Abstract
The conversion of bacterial CRISPR/Cas defense system into a simple and efficient tool for genome manipulations brought experimental biology into new dimensions. Suddenly, genome editing reached many groups most of which were interested in it but not able to employ the available time- and labor-consuming approaches of the pre-CRISPR era. In plant biology and biotechnology, CRISPR/Cas gene editing became the second most important technology after plant transformation. Actually, it relies on the available array of methods of gene delivery. While sufficient for most purposes, the classic gene transfer methods might become a problem for some experimental settings. The main obstacle is that they include DNA delivery and, frequently, its subsequent integration into cellular genome. For this reason novel methods to achieve gene editing without the need of stable transformation and even without DNA delivery were developed. These new approaches include in vitro ribonucleoprotein complexes formulations (delivered by microinjection, particle bombardment, electroporation, liposomes etc.), use of virus-like particles and employment of bacterial secretory systems for Cas/gRNA delivery. The first attempts to achieve DNA-free editing were made less than ten years ago. Later, different types of animal and plant cells were addressed. In this mini review we try to summarize the current developments and emerging trends in the field of DNA-free editing in plants.
Introduction
DNA-free gene editing became a new and fast-developing trend in biological research due to its obvious advantages. Actually, DNA-free editing opened the way to targeted genetic modification without genome disturbances [Citation1, Citation2] and raised the possibility to create modified organisms that are non-GMO in the classic terms in plant biology and biotechnology [Citation3]. In plant biology and biotechnology, DNA-free editing has encountered the same basic problems as transformation technologies before. Despite the wider arsenal of transformation approaches, the protein and RNA delivery methods to plant cells are less developed compared to animal-related techniques, and practically only particle bombardment and transfection/lipofection of protoplasts can be used. Nevertheless, attempts to repurpose other delivery methods and systems are made due to the great potential of DNA-free editing for either fundamental or practical purposes.
Historically, genome editing became available in the 1990s upon the development of efficient plant transformation techniques. The first experiments used heterologous DNA (either relatively long fragments or oligonucleotides) for allele replacement via homologous recombination. Next, artificial meganucleases based on zinc fingers or TAL allowed for more precise genomic manipulations [Citation4]. Unfortunately, due to methodological complexity these technologies were out of reach for most researchers and did not gain popularity.
The discovery of the bacterial immunity system based on RNA-guided endoDNAses opened a new possibility for precise genomic modifications. Subsequent identification of single-protein Cas enzymes (e.g. Cas9 and Cpf1) and simplified guide RNAs led to a breakthrough in the field. Gene editing became capable of reaching almost all research groups including those with minimal equipment and facilities. It is not surprising then that the first reports of successful gene editing in plants appeared soon after the introduction of this technique. Since then CRISPR/Cas has been successfully applied on a number of species and for different purposes [Citation5]. In addition, almost all main plant transformation methods have been employed [Citation6]. Soon after a new problem emerged that needed to be addressed. The main obstacle was that the original gene editing includes DNA delivery (encoding either Cas or gRNA or both) and, frequently, its subsequent integration into the cellular genome. Such integration might be a problem in some experimental settings or may preclude the commercial use of the obtained lines. Since Cas9/gRNA activity is needed for a short period of time after its introduction into the cell, novel approaches for non-DNA based delivery were developed. These new approaches include in vitro ribonucleoprotein (Cas9/gRNA) complexes formulations (delivered by microinjection, particle bombardment, electroporation, liposomes etc.), use of virus-like particles and employment of bacterial secretory systems for Cas/gRNA delivery. First attempts to achieve DNA-free editing in plants were made some five years ago [Citation7] and the current state was summarized by Metje-Sprink et al. [Citation8]. In this mini review we try to summarize the recent (during the last two years) achievements and emerging trends in the field of DNA-free editing in plants.
Targeted plant species and delivery techniques used
Soon after the first successful reports [Citation9], the DNA-free editing approach was applied on a number of species. Since the review of Metje-Sprink et al. [Citation8], researchers have achieved DNA-free editing in Nicotiana benthaminiana [Citation10], potato [Citation11], wheat and maize [Citation12, Citation13], Brassicaceae [Citation14], rice [Citation15], banana [Citation16], lettuce [Citation17], pepper [Citation18]. While two years is not a significantly long period, it is clear that commercially important species have become the main target for DNA-free genomic manipulations (i.e. [Citation19]).
