Abstract
The aim of the present study was to investigate various biological activities (antimicrobial, anti-Giardial, antimalarial, antitumor, and antioxidant) as well as the phytochemistry of the root, stem, leaves, flowers and fruit of Ludwigia erecta L. The extracts of different plant parts showed variable activity against the tested microorganisms, with the highest activity (p < 0.05) obtained from the flower chloroform: methanol extract against E. coli (17.7 ± 2.2 mm). The strongest anti-Giardial activity was that of the root extract with a half-maximal inhibitory concentration IC50 value of 41.60 ± 01 μg/mL (p < 0.05). None of the extracts was cytotoxic to Vero cell line (IC50 > 100 µg/mL), whereas the flower extract showed a cytotoxic effect against the MDA-MB231 cell lines, with an IC50 value of 30.5 ± 2.9 µg/mL. Furthermore, the highest antioxidant activity (p < 0.05) was shown by the fruits extract with IC50 50 µg/mL, whereas the same antioxidant capacity was shown by the stem, leaf, and flowers extracts with IC50 75 µg/mL. The phytochemical screening of extracts revealed the presence of flavonoids, sterols, tannins, triterpenes and saponins. The total polyphenolic contents, calculated as gallic acid equivalents (mg/L), ranged from 3428.21 ± 12.53 to 3671.50 ± 11.25 mg GAE/L, and the concentration of flavonoids, calculated as quercetin equivalents (mg/L), ranged from 1501.76 ± 16.99 to 3928 ± 219.81 QE mg/L, whereas the tannin content was calculated as the tannic acid equivalent (mg/L) with a value ranging between 9.55 ± 0.11 and 48.66 ± 2.14 TAE mg/L.
Introduction
Every year, more than 13 million deaths worldwide are caused by the emergence of new infectious diseases or the reemergence of old pathogens [Citation1]. The traditional applications of medicinal plants have significantly contributed to the development and improvement of health care. It is known that plants produce an array of structurally diverse secondary metabolites. Given the potential health risks, the incidence of multidrug resistance in human pathogenic microorganisms, and the toxicity of synthetic antioxidants, intensive studies are carried out on plants in the search for potential natural therapies and leading biomolecules for the pharmaceutical industry [Citation2,Citation3].
The Onagraceae family consists of 22 genera, divided into two subfamilies, namely, Ludwigioideae (represented by only one genus; Ludwigia) and Onagroideae (the other genera) [Citation4]. The genus Ludwigia is comprised of 82 species and is found in both temperate and tropical regions. Ludwigia spp. are either aquatic or grown in wet places around coastal regions, lakes, lagoons, rivers and water-logged areas [Citation5]. In Sudan, the genus is represented by five species, including L. adscenoens, L. erecta, L. leptocarpa, L. octovalvis and L. perennis.
Some pharmacological properties of Ludwigia spp. were demonstrated by previous reports, including the antimicrobial activity of L. adscendens, L suffruticosa, L. abyssinica, L. decurrens, L. peploides subsp. montevidensis and L. grandiflora subsp. hexapetala [Citation6–10], the anti-inflammatory activity of L. adscendens [Citation6], the antioxidant activity of L. abyssinica [Citation11], as well as the anti-acne, antioxidant and cytotoxic properties of L. peploides [Citation12]. L. octovalvis was characterized with anti-hyperglycemic [Citation13] and antiproliferative [Citation14] activities, and a capacity to inhibit α-glucosidases and pancreatic lipase [Citation15]. Moreover, three new oleanane-type triterpenes isolated from L. octovalvis were found to exhibit cytotoxic activity against two human cancer cell lines [Citation16].
In Sudan, L. erecta (L.) H. Hara (Syn. Jussiaea erecta L., Sp.) is an annual herb found along the riversides of the Nile. Massage lotions are prepared from the whole plant and used in traditional medicine as an antipyretic drug [Citation17]. There is limited data available on the biological properties of L. erecta and only the antimicrobial activity of the plant has been evaluated [Citation18,Citation19]. Therefore, the objective of this study was to evaluate the biological properties including the antimicrobial, anti-Giardial, antimalarial, antiproliferative, and antioxidant activities of the root, stem, leaves, flowers and fruit of L. erecta. Screening for phytochemicals and the determination of the phenolic content were also carried out.
