradation solution containing amino acids was adjusted to 7, which is lower than the isoelectric point of d-arginine. d-arginine was positively charged and can be combined with the strong acid cation-exchange resin 732. Finally, d-arginine was eluted with 2 mol/L ammonia water, and the eluent was decolourized with 10 g/L activated carbon. Then the activated carbon was centrifuged and filtered out. The decolourized eluent was at 55 °C. The concentrated eluent was placed into a 65 °C vacuum drying oven until d-arginine crystallized.
Q:adsorption capacity of d-arginine (mg/g)
C0:initial concentration of d-arginine in the upper column solution (g/L)
C:final concentration of d-arginine at adsorption equilibrium (g/L)
V:volume of upper column solution (L)
W:weight of wet resin (g)
Determination of chemical purity and optical purity of d-arginine
The product d-arginine was dissolved in deionized water, diluted to a suitable concentration, and the content of d-arginine was measured by HPLC. The chemical purity of the product d-arginine was determined as follows [Citation18]:
p1: Chemical purity (%)
w: d-arginine concentration (mg/L)
v: Volume(L)
m: Quality(mg)
Using deionized water as the reference solution, accurately weigh 0.1 g product d-arginine and 0.1 g pure product d-arginine, respectively, and dissolve them in 100 mL deionized water. Use a polarimeter to determine the optical rotation α, the optical purity test formula of d-arginine is as follows:
α: Optical rotation
T: Determination temperature (°C)
λ: Wavelength of light wave
L: Length of polarimeter (CM)
C: Mass of sample in 100 mL solution (g)
The optical purity of the product was calculated according to the optical rotation ratio of the measured sample. The calculation formula is as follows:
p2: Optical purity
A1: Specific rotation of sample
A2: Specific rotation of pure product
Results and discussion
Selection of oxygen-vector
A recent report showed that n-hexadecane (5%) increased the dissolved oxygen during submerged fermentation of Antrodia cinnamomea [Citation16]. ɛ-Poly-lysine synthesis was enhanced by carbon metabolism and energy metabolism with 0.5% n-dodecane in broth [Citation14]. n-Hexadecane (1%) significantly improved welan gum yield [Citation15]. The cultivation of aerobic microorganisms needs sufficient oxygen. If oxygen can be brought into the fermentation medium more efficiently, it is of great significance for the subsequent fermentation industrialization. In the present study, different oxygen-vectors were selected to increase the dissolved oxygen content in the fermentation medium based on previous reports. Different oxygen carriers had different effects on bacterial biomass and l-amino acid oxidase activity (). Except for the negative effect of n-hexane on bacteria, the overall effect was positive. When oleic acid was used as the oxygen-vector, the wet weight of biomass and l-amino acid oxygenase activity of B. subtilis HLZ-68 significantly increased. The results showed that oleic acid was the most suitable oxygen-vector.
Table 1. Effects of different oxygen-vectors on biomass wet weight and enzyme activity.
Determination of different isomers of arginine by HPLC
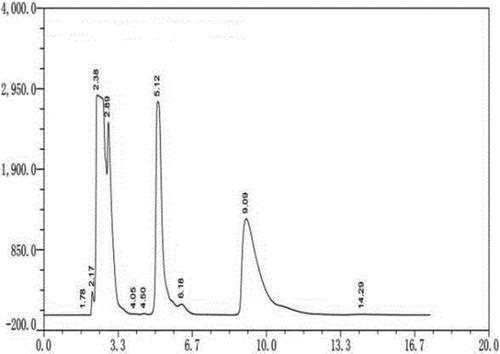
The retention time of d-arginine on elution column was 9.09 min, and that of l-arginine was 5.12 min (). Therefore, it is an effective method to detect d-arginine and l-arginine in dl-arginine by derivatization with phenyl isothiocyanate.
Figure 1. HPLC of different optical isomers of arginine. Note. The standard sample of dl-arginine was pre-column derivatized. The HPLC column was Supelco chiral column (25 cm × 4.6 mm, 5 μm); with manual injection of 20 μL, UV detection wavelength 254 nm; column temperature 25°; mobile phase A: mobile phase B: 5:1; total flow rate: 1 mL/min.