To achieve DNA-free editing, three approaches for Cas9/gRNA delivery have received main attention. The most popular one is by delivering in vitro assembled ribonucleoprotein. The second approach is to employ virus-mediated delivery of encoding RNA templates. The third approach is most intriguing. It is implementation of Agrobacterium tumefaciens Type IV secretory system for Cas9 delivery as protein into plant cells.
It should be noted that in all reported cases DNA-free editing has resulted in inheritable modifications, regardless of the delivery systems used.
CRISPR/Cas delivered as ribonucleoprotein complexes/nanoparticles
The first and still most popular approach for achieving DNA-free editing is by delivering in vitro assembled Cas9-gRNA ribonucleoprotein complexes. It relies on the inherent ability of single-molecule Cas protein members (i.e. Cas9 or Cpf1) to interact with gRNA without the need of auxiliary factors. Typically, Cas9 is expressed and purified in Escherichia coli, whereas gRNA is either chemically synthetized or produced by in vitro transcription. Next, a ribonucleoprotein complex is obtained according to the transformation protocol used [Citation20].
A variation of this approach is the formation of more complex nanostructures rather that ‘simple’ ribonucleoproteins [Citation21]. Such complexes resemble but are not identical to virus-like particles. Nanoparticles allow not only delivery of premade protein-RNA complexes but also incorporation of mRNA and gRNA for successful expression of Cas9, followed by assembly of Cas9/gRNA in the plant cell and subsequent DNA-free editing [Citation9]. The same capability was exploited by Li et al. [Citation22] to achieve targeted gene replacement using RNA template. In addition, nanoparticles also allow for improvements of cargo stability and efficiency of delivery.
Ribonucleoprotein complexes and nanoparticles are delivered in plant cells mostly by particle bombardment [Citation23] or protoplast transformation. In the latter case lipofection [Citation14, Citation24] or PEG-Ca2+ transfection [Citation13, Citation15, Citation16] is used. Electroporation is another way to deliver macromolecules across protoplast membrane and well-designed protocols are available [Citation7], but there have been no reports (to our knowledge) during the last two years.
Premade nanocomplexes were also used for the delivery of other genome-manipulating enzymes like recombinases [Citation25].
CRISPR/Cas delivered as virus-like particles
Virus vectors have been successfully employed for editing the animal cell genome by delivering the CRISPR/Cas9 system both in vivo and in vitro [Citation26]. Viruses have also been utilized in plants for virus-induced gene silencing (VIGS) by introducing small interfering RNA and have become a promising tool for DNA-free plant genome editing [Citation27, Citation28].
It has to be noted that transgenic plants expressing the Cas9 protein have already been successfully edited by introducing sgRNA, with the most recent example developed by Hu et al. [Citation12]. Engineering viruses with the CRISPR/Cas system for transgene-free plant genome editing proves to be a significant hurdle because of certain restrictions related to the viruses. First, mainly DNA and positive-strand RNA viruses have been used for editing the plant genome and their application is limited due to their cargo capacities [Citation28]. Large foreign sequences make them unstable and are prone to deletion during replication [Citation29]. These deletion mutants predominate over the parental virus vector and are rapidly selected for cell-to-cell spread processes [Citation30]. Furthermore, the CRISPR/Cas system is usually >4.5 kb, therefore the sequence size constraints would render viral infection with this large system difficult [Citation28]. However, small genome-editing reagents such as meganuclease [Citation31], zinc-finger nuclease [Citation32], and the CRISPR gRNA [Citation33], have successfully been delivered with DNA and positive-strand viral vectors [Citation27].
The delivery of the whole CRISPR/Cas9 system in plants has only until recently been made possible by using virus vectors. Ma et al. [Citation10] successfully delivered the complete CRISPR/Cas9 cassette in Nicotiana benthamiana, thus obtaining DNA-free genome edited plants with sufficiently high efficiency. This was achieved by using the negative-strand sonchus yellow net rhabdovirus (SYNV; Rhabdoviridae) as a vector. Infection with negative-strand viruses tends to be avoided as a result of the difficulties when genetically engineering this class of viruses [Citation28]. This technological breakthrough opened new possibilities for plant genome editing thanks to the larger cargo capacity for foreign sequences that negative-strand RNA viruses have.