Materials and methods
Plant material
The whole plant of L. erecta was collected from a riverbank in Khartoum State, Sudan, in May 2014. A voucher specimen (No. LE514) was deposited at the Herbarium of the Botany Department, Faculty of Science, University of Khartoum, as a reference material. The root, stem, leaves, flowers and fruit were separated, shade-dried, and coarsely powdered in a hammer mill separately.
Preparation of the extract
Fifty grams of dried powder from the different plant parts was defatted by maceration in hexane and subsequently extracted with chloroform: methanol (CM) (1:1, v/v) using a shaker apparatus at room temperature for 24 h. The filtrate of each extract was concentrated with a rotary evaporator under reduced pressure. Finally, the extracts were weighed and stored in a refrigerator until further usage.
Biological assays
Antimicrobial activity
The bacterial cultures used were Bacillus subtilis NCTC 8236, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 10145. The used fungi cultures were Aspergillus niger ATCC 9763 and Candida albicans ATCC 7596. Each extract (10 mg/disc) was tested for antimicrobial activity by the disc diffusion method [Citation20]. The standard gentamicin and nystatin at a concentration of 10 μg/disc served as the positive control for evaluation of the antibacterial and antifungal activities, respectively.
Anti-giardial activity
Giardia lamblia was obtained from Ibrahim Malik Hospital (Khartoum, Sudan) and an in vitro susceptibility assay was done according to the method of Cedillo-Rivera et al. [Citation21].
Antimalarial activity
The culture of the malaria parasite, Plasmodium falciparum K1, was obtained from Khartoum Hospital (Khartoum, Sudan), and in vitro antiplasmodial activity was determined using the candle jar method [Citation22].
Cytotoxicity
The extracts were screened for the determination of their cytotoxic effect against normal (Vero cells) and different cancer cell lines, including human breast carcinoma cells (MCF7 and MDA-MB231) and human colon adenocarcinoma cells (HT29 and HCT116) using the MTT assay [Citation23].
Antioxidant activity
The antioxidant activity of the extracts was evaluated using the in vitro DPPH radical scavenging method [Citation24].
Phytochemical analysis
A preliminary phytochemical screening was carried out using standard phytochemical methods [Citation25] to determine the presence of secondary metabolites. The total polyphenolic, flavonoid and tannin content was determined according to the methods described by Wolfe et al. [Citation26], Ordonez et al. [Citation27] and Shivakumar et al. [Citation28], respectively.
Thin-layer chromatography (TLC) autographic assay
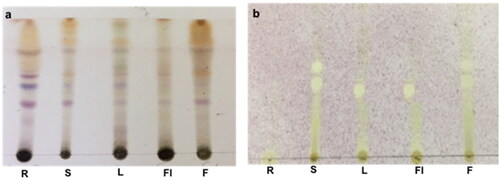
Extracts were developed in duplicate on separate TLC silica gel plates using an appropriate solvent system (Toluene: ethyl acetate: formic acid (5:4:1)). The first plate was sprayed with a Vanillin-Sulfuric acid spray reagent, whereas the other with the 2,2-diphenyl-1-picrylhydrazyl (DPPH) spray reagent. Yellow spots against a purple background showed a positive antioxidant activity when sprayed with DPPH [Citation29].
Statistical analysis
Experiments were performed in triplicate and the obtained results were expressed as the mean with standard deviation (±SD). The one-way analysis of variance (ANOVA) was performed for the determination of significant differences between the four extracts and their free-radical–scavenging activity.
Results and discussion
The bioactivity and medicinal aspects of a plant are usually correlated with the presence of various classes of phytochemicals, including phenols, flavonoids, terpenes, among others. Their presence and concentration vary in various parts of the plant which are used in Ethnobotany [Citation30]. In this study, a preliminary phytochemical screening of the different parts of L. erecta, including the root, stem, leaves, flowers, and fruit showed that the plant contained phenols, terpenes, steroids, and saponins and consequently could be a rich source of natural products.
