Abstract
The off-label use of medicines is neither prohibited nor explicitly allowed, according to the current European Union (EU) pharmaceutical legislation. This can cause confusion in physicians’ practice as well as lead to patients’ dissatisfaction. The purpose of this survey is to assess the knowledge and experience of Bulgarian physicians with the off-label use of medicines. The survey was performed at the beginning of 2020 in the biggest private hospital in South-Central Bulgaria. Ninety-six physicians from the inpatient and outpatient medical care participated in the survey and answered 10 questions. The data analysis was performed using the statistical program SPSS v19.0. The results are described as numbers and percentages for the qualitative variables and mean value (median due to their distribution) and a variance measure (range) for the quantitative ones. The distribution of the variables was tested with the Kolmogorov–Smirnov test. The evaluation of the relationships between the qualitative variables was performed using the χ2 test or the Fisher's exact test, where applicable. The results showed that most of the participants (81%) have never prescribed medicines off-label and believe it is not allowed to use medicines off-label (73%). Physicians strongly support the introduction of clear rules for the off-label use (88%). In conclusion, physicians practicing outside of the capital city demonstrated good level of knowledge, but relatively low awareness and little experience with the off-label use of medicines. They feel also, uncertainty in their prescribing activities when they use medicines off-label and strongly support introduction of clear rules for the off-label use.
Keywords:
Introduction
When a medicine is used ‘off-label’, it means it is used not in accordance with the conditions specified in the product information (i.e. the summary of product characteristics [SmPC]). In other words, the off-label use refers to the use of an authorized medicinal product in an unauthorized manner. This raises many legal, ethical and safety issues, especially when taking into account the prevalence of this practice. Data from the literature show that in Europe 13–69% of medicines are used off-label, especially in some medical fields such as paediatrics, oncology, neurology, orphan diseases, cardiology and ophthalmology. [Citation1–4]. In Bulgaria, our previous studies reveal that approximately one third of the medicines in both the younger and older population are used off-label (39% in paediatric and 32% in neurology patients) [Citation5,Citation6].
According to European Union (EU) pharmaceutical legislation, it is prohibited to place on the market a medicinal product without a marketing authorization [Citation7]. As an exception, an unauthorized medicinal product can be used in order to meet the individual need of a particular patient or group of patients, but there is an explicit condition that the medicinal product is in the authorization procedure or at least in the clinical trial phase [Citation8]. In Bulgaria, the conditions for use of an unauthorized medicine are laid down in the Law on Medicinal Products in Human Medicine (LMPHM). Most often, an unauthorized medicine is used according to the conditions of Art. 266a of the Law [Citation9].
The lack of rules for off-label use has led some EU countries to further clarify, regulate and control this practice through guidelines or legislative changes (e.g. The United Kingdom, Italy, France, Spain) [Citation10–14]. Since July 2020, Bulgaria has joined the group of countries with off-label regulation and through legislative change, the off-label use is now allowed under certain conditions [Citation15]. The introduction of regulation by more and more EU Member States is a positive trend, but unfortunately, they all have different approaches, which increases the need for harmonization at EU level [Citation16].
The purpose of this survey is to assess the knowledge and experience of a sample sized Bulgarian physicians with the off-label use of medicines. This will supplement the available data for a more comprehensive and clear picture of the real experience and current knowledge of physicians in Bulgaria in regard to the off-label use. The survey also reveals some problematic aspects associated with the current practice. It remains to be seen whether they will be resolved with the new amendments to the Bulgarian drug legislation.
Methods
This prospective, face-to-face questionnaire-based survey was performed at the beginning of 2020 in the biggest private hospital in the South-Central region of Bulgaria. A hundred and twenty physicians were invited and 96 physicians (80% response rate) from the inpatient and outpatient medical care participated in the survey and answered 10 questions. The questionnaire was specially developed to evaluate the knowledge and experience of physicians in regard to off-label use and to reveal whether it is legal according to them.
The questions were closed-ended or multiple-choice questions in order to be easy to answer and time-saving for participants. Inclusion criteria were having a master's degree in medicine and currently practicing. The questionnaire was paper based. It was provided personally, by an interviewer. All physicians participated anonymously and voluntarily. For the purposes of the survey, the following variables were collected: gender, age and medical specialty of physicians. Data about patients and personal data of respondents were not collected.
The data analysis was performed using the statistical program SPSS v19.0. The results are described as numbers and percentages for the qualitative variables and mean value (median due to their distribution) and a variance measure (range) for the quantitative ones. The distribution of the variables was tested with the Kolmogorov–Smirnov test. All p values < .05 are considered significant. The evaluation of the relationships between the qualitative variables was performed using the χ2 test or Fisher's exact test, where applicable.
