Abstract
Visual cortex functional deficits can be observed in schizophrenia patients and in individuals at high risk of schizophrenia. However, to date, few studies have investigated methods to improve these functional deficits. This study aimed to investigate the pathological change in the primary visual cortex of a prenatal MK-801-induced high-risk mouse model of schizophrenia (HRMMS) and to test the effect of paroxetine on visual cortex activity. Pregnant mice were given a systemic injection of MK-801, and male offspring that did not present schizophrenia-like behaviors in early adulthood were defined as HRMMS. Some of the HRMMS mice were treated with pharmacological agents beginning at 4 weeks of age. After 4 weeks of treatment with risperidone and/or paroxetine, two-photon calcium imaging was performed to analyze the primary visual cortex activity. The sucrose preference test and the prepulse inhibition (PPI) apparatus test were used to assess the cognitive and behavioral performance. HRMMS mice with or without risperidone treatment had impairments in the primary visual cortex as observed by reduced neuronal calcium activity. Risperidone plus paroxetine and paroxetine alone treatments increased the neuronal calcium activity in the primary visual cortex. Notably, the neuronal calcium activity was higher in mice treated with paroxetine alone. Treatment with paroxetine alone also improved the cognitive and behavioral performance better than treatment with risperidone plus paroxetine. Our pioneering animal model showed that treatment with paroxetine alone improves visual cortex impairments in HRMMS mice better than treatment with risperidone plus paroxetine, indicating that antipsychotics cannot normalize visual cortex impairments.
Introduction
Numerous studies have reported that more than 50% of patients with schizophrenia have visual perception disturbances, including visional hallucination and visional distortions [Citation1], which can be observed at any schizophrenia disease stage. In addition, visual perception disturbances can be observed in individuals with a high risk of schizophrenia [Citation2,Citation3]. The visual disturbances can lead to other symptoms, such as auditory impairments, cognitive deficits and memory dysfunctions [Citation4–6]. However, the strategies to improve visual disturbances in schizophrenia patients remain limited [Citation7,Citation8]. By exploring methods to protect from visual disturbances in individuals at high risk of schizophrenia, the downstream disease symptoms may be prevented [Citation9,Citation10].
During the last decade, many studies have linked the visual perception disturbances in schizophrenia patients and in individuals at high risk of schizophrenia to structural and functional impairments in the primary visual cortex. Therefore, many studies have suggested that the visual pathway, especially the primary visual cortex, must be protected in individuals with a high risk of schizophrenia. Simultaneously, some studies have investigated the effects of pharmacological agents on visual perception disturbances and the corresponding alterations in brain activity, and this research must continue to develop early disease prevention and management strategies [Citation9].
Thus, we designed a pilot animal study to investigate the functional changes in the primary visual cortex induced by pharmacological agents. Previous MRI analyses showed that paroxetine, an antidepressant, can improve visual cortex activity [Citation10–12], but it is unknown whether antipsychotic agents can improve visual cortex activity [Citation9]. Using our novel high-risk mouse model of schizophrenia (HRMMS), we aimed (1) to determine whether the offspring of high-risk mice had primary visual cortex functional impairments when compared to healthy control offspring and (2) to determine whether early treatment with pharmacological agents can improve the primary visual cortex functional activity.
Materials and methods
Development of the HRMMS
Pregnant C57BL/6 mice (12 ∼ 15 weeks age, 25 ∼ 28 g body weight) were housed in an animal facility with food and water ad libitum. To create the schizophrenia mouse model, a single intraperitoneal injection of MK-801, also known as dizocilpine, a non-competitive N-methyl-D-aspartic acid (NMDA) receptor antagonist that induces schizophrenia-like symptoms, was administered to pregnant female mice on the 14th day of pregnancy. After the birth of the offspring mice, we selected the surviving male offspring mice as study subjects.
Four weeks after birth, the study animals were given the sucrose preference test and the prepulse inhibition (PPI) apparatus test [Citation13]. The novel object recognition test was further employed to evaluate the cognitive function of animals as previously described [Citation14]. The sucrose preference test was given as described previously [Citation14]. For the PPI test, a 40 ms, 120 dB startle (PA) was applied after a 20 ms, 75 dB prepulse (PP) with a time interval of 100 ms. The background noise was maintained a 65 dB. The inter-trial time was set at 30 s. The PPI test was performed three times and the scores were averaged.