To achieve transgene-free plants (Citation10,] inserted the Streptococcus pyogenes Cas9 (SpCas9) and gRNAs into the SYNV genome. In order to confirm that the gRNA would be cut and expressed correctly, a (tRNA-gRNA-tRNA)-Cas9 cassette was constructed, relying on tRNA processing enzymes to cleave precisely the transcript and release an authentic gRNA. First, the system was tested by targeting two sites within the GFP gene of transgenic Nicotiana benthamiana plants [Citation10]. PCR-restriction digestion (PCR_RE) assays showed 77 and 91% mutagenesis frequencies respectively. The next target for mutagenesis were 3 endogenous N. benthamiana genes: phytoene desaturase (PDS), RNA-dependent RNA polymerase 6 (RDR6) and Suppressor of Gene Silencing 3 (SGS3) [Citation10]. The mutagenesis frequencies remained high (40–91%). Furthermore, the multiplex editing ability was tested by designing a construct containing 2 gRNAs (tRNA-gRNA1-tRNA-gRNA2-tRNA). Their results showed that multiplexed gRNA expression did not lower the genome-editing efficiency of a single gRNA.
Although this new way of DNA-free plant genome editing offers many possibilities, the method has its drawbacks. The SYNV vector still requires plant tissue culture and plant regeneration because plant rhabdoviruses do not usually invade plant germline or meristem cells [Citation28]. Plant regeneration is genotype- and species-dependent, thus restricting the application of this vector. One possible solution to this problem is employing a mobile RNA sequence like the FLOWERING LOCUS T (FT) fused to the gRNAs [Citation34]. This would make it possible for gRNAs to enter meristemic and germline cells, thereby allowing genome-editing in them [Citation28]. It may be possible to lift the species and genotype restriction by using such a mobile RNA sequence together with the SYNV vector in order to achieve genome editing completely independently of plant tissue [Citation28].
Another recent example of virus-based DNA-free editing was demonstrated by Ariga et al. [Citation29]. Potato Virus X was used for delivering both Cas9 and gRNA. The main advantage of the proposed vector is the possibility to encode and express a full-length Cas9 gene, albeit at low levels. Also, gRNA was contained within the same transcript and processed simultaneously by Cas9 and cellular factors. This native in vivo processing approach developed by Cody and Scholthof [Citation35] might be the keystone for designing novel DNA-free editing methods.
Hu et al. [Citation12] employed barley stripe mosaic virus‐based vector to deliver gRNA in wheat. Since Cas9 was previously introduced into the wheat genome, this work is a good illustration of the shortcomings of classic gene editing approaches.
CRISPR/Cas delivery by Agrobacterium
Agrobacterium-mediated plant transformation is the most widely used method for the delivery of the CRISPR/Cas9 cassette [Citation36]. The exogenous DNA carrying Cas9 and the gRNA is called T-DNA and is located on a Ti plasmid [Citation37]. This method has been employed successfully in more than 20 plant species [Citation36]. Furthermore, it is possible to use various explants as targets for transformation such as callus, leaf and floral organs of plants [Citation36].
It is also possible to eliminate transgenic plants by genetic segregation. In T0 (the first plant generation) the transgene locus is usually heterozygous [Citation38]. The plants carrying the T-DNA would segregate according to Mendelian genetics in the next generation (T1) [Citation38]. The transgene-free plants can therefore be selected by PCR-based methods. However, this method works only for sexually propagated plants and is rarely applicable for vegetatively propagated perennial plants because of the long time needed to reach sexual maturity [Citation39]. Furthermore, sexual reproduction would hinder the expression of genes responsible for many important traits in the plants because they are highly heterozygous for them [Citation40]. This would lead to transgene-free but also mutant progeny having undesirable traits [Citation40]. For this reason, it is imperative to develop new methods for the delivery of the CRISPR/Cas9 system in vegetatively propagated plants.
In the absence of an antibiotic, Agrobacterium infection can be used for the transient production of Cas9 and gRNA, thus avoiding the integration of T-DNA into the plant genome and generating DNA-free plants [Citation38]. Using this method Chen et al. [Citation39] targeted the phytoene desaturase (PDS) gene in tobacco plants and successfully obtained transgene-free plants with a mutagenesis frequency of 8.2% without the need for sexual segregation. This strategy has its advantages compared to other methods such as the delivery of pre-assembled CRISPR/Cas9 ribonucleoproteins and particle bombardment. These methods rely on inserting the editing components in certain plant tissues which restricts their application to only some species.