Results and discussion
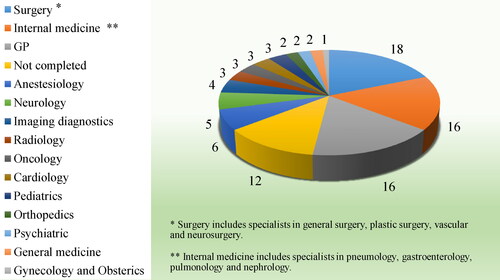
The majority of the participants interviewed in this survey were physicians with specialty (83%) with an average age of 48 years (age range: from 28 to 80 years). The participants were equally distributed by gender (50% male and 50% female), which corresponds to their distribution in the population. The distribution of participants by their specialty is shown in .
The analysis of the results showed that 7 out of 10 physicians have knowledge in regard to the meaning of the term off-label when applied to the specific use of a certain medication, which is comparable to the results from other EU surveys [Citation17,Citation18]. However, two-thirds of respondents (72%) associated the off-label use with the use for ‘another indication’ and not to the other terms specified in the SmPC (i.e. dosage, method of administration, age). Only 6% of the respondents gave the right definition for off-label use: ‘when used not in accordance with the authorized product information’. The answers to the questions posed in the survey, differentiating between General practitioners (GPs) and Specialist, are shown in .
Table 1. Responses to the survey.
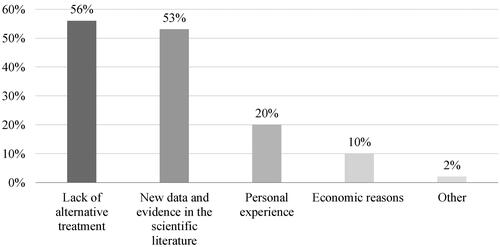
The majority of respondents stated they had never prescribed off-label medicine in their medical practice (81%). Two-thirds of respondents (66%) stated they had not experienced any trouble when prescribing off-label (e.g. refusal of funding, patient doubts, sanctions, bans, comments from colleagues). The lack of personal experience among the physicians in our survey is in contradiction with other surveys conducted in the EU, where 40–75% of physicians admit they prescribe medicines off-label in their everyday practice [Citation17–20]. A list of the possible reasons that in certain situations could lead our respondents to prescribe a medicine off-label is shown in .
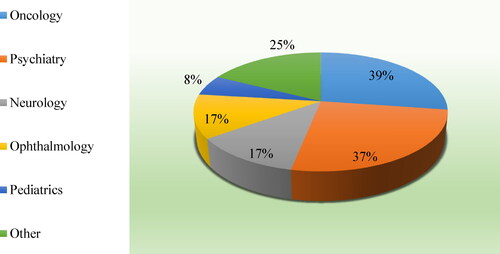
The possible reasons for the off-label use are analysed in a study commissioned by the European Commission [Citation4]. They are generally related to the lack of alternative treatment, economic reasons, but also to the expensive and time-consuming process of drug development, as well as the insufficient interest of the marketing authorization holders to expand medicines’ indications. Respondents from this survey also identified lack of alternative treatment and economic reasons as possible reasons for off-label use, but they pointed out the availability of new scientific data as the main reason (63%) that would make them prescribe a medicine off-label. To a larger extent (86%) the respondents did not believe the off-label use is a common practice. The main off-label medical areas, as per the respondents, are shown in . The respondents’ opinion is in line with the data from other studies conducted in the EU, which point out oncology, psychiatry, paediatrics, but also rheumatology and haematology, as medical areas with more frequent off-label use of medicines [Citation4].
The majority of the respondents believed that this practice is not allowed by the law (74%) and to a large extent they also believe that there should be clear rules and guidelines for the off-label use (88%). The lack of clear rules can cause confusion in physicians’ practise and can lead to patients’ dissatisfaction. Uncertainty and confusion could be avoided only by introducing clear rules, guidance and access to scientific information. Respondents in some EU studies stated that they do not have access to enough information in regard to the off-label prescription of medicines [Citation18,Citation20]. This coincides with the response of our respondents, 64% of whom believe they do not have access to sufficient scientific literature in this regard.
The data from this survey complement the results from another survey conducted in the capital city (Sofia) for better and more comprehensive understanding of this practice in Bulgaria. The comparative analysis showed that the answers overlap to a large extent, but some of them show significant differences (p < .05) [Citation19]. They are outlined in .
Table 2. Significant differences in answers between medical doctors in the capital city and in a provincial hospital.
These significant differences show a trend for more confident off-label prescribing of medicines by physicians in the capital city and greater caution in the provincial areas. This also shows gaps in the level of awareness between doctors in the country versus those in the capital. More information on off-label use may be helpful to increase physicians’ awareness, to magnify the appropriateness of prescribing and to improve safety.