We selected the male offspring mice without schizophrenic behaviors and defined them as the high-risk mice model of schizophrenia (HRMMS). We then divided the HRMMS mice into four groups (n = 8 each group): (1) no prevention, (2) early risperidone prevention from 4–8 weeks after birth, (3) early risperidone plus paroxetine prevention, and (4) early paroxetine prevention.
Ethics statement
All of the animal experiments were approved by the joint Animal Ethics Committee of the Jining Medical University - The First Hospital of Shanxi Medical University – Tianjin Mental Health Centre joint research group (IRB number: JSTEBSRA-001).
In vivo calcium recordings and analysis
We recorded the neuronal activity in the prefrontal cortex (PFC) as described previously [Citation15] with slight modifications. In brief, a chronic cranial window was created in anesthetised mice. Then, 200 nL of AAV2/9-syn-GCaMP6s virus (2 × 1013 genome copies/mL; University of Pennsylvania Vector Core) was injected bilaterally into the prelimbic cortex using the following coordinates: +2.8 ± 0.5 mm from the bregma. The imaging window was covered by a circular coverslip, and the skull was sealed using dental cement. A customized steel bar was embedded into the skull to fix the position of the mouse head during the imaging session.
We performed two-photon in vivo imaging as previously described [Citation16]. A two-photon microscope (LSM780; Zeiss, Germany) with a 16X, 0.8 numerical aperture (NA) water immersion objective was used. Time-series images were recorded at 1.96 Hz for 150 s periods at an excitation wavelength of 950 nm. Captured images were analysed by ImageJ software (National Institutes of Health, Bethesda, MD, USA). Regions of interest (ROIs) were selected manually using ImageJ with the FIJI plug-in package [Citation17], and then detection and normalization of calcium transients were performed.
Statistical analysis
All experimental data are presented as the mean with standard error of the mean (±SEM) unless otherwise specified. The two-sample Student’s t-test or the non-parametric K-S test was used to compare the means between two groups [Citation15]. For multi-group comparisons, one-way analysis of variance (ANOVA) was performed followed by Tukey’s post hoc comparison [Citation15]. GraphPad Prism 7.0 was used for statistical analyses and data plots [Citation15].
Results and discussion
Survival of HRMMS mice
HRMMS mice were divided into four groups: (1) no prevention, (2) early risperidone prevention for 4–8 weeks after birth, (3) early risperidone plus paroxetine prevention, and (4) early paroxetine prevention. Only 46 mice survived to the 8-week timepoint. Fourteen out of 15 mice in the no prevention group survived, eight out of 15 mice in the early risperidone prevention group survived, 12 out of 15 mice in the early risperidone plus paroxetine prevention group survived, and 12 out of 15 mice in the early paroxetine prevention group survived.
Dysregulation of visual cortical activities in HRMMS
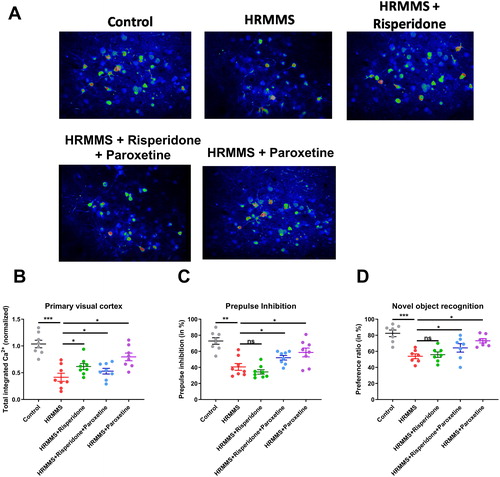
Utilizing in vivo two-photon imaging, the calcium activities in layer 2/3 pyramidal neurons in the primary visual cortex were recorded in awake and head-fixed mice that had been transfected with the genetically encoded calcium indicator GCaMP6s using an adeno-associated virus (AAV) vector. We successfully imaged the brains of seven mice in the early risperidone prevention group, 10 mice in the early risperidone plus paroxetine prevention group, 10 mice in the early paroxetine prevention group, 14 mice in the no prevention group, and 15 healthy control mice. Time-lapse recordings of neuronal activity demonstrated that all of the groups of HRMMS mice (with or without intervention) had significantly lower calcium activities compared to healthy control mice (). No significant difference was observed between mice with and without the early antipsychotic intervention ().