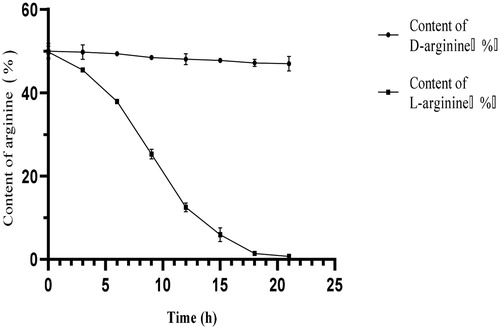
Degradation of l-alanine from dl-alanine mixture
Bacillus subtilis HLZ-68 can produce l-amino acid oxidase. In order to verify that the wet bacterial biomass can asymmetrically degrade l-arginine in dl-arginine and retain d-arginine, we added bacterial cells in the initial degradation solution containing dl-arginine. shows that the initial percentage content of d-arginine and l-arginine in the shake-flask cultures was 50% each. The percentage of l-arginine decreased rapidly with 37.4% at 12 h, and further with 11.9% from 12 to 21 h. After 21 h of reaction, the percentage content of l-arginine was 0.7%, while that of d-arginine was 47%, indicating that the viable bacteria had the ability of asymmetric degradation of dl-arginine. Therefore, wet bacterial biomass could consume l-arginine in dl-arginine and produce d-arginine under certain conditions.
Degradation of dl-arginine in a 5-L fermenter
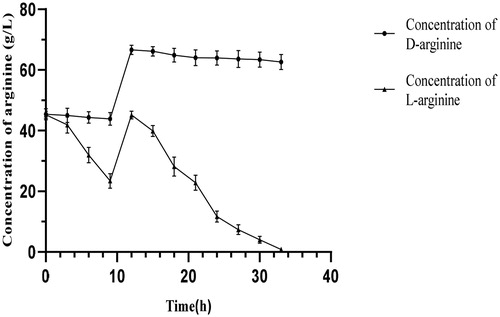
In order to improve the utilization of wet bacterial biomass when live bacteria degrade dl-arginine asymmetrically and reduce production costs, we studied the dynamics of dl-arginine degradation in batch cultures (). That the initial concentrations of d-arginine and l-arginine were about 45 g/L. Following degradation by the viable bacteria for 9 h, the l-arginine concentration was 23.4 g/L, i.e. the initial l-arginine concentration dropped by 48.2%. At this point, when 45 g of dl-arginine was fed, the bacteria could still maintain the rapid degradation of l-arginine because the bacteria were fully adapted to the degradation environment. During the degradation process, the amount of d-arginine hardly decreased, whereas the concentration of l-arginine decreased rapidly. After 30 h of degradation, the l-arginine was basically completely degraded, and the concentration of the remaining d-arginine was 65 g/L, indicating that the fed-batch experiment made preliminary progress and could improve the utilization of wet bacterial biomass. Fed-batch fermentation in a 5-L fermentor can obtain large quantities of B. subtilis HLZ-68 cells, thereby improving the efficiency of degradation of dl-arginine into d-arginine.
Figure 3. Degradation of dl-arginine in a 5-L fermenter. Note: Bacillus subtilis HLZ-68 cells (100 g of wet weight) were suspended in 2 L of reaction solution containing 180 g of dl-alanine in a jar fermenter. After 9 h of incubation, 90 g of dl-alanine dissolved in small amount of distilled water were added. The reaction was performed at 30 °C with pH 6.0, aeration of 1.0 vvm, and agitation of 400 r/min.
Separation and purification of d-arginine
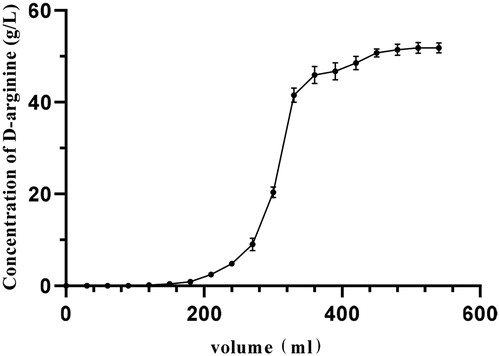
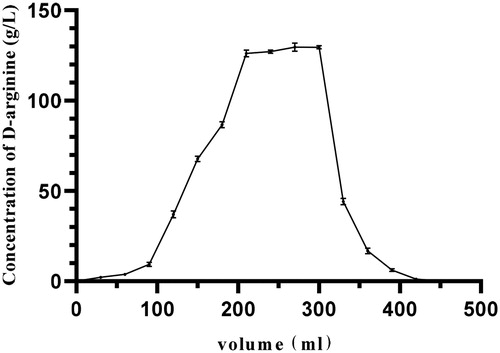
There was no d-arginine in the first 160 mL of effluent from the chromatographic column, and the content of d-arginine began to increase with the increase of the volume of the eluent after 160 mL. When the volume of the eluent exceeded 160 mL, the ion exchange resin began to adsorb d-arginine (). At 510 mL of effluent, the adsorption of d-arginine by the resin reached a saturation state. Therefore, 510 mL of upper column solution was added to each 150 mL ion exchange resin.