Extraction yield and phytochemical screening
As depicted by the results in , the highest extraction yield was obtained in the fruit extract (8.67%), followed by the flower (8.07%), leaf (5.43%), stem (3.54%), and root (2.77%) extracts, respectively. Moreover, the presence of flavonoids, sterols, tannins, triterpenes, and saponins was revealed by the phytochemical screening of different extracts. All extracts were devoid of alkaloids and anthraquinones in contrast to Ludwigia octovalvis and Ludwigia hyssopifolia [Citation31–33].
Table 1. Yield and phytochemical screening of different parts of Ludwigia erecta.
Quantitative determination of the total polyphenol, flavonoid, and tannin content
The total polyphenolic, flavonoid and tannin content of the CM extracts of the different parts of L. erecta was determined and the results are presented in . The total polyphenolic content in CM extracts, calculated as the GAE (mg/L), ranged from 3428.21 ± 12.53 to 3671.50 ± 11.25 mg GAE/L. The highest concentration was obtained in the fruit extract, followed by the root, flower, leaf and stem extracts with values of 3671.50 ± 11.25, 3648.79 ± 3.00, 3562.75 ± 11.25, 3466.42 ± 3.33, and 3428.21 ± 12.53 mg GAE mg/L, respectively. The concentration of flavonoids, calculated as the quercetin equivalent (QE, mg/L), ranged from 1501.76 ± 16.99 to 3928 ± 219.81 QE mg/L. Moreover, the fruit extract was identified to contain the highest flavonoid content, followed by the flower, root, leaf, and stem extracts with values of 3928 ± 219.81, 3349.91 ± 55.56, 2946.72 ± 104.25, 1895.38 ± 109.42, and 1501.76 ± 16.99 QE mg/L, respectively. The tannin content was calculated as the tannic acid equivalent (TAE, mg/L) and values ranged between 9.55 ± 0.11 to 48.66 ± 2.14 TAE mg/L. The fruit extract was identified to contain the highest tannin content, followed by the leaf, root, flowers and stem with values of 48.66 ± 2.14, 37.94 ± 2.27, 37.62 ± 1.41, 15.32 ± 0.15, and 9.55 ± 0.11 TAE mg/L, respectively. All the studied plant organs displayed a high polyphenolic and flavonoids content as well as low tannin content with 48.66 ± 2.14 mg TAE/L as the highest concentration detected in the fruit. Furthermore, the plant contained higher phenolic content in comparison with that reported for other Ludwigia spp [Citation12, Citation34].
Table 2. Total polyphenolic, flavonoid and tannin content of the extracts of different plant parts of Ludwigia erecta.
Antimicrobial activity
The antimicrobial activity of CM extracts from the root, stem, leaves, fruit and flowers of L. erecta was determined against gram-negative and gram-positive bacteria as well as against two species of fungi. The results are presented in . The extracts of the different parts showed a variable inhibitory activity against the tested microorganisms. Based on the results of inhibition zones, the highest activity was obtained from the flower CM extract against E. coli (17.7 ± 2.2 mm). With fewer inhibition zones (13 ± 1.4-14.7 ± 1.3 mm), B. subtilis and S. aureus were the most susceptible to the root and flower extracts, as well as P. aeruginosa to the flower extract (13.7 ± 0.8 mm). Furthermore, the antifungal activity of all extracts was stronger against C. albicans than A. niger, with the highest inhibition zone (12 ± 1.7 mm) obtained from the root extract.
Table 3. Antimicrobial activity of the extracts of different plant parts of Ludwigia erecta against standard microorganisms.
Except for the flower extract, either moderate or weak antimicrobial activity was revealed by all extracts, with the highest antibacterial activity observed in the flower extract against E. coli. The chloroform extract of the whole plant was active only against S. aureus [Citation18]. Besides, El-Egami et al. [Citation19] demonstrated that the methanolic extract of the whole plant exerted a higher antimicrobial activity than the one that included a chloroform extract, against the four tested bacteria and C. albicans. This observation suggested that the plant as a whole possesses better antimicrobial activity than its separated organs, implying a synergistic interaction between the bioactive constituents present in the various organs of the plant, in contrast to Ludwigia parviflora which showed similar activities but through fruit extract [Citation35] and Ludwigia octovalvis leaf extract [Citation32].