Figure 1. Neuronal activity in visual cortex and behavioural phenotyping. (A) Representative pseudo-coloured images showing the calcium activity in the visual cortex. (B) Quantification of total integrated calcium activities in the primary visual cortex. (C) Prepulse inhibition (in %) from different treatment groups. (D) Novel object recognition ratios of all animals. Note: ns, non-significant (p > 0.05); *p < 0.05, **p < 0.01, ***p < 0.001.
Effectiveness of pharmacological agents in the early prevention of HRMMS
The 2-photon in vivo imaging showed that the early paroxetine prevention and early risperidone plus paroxetine prevention groups had better primary visual cortex activity when compared to the risperidone prevention and no prevention groups. However, although the primary visual cortex activity improved in the early paroxetine prevention and early risperidone plus paroxetine prevention groups, neural activities in these groups remained lower than those in the healthy control group (). Moreover, behavioural test using PPI also supported the idea that paroxetine or paroxetine plus risperidone effectively improved sensory gating deficits (). Similar phenotypes occurred when examining the cognitive functions using the novel object recognition task ().
To the best of our knowledge, this pilot study is the first to investigate the effects of pharmacological agents on primary visual cortex activity using two-photon imaging on HRMMS mice. In this study, we found neural activity impairments in primary visual cortices of HRMMS mice indicating that impairments in brain function occur before psychotic episodes occur. Similarly, many human fMRI studies have shown structural and functional impairments in the visual cortices of high-risk schizophrenia patients [Citation18–21]. However, in our study, we found that early intervention with risperidone alone did not restore the normal function of the primary visual cortex or improve the cognitive and behavioural performance in HRMMS mice. Some MRI studies have shown that early interventions with antipsychotic agents prevented brain visual cortex impairments in individuals at high risk of schizophrenia [Citation22,Citation23]. The results of this study do not support the suggestion that antipsychotic agents can prevent brain visual cortex impairments. Future cohort studies are needed clarify the role of antipsychotics on brain visual cortex impairments.
Importantly, early intervention with paroxetine improved visual cortex activity the most. Early intervention with risperidone plus paroxetine also improved neural activity, but not as much as paroxetine alone. Although the intervention with paroxetine improved the visual cortex activity, the activity was not restored to the normal level as in the healthy control group. These findings indicate that the deficit in visual cortex activity in HRMMS cannot be fully normalized with the pharmacological agents tested in this study.
Because risperidone alone cannot improve neural activity, but risperidone and paroxetine can improve neural activity, perhaps paroxetine antagonizes risperidone in the HRMMS visual cortex. This idea is consistent with the suggestion that the 5-HT serotonin system interacts reciprocally with the dopamine system in the human visual cortex to improve visual cortex activity [Citation10–12].
Limitations
There are some limitations in our pilot study. First, because the survival rate of the model mice was low, survivor bias may affect interpretation of the results. Second, the hypothesis that 5-TH interacts reciprocally with the dopamine system to improve human visual cortex activity was based solely on MRI data from a few studies, thus the explanations of our findings are limited and our conclusions need to be taken with caution. Further studies using the tissue section technique and neurotransmitter detection technology are needed to clarify our results. Most importantly, fundamental difference may exist between animal models and human psychiatric disease in the real world. As the results, the extrapolation from animal models to clinical diseases should be carefully considered [Citation24].
Conclusions
Although there are limitations, our pilot study showed that there are impairments in the visual cortices of HRMMS mice and that these functional impairments can be improved by early treatment with paroxetine, but cannot be improved by treatment with antipsychotics. Our findings indicate that visual cortex impairments may be a marker of schizophrenia and may be improved by early treatment with pharmacological agents, thus further studies are needed to identify optimal strategies to improve visual disturbances in schizophrenia patients and in individuals at high risk of schizophrenia.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
The datasets generated and analysed during the present study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(Suppl 4):S233–S245.