However, the first generation of plants (T0) would include at least 3 populations: transgenic plants containing T-DNA, untransformed plants and the transiently transformed plants of interest [Citation38]. Chen et al. [Citation39] used a labor-intensive selection method - next-generation sequencing together with high-resolution melting analysis (HRM). There is still a need for non-labor intensive methods for the selection of the DNA-free plants and optimization of the current protocols in order to produce the desired ratios of the different populations.
Another study [Citation41] also focused its attention on vegetatively propagated plants. They used a cytidine based editor (CBE) to edit the acetolactate synthase (ALS) gene in potatoes and tomatoes via Agrobacterium infection. Point mutations in the ALS gene can lead to different types of resistance in plants [Citation41, Citation42]. They successfully produced transgene-free (12.9%) chlorsulfuron-resistant tomatoes with a very high base editing efficacy (up to 71%). In potatoes, the transgene frequency was a little bit lower (10%) [Citation41]. The main drawback of this method was the off-target effects, therefore there is a need for further protocol optimization.
The use of the bacterial secretory system for Cas9 delivery is the most intriguing and promising achievement in plant gene editing. Agrobacterium is a well-known agent, widely used for plant transformation. Bacteria deliver a linear single-stranded DNA fragment via a specialized type IV secretory system. Along with DNA transfer, the secretory system also transfers some auxiliary proteins. The signals required for protein transfer by T4SS are studied and might be linked to other proteins of interest, and the option was already exploited to deliver Cas9 into plant cells [Citation43]. Unfortunately, the method has exposed some drawbacks. One is low editing efficiency as compared to other delivery approaches. However, this might be improved by the engineering of bacterial delivery mechanisms. A more critical drawback is the necessity to deliver gRNA by other means since it is not a transferable substrate for T4SS.
Nevertheless, the use of Agrobacterium for DNA-free delivery of at least the protein component is a fascinating achievement with great potential. Its use to deliver base editor for gene editing is yet another confirmation of the new possibilities in DNA-free editing [Citation41].
Emerging trends
Recent advances in CRISPR/Cas delivery strategies and approaches were summarized in several excellent reviews recommended for further reading [Citation2, Citation26, Citation36, Citation38, Citation44, Citation45]. It should be noted that these articles are not focused entirely on DNA-free delivery but on the genome editing as whole.
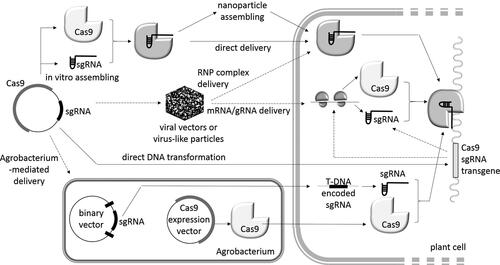
As it was mentioned before, gene editing relies on various delivery methods (). The oldest transformation approach used the delivery of vector DNA either in ‘pure form’ (i.e. electroporation) or with minimal treatment (typically Ca2+ and/or PEG condensation; i.e. particle bombardment, transfection). In this case vector DNA can be integrated into the plant genome (with some efficiency).
Modifications of this approach allowed for assembling vector DNA into nanoparticles. Further development of the idea was to include in nanoparticles a premade ribonucleoprotein complex (Cas9-sgRNA) or corresponding RNAs (mRNA and gRNA) instead of encoding DNA. In this case Cas9 was first expressed and purified and then mixed with synthetic gRNA to form the editing ribonucleoprotein. The successful realization of this idea outlined the first DNA-free editing experiments.
The use of viral vectors and virus-like particles, albeit not in the mainstream, has long been used for plant transformation. RNA viruses, in particular, turned out to be appropriate carriers for achieving DNA-free editing.
Agrobacterium-mediated transformation is the second oldest plant transformation technology. The Type IV secretory system transfers into plant cell single-stranded linear DNA fragment as well as some auxiliary proteins. This opportunity was already used for Cas9 as well as base editor in plant cells. Unfortunately, gRNA must be delivered by other means since it is not a substrate for T4SS [Citation43].
When comparing already exploited approaches, some trends might be noted. First, there is interest in the development of nanoparticles mostly due to improved delivery efficiency, stability as well as loading different types of cargo molecules.
Viral vectors based on RNA viruses are also recognized as appropriate tools for DNA-free editing. These vectors deliver RNA, which is only translated but not integrated. Along with the report that RNA can be used as template for allele replacement, viral vectors and nanoparticles might gain better popularity in near future.