Figure 4. Dynamics of d-arginine adsorption. Note. A strong acid ion exchange resin 732 was used to adsorb d-arginine. The chromatographic column was packed with 150 mL resin. The flow rate of the pump was 2 mL/min. A tube of effluent was collected every 15 min.
There was no d-arginine in the first 60 mL eluate (), indicating that deionized water had no effect on the elution. Within the eluate volume rage of 60–420 mL, the content of d-arginine in the eluate increased initially to form a peak, and then gradually decreased. When the volume of the eluate was 420 mL, the content of d-arginine gradually tended to zero. At this time, the d-arginine was completely eluted. Therefore, after the adsorption of 150 mL, the ion exchange resin was saturated, and 420 mL of ammonia water was needed for elution.
Figure 5. Dynamic elution curve of d-arginine. After the adsorption of d-arginine by ion exchange resin reached a saturation state, d-arginine was eluted. The flow rate of the peristaltic pump was set at 2 mL/min, and the excess impurities in the chromatographic column were washed with 350 mL deionized water; then d-arginine was eluted with 480 mL of 2 mol/L ammonia water at the same flow rate. A tube of eluent was collected every 15 min.
Identification of d-arginine
d-arginine crystals were obtained by decolourizing, concentrating and removing ammonia, vacuum drying. The identification results are shown in . The simple procedure for isolation of d-arginine from the reaction mixture resulted in a yield of 91.58% after cation exchange and drying. The specific rotation of the obtained d-arginine was at 20 °C. The chemical purity of the isolated and purified d-arginine was 92.68% and the optical purity was 97.46%.
Table 2. Identification of d-arginine.
Prospective application in practice
As an important chiral compound, arginine has been used in many fields, such as medicine, food, agriculture and so on. At present, the market price of d-arginine is very high. In this study, B. subtilis HLZ-68 was used for production of d-arginine by asymmetric degradation of dl-arginine. There are two factors that affect the asymmetric degradation of d-arginine by wet bacterial biomass: less wet biomass and lower enzyme activity. Therefore, it is urgent to develop a simple and efficient production method of d-arginine. B. subtilis HLZ-68 possesses l-amino acid oxidase and not d-amino acid oxidase and can carry out asymmetric oxidative degradation of l-arginine from dl-arginine. In addition, B. subtilis HLZ-68 does not exhibit racemase activity, so it cannot transform l-arginine into d-arginine. As a result, this strain just utilizes l-arginine, leaving d-arginine in the medium. A similar approach has also been reported by [Citation12] using Aspergillus fumigatus. In order to obtain large bacterial cell counts in the wet biomass, oxygen-vectors can be added to the initial fermentation broth to increase the dissolved oxygen in the fermentation broth and solve the problem of low cell reproduction caused by insufficient oxygen in the fermentation process [Citation19]. Blaga et al. [Citation20] increased the ergosterol production by S. cerevisiae fed-batch fermentation with n-dodecane as the oxygen-vector. Ciobanu et al. [Citation21] achieved enhanced growth and β-galactosidase production by Escherichia coli using oxygen-vectors. Xia et al. [Citation22] enhanced the production of antroquinonol during batch fermentation using pH control coupled with an oxygen-vector. The oxygen-vectors commonly used in industrial production are n-hexadecane, n-dodecane, oleic acid, paraffin and n-hexane. In the present study, oleic acid was selected as the optimal oxygen-vector. The biomass increased by 66.83%, and the activity of l-amino acid oxidase increased by 38.88%. After cation exchange and drying, the chemical and optical purity of the obtained d-arginine were 92.68% and 97.46%.
Conclusions
l-arginine in dl-arginine can be degraded asymmetrically by B. subtilis HLZ-68 to produce d-arginine. Compared with other methods, adding an oxygen-vector solves the high cost problem of oxygen transfer in fermentation tanks, which is economical, simple and practical.
| Abbreviations | ||
| l-AAO | = | l-amino acid oxidase |
| HLPC | = | high-performance liquid chromatography |
Supplemental Material
Download PDF (124.4 KB)Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors. Peng Xu and Changpei Pan contributed equally to this work. Peng Xu and Changpei Pan conducted the experiments and analyzed the data. Gongcheng Cui and Chunyan Wei conceived and designed the study. Lijuan Wang and Yanting Li contributed to the experimental design and statistical analysis. Peng Xu and Changpei Pan wrote the first draft of the manuscript. Xiangping Li and Shihai Huang revised the manuscript completely. The manuscript was read and approved by all authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hoffer LJ. Human protein and amino acid requirements. J Parent Enteral Nutr. 2016;40(4):460–474.