- Mittal VA, Gupta T, Keane BP, et al. Visual context processing dysfunctions in youth at high risk for psychosis: resistance to the Ebbinghaus illusion and its symptom and social and role functioning correlates. J Abnorm Psychol. 2015;124(4):953–960.
- Hebert M, Gagne AM, Paradis ME, et al. Retinal response to light in young nonaffected offspring at high genetic risk of neuropsychiatric brain disorders. Biol Psychiatry. 2010;67(3):270–274.
- Grano N, Salmijarvi L, Karjalainen M, et al. Early signs of worry: psychosis risk symptom visual distortions are independently associated with suicidal ideation. Psychiatry Res. 2015;225(3):263–267.
- Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204.
- Green MF, Hellemann G, Horan WP, et al. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69(12):1216–1224.
- Guidotti A, Grayson DR. A neurochemical basis for an epigenetic vision of psychiatric disorders (1994-2009). Pharmacol Res. 2011;64(4):344–349.
- Fernandes TMP, Silverstein SM, Butler PD, et al. Color vision impairments in schizophrenia and the role of antipsychotic medication type. Schizophr Res. 2019;204:162–170.
- Silverstein SM. Visual perception disturbances in schizophrenia: a unified model. Nebr Symp Motiv. 2016;63:77–132.
- Cohen L, Ponchel A, Kas A, et al. Recovery from cortical blindness with mepivacaïne. Ann Clin Transl Neurol. 2019;6(8):1541–1545.
- Schneier FR, Pomplun M, Sy M, et al. Neural response to eye contact and paroxetine treatment in generalized social anxiety disorder. Psychiatry Res. 2011;194(3):271–278.
- Parsons B, Roxas A, Jr., Huang YY, et al. Regional studies of serotonin and dopamine metabolism and quantification of serotonin uptake sites in human cerebral cortex. J Neural Transm Gen Sect. 1992;87(1):63–75.
- Haß K, Bak N, Szycik GR, et al. Deficient prepulse inhibition of the startle reflex in schizophrenia using a cross-modal paradigm. Biol Psychol. 2017;128:112–116.
- Zieba J, Morris MJ, Weickert CS, et al. Behavioural effects of high fat diet in adult Nrg1 type III transgenic mice. Behav Brain Res. 2020;377:112217.
- Chen K, Zheng Y, Wei JA, et al. Exercise training improves motor skill learning via selective activation of mTOR. Sci Adv. 2019;5(7):eaaw1888.
- Forster D, Dal Maschio M, Laurell E, et al. An optogenetic toolbox for unbiased discovery of functionally connected cells in neural circuits. Nat Commun. 2017;8(1):116.
- Hutchinson EB, Stefanovic B, Koretsky AP, et al. Spatial flow-volume dissociation of the cerebral microcirculatory response to mild hypercapnia. Neuroimage. 2006;32(2):520–530.
- Silverstein SM, All SD, Kasi R, et al. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol Med. 2010;40(7):1159–1169.
- Silverstein SM, Berten S, Essex B, et al. An fMRI examination of visual integration in schizophrenia. J Integr Neurosci. 2009;8(2):175–202.
- Nagel M, Sprenger A, Nitschke M, et al. Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: an fMRI study. Neuroimage. 2007;34(1):300–309.
- Allen P, Moore H, Corcoran CM, et al. Emerging temporal lobe dysfunction in people at clinical high risk for psychosis. Front Psychiatry. 2019;10:298.
- Iwashiro N, Koike S, Satomura Y, et al. Association between impaired brain activity and volume at the sub-region of Broca's area in ultra-high risk and first-episode schizophrenia: a multi-modal neuroimaging study. Schizophr Res. 2016;172(1-3):9–15.
- Kasparek T, Prikryl R, Schwarz D, et al. Gray matter morphology and the level of functioning in one-year follow-up of first-episode schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1438–1446.
- Stoyanov D. Perspectives in applied neuroscience research - part I. Curr Top Med Chem. 2018;18(19):1619–1620.