The fast-developing field of DNA-free editing requires repurposing of the tools for macromolecular delivery [Citation46]. Among them might be the use of phytopathogenic bacteria for delivery of CRISPR/Cas components. Bacteria like Xanthomonas and Pseudomonas deliver a set of proteins into the plant cells via modified Type III secretory systems. While these systems work well with TALEN, they are not capable of transferring Cas9 for unknown reasons (probably cell toxicity; unpublished results). Unfortunately, these promising protein-delivering bacteria cannot be used at this stage for CRISPR/Cas gene editing. At the moment the only viable perspective to employ native protein delivery mechanisms is the one of Agrobacterium.
On the other hand, one recent report is rather interesting in terms of DNA-free editing [Citation35]. It deals with gRNA processing; for maximum efficiency, gRNA must not contain excess bases at both ends. This imposes some problems onto the experimental design. The work of Cody and Scholthof [Citation35] revealed that in plants excess bases at the 5′ end can be trimmed by plant exonucleases even when gRNA forms a complex with Cas9. Such result might significantly improve vector designs, especially ones for multiple gRNA cassettes. Future work on this problem might reveal novel mechanisms for gRNA delivery by bacterial secretion systems.
Another issue of these emerging approaches is related to the avoidance of extensive genetic modifications (non-GMO progeny) by DNA-free editing, especially for industrial crops. Current drawbacks of gene editing are not technical but public concerns and acceptance, enhanced by heavy regulatory affairs (the ban imposed by EC and cumbersome regulatory procedures adopted by the USA, Australia and Canada). Since the main concerns are related to the biosafety of genome edited crops, there is need for novel technological solutions to overcome negative public perception and to ease the regulatory barriers.
There are several bottlenecks regarding the efficient application of DNA-free editing. The first one is related to the properties of Cas9, especially the off-target effects. Novel Cas9 orthologs like Cpf1, Cas13a and Cas14 with lower molecular weight, broader PAM sites and reduced off-target effects might stand as a better alternative especially for commercial purposes. Actually, Cpf1 has already been used for DNA-free editing [Citation47].
The second bottleneck deals with the delivery of editing moieties. Obviously, all methods that do not deliver DNA fragments along with the editors are suitable. Nanoparticles containing premade Cas9/gRNA complexes or mRNA/gRNA only, appear most reliable in terms of non-GMO production. The use of Agrobacterium or viruses also seems appropriate but theoretically might impose some unexpected problems. On the other hand, gene editing often requires not only simple indels but sequence replacements that need a template of some kind. There are several possibilities that might be exploited. Regarding the nanoparticles, co-delivery of ss- or dsDNA with nanoparticles in theory might lead to its integration into the genome and will require additional efforts to eliminate such undesirable events. The use RNA as a template integrated in nanoparticles or viral vectors is a viable opportunity to alleviate the problem [Citation9, Citation22].
Another option is to further modify Agrobacterium to deliver the ssDNA fragment only as a template along with Cas9 as protein. In this case a modification of VirD2 might be necessary in order to decrease (or eliminate) T-DNA integration into the genome.
The third bottleneck is related to the confirmation of targeted editing only with no off-target effects or other unwanted genome changes. The ultimate solution is the full genome sequencing, but for large-scale screening, it is still applicable to relatively small genomes like fungi [Citation48]. A viable alternative is the adaptation of ‘classic’ molecular marker techniques (like AFLP, SSR SCAR, retrotransposon-based, microarrays, etc.) for this task [Citation49]. Another interesting approach was developed by Aliaga-franco et al. [Citation50]. The authors included DsRED protein as a visual marker for allele segregation after gene editing and confirmed its efficiency on dry seeds in the progeny. While not directly related to DNA-free editing, the proposed approach might be used as an additional tool for confirmation of the GM-free status of the lines especially when a DNA template was co-delivered.
Conclusions
DNA-free genome editing is a new but fast-developing field in plant biology and biotechnology. Most likely, the main approach will rely on delivering premade ribonucleoprotein complexes or nanoparticles. Viral vectors are gaining popularity and some new opportunities might bring them into focus. Agrobacterium-mediated macromolecule delivery just emerges and, to our opinion, is the most promising and viable technology for DNA-free genome editing in plants. Of course, there is always a chance that continuous development in biological sciences might reveal a currently unknown delivery mechanism which will put all current tools onto the dusty shelf of history.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Malzahn A, Lowder L, Qi Y. Plant genome editing with TALEN and CRISPR. Cell Biosci. 2017;7:21.