Anti-Giardial activity
The anti-Giardial activity of different CM extracts of different parts of L. erecta against G. lamblia was investigated using three different concentrations (125, 250 and 500 μg/mL) . The anti-Giardial activity of all extracts was both concentration- and time-dependent (). The root and fruit extracts were the most effective against G. lamblia with 85% mortality observed at the highest concentration (500 μg/mL) after 72 h. This was followed by the stem and leaf extracts which showed 80% and 77% mortality, respectively, at 500 μg/mL after 72 h. Furthermore, the IC50 values were calculated for the anti-Giardial activity of the extracts after 72 h, and the results are given in . The ranking order of the extracts with the highest anti-Giardial activity regarding the IC50 values was the root (41.60 ± 01 μg/mL) > fruit (109.89 ± 14 μg/mL) > stem (168.46 ± 20 μg/mL) > leaf (212.26 ± 15 μg/mL).
Figure 1. Anti-Giardial activity of the different parts of the CM extracts of Ludwigia erecta against Giardia lamblia. Note: positive control “ornidazole”
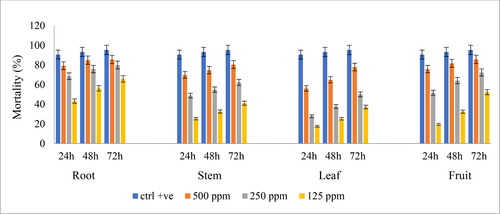
Table 4. Half-maximal inhibitory concentration (IC50) of anti-Giardial, antimalarial, and antioxidant activities of the extracts of the different plant parts of Ludwigia erecta.
The results of anti-Giardial activity showed that all extracts displayed considerable anti-Giardial activity depending on concentration and time. The root (IC50 41.60 ± 01 μg/mL) extract was the most effective against G. lamblia. However, the stems of Fuchsia microphylla, the only species that belonged to the family Onagraceae, were investigated for its anti-Giardia property and the obtained results showed inactivity (IC50 > 500) [Citation36]. In a literature review on a natural chemical molecule with anti-Giardia activity, Amaral et al. [Citation37] reported that flavonoids, especially flavonols, isoflavones, triterpenes, with quassinoids being the most representative alkaloids, including mainly indole alkaloids, sesquiterpenes, steroids, phenolic acids, esters, lignin, and amine were among the classes of metabolites that contained bioactive compounds against giardia.
Antimalarial activity
The antimalarial activity assay of the CM extracts of different parts of L. erecta against P. falciparum showed that the highest activity (IC50 321 ± 11 ppm) was displayed by the root, followed by the stem (IC50 328 ± 21 ppm), fruit (IC50 458 ± 11 ppm) and leaf (IC50 value was 942 ± 19 ppm) extracts (). The results of the antiplasmodial activity revealed that the different parts of L. erecta exhibited either weak or no antimalarial activity.
Antiproliferative activity
CM extracts from different parts of L. erecta were tested in vitro for their potential cytotoxic effects against the HT29, HCT116, MCF7 and MDA-MB231 cancer cell lines as well as normal Vero cell line (). None of the extracts were toxic to the Vero cell line (IC50 > 100 µg/mL), and only the flower extract demonstrated cytotoxic activity against the MDA-MB231 cell lines with an IC50 value 30.5 ± 2.9 µg/mL. All other extracts had no anticancer effect on the tested cancer cell lines (IC50 value > 50 µg/mL). Although previous studies on other Ludwigia spp., including L. hyssopifolia [Citation33], L. peploides [Citation12] and L. octovalvis [Citation14, Citation16], showed a high antiproliferative activity against many cancer cell lines, in this study, the flower extract was the only one to demonstrate moderate antiproliferative activity against MDA-MB231 cell lines with an IC50 value of 30.5 ± 2.9 μg/mL (). Three oleanane-type triterpenes isolated from L. octovalvis were found to exhibit a cytotoxic effect against two human cancer cell lines [Citation16].
Table 5. Antiproliferative activity of the extracts of the different plant parts of Ludwigia erecta against normal and carcinoma cells.