Despite the limitations and possible biases (relatively small sample size, untrustworthy responses, etc.), the authors of the present work consider the results of this survey informative and valuable. They complement the results from other studies on the off-label practice and reflect the current local situation in which the off-label use of medicines is real, but at the same time a legally unclearly regulated practice.
Conclusions
Physicians practicing outside of the capital city demonstrated good level of knowledge, but relatively low awareness and little experience with the off-label use of medicines. They feel also uncertainty in their prescribing activities when they use medicines off-label and strongly support introduction of clear rules for the off-label use.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chalumeau M, Tréluyer JM, Salanave B, et al. Off label and unlicensed drug use among French office based paediatricians. Arch Dis Child. 2000;83(6):502–505.
- Ekins-Daukes S, Helms PJ, Simpson CR, et al. Off-label prescribing to children in primary care: retrospective observational study. Eur J Clin Pharmacol. 2004; 60(5):349–353.
- 9th European Conference on Rare Diseases & Orphan Products (ECRD Vienna 2018). Orphanet J Rare Dis. 2018;13:167.
- Weda M, Hoebert J, Vervloet M, et al. Study on off-label use of medicinal products in the European Union. European Commission. 2017. Available from: https://op.europa.eu/en/home
- Drenska M, Grigorova V, Elitova S, et al. The off-label use of medicines in pediatric outpatients in Bulgaria based on an analysis of their prescription data. Drugs Ther Perspect. 2019;35(8):391–395.
- Drenska M, Elitova S, Grigorova V, et al. Analysis of primary outpatient data for off-label use of medicines in neurology. PHAR. 2019;66(4):165–170.
- Council of the European Union. Directive 2001/83/EU of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. 2001; OJ L 311, 28.112001; p. 67–128. Available from: https://ec.europa.eu/health/documents/eudralex/vol-1_en
- Council of the European Union. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. 2004. OB L 136, 30.04.2004; p. 1–33. Available from: https://ec.europa.eu/health/documents/eudralex/vol-1_en
- Sotirova Y, Issaev S, Grigorov E. Possibilities for conducting treatment with unauthorized medicinal products in Bulgaria. Ann Hosp Pharm. 2020;6(1):49–57.
- Degrassat-Théas A, Bocquet F, Sinègre M, et.al. The "temporary recommendations for use": a dual-purpose regulatory framework for off-label drug use in France. Health Policy. 2015;119(11):1399–1405.
- Prada М, Bertozzi C, Proietti B, et al. The Italian 648/96 list: approvals, rejections and methods in Aifa’s evaluation process between January 2013 and May 2015. Value Health. 2015;18(7):A680.
- Faus J, Moliner X, Suarez J, et al. Medicinal product regulation and product liability in Spain: overview. [Web Page]. Thomson Reuters - Practical Law; 2018. Available from: https://uk.practicallaw.thomsonreuters.com/8-500-4409?navId=8EC5F403350E364FC5BAEE5763E7F8FE&comp=pluk&transitionType=Default&contextData=%28sc.Default%29
- Obermann S. Off-Label Use of Medicines – General Aspects, Challenges and Strategies. PhD Thesis. Bonn: Angefertigt mit Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn; 2013. Available from: https://dgra.de/deutsch/studiengang/master-thesis/2013-9075
- General Medical Council (GMC). Prescribing and managing medicines and devices. [Revised 2019]. GMC; 2013. Available from: https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/prescribing-and-managing-medicines-and-devices
- Law on Medicinal Products in Human Medicine. Bulgaria. SG. 31/13 April 2007, amend and suppl. SG. 67/28 Jul 2020. Available from: https://dv.parliament.bg
- Drenska M, Getov I. Research on approaches for regulation of the "off-label" use of medicinal products in the European Union. Acta Medica Bulg. 2017;44(1):17–21.
- Pérez RP, Antorán MR, Solá CA, et al. Results from the 2012-2013 paediatric national survey on off-label drug use in children in Spain (OL-PED study). An Pediatr (Barc). 2014;81(1):16–21.
- Hagemann V, Bausewein C, Rémi C. Off-label-prescriptions in daily clinical practice – a cross-sectional national survey of palliative medicine physicians. Prog Palliat Care. 2019;27(4):154–159.
- Drenska M, Naseva E, Getov I. The knowledge and experience with the off-label use – results of a survey. Pharmacia 2019;66(4):217–221.
- Saullo F, Saullo E, Caloiero M, et al. A questionnaire-based study in Calabria on the knowledge of off-label drugs in pediatrics. J Pharmacol Pharmacother. 2013;4(Suppl 1):S51–S54.