- Mao Y, Botella JR, Liu Y, et al. Gene editing in plants: progress and challenges. National Science Review. 2019;6(3):421–437.
- Wolter F, Puchta H. Knocking out consumer concerns and regulator's rules: efficient use of CRISPR/Cas ribonucleoprotein complexes for genome editing in cereals. Genome Biol. 2017;18(1):43.
- Boettcher M, McManus MT. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol Cell. 2015;58(4):575–585.
- Arora L, Narula A. Gene editing and crop improvement using CRISPR-Cas9 system. Front Plant Sci. 2017;8:1932.
- Ran Y, Liang Z, Gao C. Current and future editing reagent delivery systems for plant genome editing. Sci China Life Sci. 2017;60(5):490–505.
- Woo JW, Kim J, Kwon SI, et al. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol. 2015;33(11):1162–1164.
- Metje-Sprink J, Menz J, Modrzejewski D, et al. DNA-free genome editing: past, present and future. Front Plant Sci. 2019;9:1957.
- Miller JB, Zhang S, Kos P, et al. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed. 2016;55:1–6.
- Ma X, Zhang X, Liu H, et al. Highly efficient DNA-free plant genome editing using virally delivered CRISPR-Cas9. Nat Plants. 2020;6(7):773–779.
- Andersson M, Turesson H, Olsson N, et al. Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol Plant. 2018;164(4):378–384.
- Hu J, Li S, Li Z, et al. A barley stripe mosaic virus-based guide RNA delivery system for targeted mutagenesis in wheat and maize. Mol Plant Pathol. 2019;20(10):1463–1474.
- Sant’Ana RRA, Caprestano CA, Nodari RO, et al. PEG-delivered CRISPR-Cas9 ribonucleoproteins system for gene-editing screening of maize protoplasts. Genes (Basel). 2020;11(9):1029.
- Murovec J, Guček K, Bohanec B, et al. DNA-free genome editing of Brassica oleracea and B. rapa protoplasts using CRISPR-Cas9 ribonucleoprotein complexes. Front Plant Sci. 2018;9:1594.
- Toda E, Koiso N, Takebayashi A, et al. An efficient DNA- and selectable-marker-free genome-editing system using zygotes in rice. Nat Plants. 2019;5(4):363–368.
- Wu S, Zhu H, Liu J, et al. Establishment of a PEG-mediated protoplast transformation system based on DNA and CRISPR/Cas9 ribonucleoprotein complexes for banana. BMC Plant Biol. 2020;20(1):425.
- Park J, Choi S, Park S, et al. DNA-free genome editing via ribonucleoprotein (RNP) delivery of CRISPR/Cas in lettuce. Methods Mol Biol. 2019;1917:337–354.
- Kim H, Choi J, Won KH. A stable DNA-free screening system for CRISPR/RNPs-mediated gene editing in hot and sweet cultivars of Capsicum annuum. BMC Plant Biol. 2020;20(1):449.
- González MN, Massa GA, Andersson M, et al. Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Front Plant Sci. 2019;10:1649.
- Park J, Choe S. DNA-free genome editing with preassembled CRISPR/Cas9 ribonucleoproteins in plants. Transgenic Res. 2019;28(Suppl 2):61–64.
- Wang P, Zhao F-J, Kopittke PM. Engineering crops without genome integration using nanotechnology. Trends Plant Sci. 2019;24(7):574–577.
- Li S, Li J, He Y, et al. Precise gene replacement in rice by RNA transcript-templated homologous recombination. Nat Biotechnol. 2019;37(4):445–450.
- Liang Z, Chen K, Zhang Y, et al. Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat Protoc. 2018;13(3):413–430.
- Liu W, Rudis MR, Cheplick MH, et al. Lipofection-mediated genome editing using DNA-free delivery of the Cas9/gRNA ribonucleoprotein into plant cells. Plant Cell Rep. 2020;39(2):245–257. Jr.
- Martin-Ortigosa S, Trewyn BG, Wang K. Nanoparticle-mediated recombinase delivery into maize. Methods Mol Biol. 2017;1642:169–180.
- Yip BH. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules. 2020;10(6):839.
- Zaidi SS, Mansoor S. Viral vectors for plant genome engineering. Front Plant Sci. 2017;8:539.