Antioxidant activity
The antioxidant capacity of CM extracts of different parts of L. erecta was first investigated through TLC bioautography. The developed TLC plate was sprayed with DPPH reagent and samples producing yellowish spots on the purple background were considered to possess radical scavenging activity. In addition, the stem and fruit extracts displayed two distinct antioxidant spots, while the leaf and flower gave only one major spot. The root extract did not react with the DPPH reagent (). Furthermore, the scavenging effect of the extracts on the DPPH radicals was calculated in regard to the IC50 values. Moreover, the quantitative determination of the DPPH scavenging activity revealed that the fruit extract displayed the highest antioxidant activity with IC50 50 μg/mL (), a value lower than that obtained from the standard propyl gallate (IC50 77 ± 0.01 μg/mL), whereas other organs also displayed good antioxidant activity with IC50 values (75 μg/mL) comparable to the standard, suggesting that the plant is very rich in antioxidant molecules. Moreover, the antioxidant activity bioautograms showed that all extracts except that of the root displayed a distinct antioxidant spot, suggesting the presence of major molecules responsible for the observed scavenging activity. Although the stem displayed an additional antioxidant spot, it did not increase its antioxidant capacity as observed in the fruit, this could be attributable to a lower level of antioxidants. Moreover, the high polyphenolic and flavonoid contents of L. erecta could play a major role in the observed potent antioxidant activity similar to Ludwigia leptocarpa and Ludwigia hyssopifolia [Citation33, Citation38].
Conclusions
This was the first study conducted to evaluate the anti-Giardia, antimalarial, cytotoxic and antioxidant potential of L. erecta. The results showed that the different parts of the plant were not toxic and exerted different biological activities. Furthermore, the highest antibacterial activity with the best sensitivity against E. coli and the only organ to possess antiproliferative activity against the MDA-MB231 cell line was the flower. In addition, the root displayed the highest anti-Giardial activity. Besides, all parts exerted potent free-radical–scavenging activity, with the fruit showing the highest capacity. Thus, L. erecta could be a natural source for bioactive agents, although further scientific research is needed to determine the active molecules.
Disclosure statement
The authors declare that they have no conflict of interest.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group Project no. RG-1441-472.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Kourtesi C, Ball AR, Huang Y-Y, et al. Suppl 1: microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol J. 2013;7:34–52.
- Wang F, Miao M, Xia H, et al. Antioxidant activities of aqueous extracts from 12 Chinese edible flowers in vitro and in vivo. Food Nutr Res. 2017;61(1):1265324
- Abreu AC, Coqueiro A, Sultan AR, et al. Looking to nature for a new concept in antimicrobial treatments: isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci Rep. 2017; 7(1):1–16.
- Wagner WL, Hoch PC, Raven PH. Revised classification of the Onagraceae. Systematic Botany Monographs 2007. USA: American Society of Plant Taxonomists.
- Oziegbe M, Faluyi JO, Oluwaranti A. Effect of seed age and soil texture on the germination of some Ludwigia species (Onagraceae) in Nigeria. Acta Botanica Croatica. 2010;69(2):249–257.
- Selim M. Phytochemical and pharmacological screening of Ludwigia adscendens LB Pharm project report submitted to pharmacy discipline. J Bangladesh: Khulna Univ. 2003;16.
- Ahmed F, Selim MS, Shilpi JA. Antibacterial activity of Ludwigia adscendens. Fitoterapia. 2005;76(5):473–475.
- Aliyu A, Musa A, Abdullahi M, et al. Phytochemical and antibacterial properties of Ludwigia suffruticosa (Willd.) Oliv. ex. O. Ktze (Onagraceae). Int J Appl Sci. 2008; 2(4):1–5.
- Oyedeji O, Oziegbe M, Taiwo FO. Antibacterial, antifungal and phytochemical analysis of crude extracts from the leaves of Ludwigia abyssinica A. Rich. and Ludwigia decurrens Walter. J Med Plants Res. 2011; 5(7):1192–1199.
- Smida I, Charpy-Roubaud C, Cherif SY, et al. Antibacterial properties of extracts of Ludwigia peploides subsp montevidensis and Ludwigia grandiflora subsp hexapetala during their cycle of development. Aquat Bot . 2015; 121:39–45.
- Fodouop SPC, Gatsing D, Tangue BT, et al. Effect of Salmonella typhimurium infection on rat's cell oxidation and in vivo antioxidant activity of Vitellaria paradoxa and Ludwigia abyssinica aqueous extract. Asian Pacific J Trop Dis. 2015; 5(1):38–46.