- Griselda CM. d-arginine action against neurotoxicity induced by glucocorticoids in the brain. Neurosci Biobehav Rev. 2011;35(6):1353–1362.
- Wolkart G, Stessel H, Brunner F. In vivo administration of d-arginine: effects on blood pressure and vascular function in angiotensin II-induced hypertensive rats. Atherosclerosis. 2004;176(2):219–225.
- Suessenbacher A, Lass A, Mayer B, et al. Antioxidative and myocardial protective effects of l-arginine in oxygen radical-induced injury of isolated perfused rat hearts. Naunyn-Schmiedeberg's Arch Pharmacol. 2002;365(4):269–276.
- Martin S, Desai K. The effects of oral arginine on its metabolic pathways in Sprague-Dawley rats. Br J Nutr. 2020;123(2):135–148.
- Koohikamali S, Kamal SMM. Enhanced oxidative stability and water miscibility of conjugated linoleic acid complexed with lysine and arginine. Eur J Lipid Sci Technol. 2015;117(5):637–645.
- Aso K, Nishigawa T, Nagamachi S, et al. Orally administrated d-arginine exhibits higher enrichment in the brain and milk than l-arginine in ICR mice. J Vet Med Sci. 2020; 82(3):307–313.
- Moozeh K, So SM, Chin J. Catalytic stereoinversion of l-Alanine to deuterated d-Alanine. Angew Chem. 2015;54(32):9381–9385.
- Radkov AD, Moe LA. Bacterial synthesis of d-amino acids. Appl Microbiol Biotechnol. 2014;98(12):5363–5374.
- Takahashi E, Furui M, Shibatani T. d-Amino acid production from racemic amino acids by microbial asymmetric degrading activity of Candida maltisa. Biotechnol Tech. 1997;11(12):913–916.
- Umemura ISAO, Yanagiya KOJI, Komatsubara S, et al. Alanine production by using asymmetric degrading activity of Candida maltosa. Ann NY Acad Sci. 1990;613(1):659–662.
- Singh S, Gogoi BK, Bezbaruah RL. Optimization of medium and cultivation conditions for l-amino acid oxidase production by Aspergillus fumigatus. Can J Microbiol. 2009;55(9):1096–1102.
- Cascaval D, Galaction A-I, Folescu E, et al. Comparative study on the effects of n-dodecane addition on oxygen transfer in stirred bioreactors for simulated, bacterial and yeasts broths. Biochem Eng J. 2006;31(1):56–66.
- Xu Z, Bo F, Xia J, et al. Effects of oxygen-vectors on the synthesis of epsilon-poly-lysine and the metabolic characterization of Streptomyces albulus PD-1. Biochem Eng J. 2015;94(94):58–64.
- Zhu P, Dong S, Li S, et al. Improvement of welan gum biosynthesis and transcriptional analysis of the genes responding to enhanced oxygen transfer by oxygen vectors in Sphingomonas sp. Biochem Eng J. 2014;90(90):264–271.
- Wei Y-Y, Kevin C-CC, Yang S-H, et al. Oxygen vector accelerates farnesylation and redox reaction to promote the biosynthesis of 4-acetylantroquinonol B and antroquinonol during submerged fermentation of Antrodia cinnamomea. Food Bioprod Process. 2020;120:80–90.
- Ozdal M, Gurkok S, Ozdal OG, et al. Enhancement of pyocyanin production by Pseudomonas aeruginosa via the addition of n-hexane as an oxygen vector. Biocatal Agric Biotechnol. 2019;22:101365.
- Zhang Y, Li X, Zhang C, et al. Production of d-alanine from dl-alanine using immobilized cells of Bacillus subtilis HLZ-68. World J Microbiol Biotechnol. 2017;33(9):176–183.
- Wei LZ, Abdul R R, Rosfarizan M. Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed. J Biosci Bioeng. 2012;114(3):286–291.
- Blaga AC, Ciobanu C, Caşcaval D, et al. Enhancement of ergosterol production by Saccharomyces cerevisiae in batch and fed-batch fermentation processes using n -dodecane as oxygen-vector. Biochem Eng J. 2018;131:70–76.
- Ciobanu CP, Blaga AC, Froidevaux R, et al. Enhanced growth and β-galactosidase production on Escherichia coli using oxygen vectors. 3 Biotech. 2020;10(7):298
- Xia Y, Chen Y, Liu X, et al. Enhancement of antroquinonol production during batch fermentation using pH control coupled with an oxygen vector. J Sci Food Agric. 2019;99(1):449–456.