- Liu H, Zhang B. Virus-based CRISPR/Cas9 genome editing in plants. Trends Genet. 2020;36(11):810–813.
- Ariga H, Toki S, Ishibashi K. Potato Virus X vector-mediated DNA-free genome editing in plants. Plant Cell Physiol. 2020;pcaa123 (in press).doi: 10.1093/pcp/pcaa123/5912940.
- Miyashita S, Ishibashi K, Kishino H, et al. Viruses roll the dice: the stochastic behavior of viral genome molecules accelerates viral adaptation at the cell and tissue levels. PLoS Biol. 2015;13(3):e1002094.
- Honig A, Marton I, Rosenthal M, et al. Transient expression of virally delivered meganuclease in planta generates inherited genomic deletions. Mol Plant. 2015;8(8):1292–1294.
- Marton I, Zuker A, Shklarman E, et al. Nontransgenic genome modification in plant cells. Plant Physiol. 2010;154(3):1079–1087.
- Cody WB, Scholthof HB. Plant virus vectors 3.0: transitioning into synthetic genomics. Annu Rev Phytopathol. 2019;57:211–230.
- Ellison EE, Nagalakshmi U, Gamo ME, et al. Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat Plants. 2020;6(6):620–624.
- Cody WB, Scholthof HB. Native processing of single guide RNA transcripts to create catalytic Cas9/Single guide RNA complexes in planta. Plant Physiol. 2020;184(2):1194–1206.
- Sandhya D, Jogam P, Allini VR, et al. The present and potential future methods for delivering CRISPR/Cas9 components in plants. J Genet Eng Biotechnol. 2020;18(1):25.
- Gelvin BS. Agrobacterium-mediated plant transformation: the biology behind the “Gene-Jockeying” tool. Microbiol Mol Biol Rev. 2003;67(1):16–37.
- He Y, Zhao Y. Technological breakthroughs in generating transgene-free and genetically stable CRISPR-edited plants. aBIOTECH. 2020;1(1):88–96.
- Chen L, Li W, Katin-Grazzini L, et al. A method for the production and expedient screening of CRISPR/Cas9-mediated non-transgenic mutant plants. Hortic Res. 2018;5:13.
- Norelli JL, Wisniewski M, Fazio G, et al. Genotyping-by-sequencing markers facilitate the identification of quantitative trait loci controlling resistance to Penicillium expansum in Malus sieversii. PLoS One. 2017;12(3):e0172949.
- Veillet F, Perrot L, Chauvin L, et al. Transgene-free genome editing in tomato and potato plants using Agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. IJMS. 2019;20(2):402.
- Danilo B, Perrot L, Mara K, et al. Efficient and transgene-free gene targeting using Agrobacterium-mediated delivery of the CRISPR/Cas9 system in tomato. Plant Cell Rep. 2019;38(4):459–462.
- Schmitz DJ, Ali Z, Wang C, et al. CRISPR/Cas9 mutagenesis by translocation of Cas9 protein into plant cells via the Agrobacterium type IV secretion system. Front Genome Ed. 2020;2:6.
- Montecillo JAV, Chu LL, Bae H. CRISPR-Cas9 system for plant genome editing: current approaches and emerging developments. Agronomy. 2020;10(7):1033.
- Van Eck J. Applying gene editing to tailor precise genetic modifications in plants. J Biol Chem. 2020;295(38):13267–13276.
- Que Q, Chilton MM, Elumalai S, et al. Repurposing macromolecule delivery tools for plant genetic modification in the era of precision genome engineering. Methods Mol Biol. 2019;1864:3–18.
- Kim H, Kim S-T, Ryu J, et al. CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat Commun. 2017;8:14406.
- Al Abdallah Q, Souza ACO, Martin‑Vicente A, et al. Whole-genome sequencing reveals highly specifc gene targeting by in vitro assembled Cas9-ribonucleoprotein complexes in Aspergillus fumigatus. Fungal Biol Biotechnol. 2018;5:11.
- Nadeem MA, Nawaz MA, Shahid MQ, et al. DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnol Biotechnol Equip. 2018;32(2):261–285.
- Aliaga-Franco N, Cunjin Z, Presa S, et al. Identification of transgene-free CRISPR-edited plants of rice, tomato, and Arabidopsis by monitoring DsRED fluorescence in dry seeds. Front Plant Sci. 2019;10:1150.