- Smida I, Sweidan A, Souissi Y, et al. Anti-Acne, Antioxidant and Cytotoxic Properties of Ludwigia peploides Leaf Extract. Int J Pharmacogn Phytochem Res. 2018; 10(7):271–278.
- Lin WS, Lo JH, Yang JH, et al. Ludwigia octovalvis extract improves glycemic control and memory performance in diabetic mice. J Ethnopharmacol. 2017; 207:211–219.
- Wu SJ, Ng LT, Wang GH, et al. Chlorophyll a, an active anti-proliferative compound of Ludwigia octovalvis, activates the CD95 (APO-1/CD95) system and AMPK pathway in 3T3-L1 cells. Food Chem Toxicol. 2010; 48(2):716–721.
- Morales D, Ramirez G, Herrera-Arellano A, et al. Identification of digestive enzyme inhibitors from Ludwigia octovalvis (Jacq.) P.H.Raven. Evid Based Complement Alternat Med. 2018;2018:8781352:
- Chang C-I, Kuo C-C, Chang J-Y, et al. Three New Oleanane-Type Triterpenes from Ludwigia octovalvis with cytotoxic activity against two human cancer cell lines. J Nat Prod. 2004; 67(1):91–93.
- El-Ghazali GE, El-Tohami MS, El-Egami AA. Medicinal plants of the Sudan. Sudan: National Centre for Research, Medicinal & Aromatic Plants Research Institute;1986.
- Almagboul A, Bashir A, Farouk A, et al. Antimicrobial activity of certain sudanese plants used in folkloric medicine. Screening for antibacterial activity. IV. Fitoterapia. 1985; 56: 103-109.
- El-Egami AA, El-Tohami MS, El-Nima EI, et al. In vitro antimicrobial activities of Nymphaea lotus and Jussiaea erecta. Omdurman J Pharmaceut Sci. 2005; 1(1):117–123.
- Kil HY, Seong ES, Ghimire BK, et al. Antioxidant and antimicrobial activities of crude sorghum extract. Food Chem. 2009; 115(4):1234–1239.
- Cedillo-Rivera R, Chávez B, González-Robles A, et al. Yepez-Mulia L: In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. J Eukaryot Microbiol. 2002; 49(3):201–208.
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2):55–63.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10):1231–1237.
- Evans W. Trease and Evans Pharmacognosy. 15th ed. Singapore: Sanders Co. Ltd.; 2002.
- Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003; 51(3):609–614.
- Ordonez AAL, Gomez JD, Vattuone MA, et al. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem . 2006; 97(3):452–458.
- Shivakumar B, Ramaiah M, Hema M, et al. Quantitative determination of total content of phenol, flavonoid and tannin in leaf extract of Barlaria Buxifolia Linn. Amer J PharmTech Research. 2012; 2(5):418–422.
- F R. T n, A M. M n, A N. O a. Antioxidant and free radical scavenging activities of plant extracts used in traditional medicine in Mexico. Afr J Biotechnol. 2008; 7(12):1886–1893.
- S, Kumar J, Krishna Chaitanya M, Andrew J, et al. Indigenous knowledge of medicinal plants used by ethnic communities of South India. Ethnobotany Res Appl. 2019; 18(4): 1–112.
- Coe FG, Parikh DM, Johnson CA, et al. Bioactivity of 68 Species of medicinal plants of Eastern Nicaragua. J Pharmacogn Phytochem. 2020; 9(3):101–112.
- Aung LW, Chaw DKE. Study on morphology, anatomy, preliminary phytochemical test, nutritional values and antimicrobial activities of leaves of Ludwigia octovalvis (Jacq.) Raven. Dagon Univ Commemorat 25th Anniv Silver Jubilee Res J. 2019, 9(2):321–327.
- Deepak B, Kundan S, Ujwal H, et al. Phytopharmacological activities of Ludwigia hyssopifoliA (G. DON) EXELL: a review. Asian J Res Chem Pharmaceut Sci. 2019; 7(2):781–789.
- Yakob HK, Sulaiman SF, Uyub AM. Antioxidant and antibacterial activity of Ludwigia octovalvis on Escherichia coli O157: H7 and some pathogenic bacteria. World Appl Sci J. 2012; 16:22–29.
- Gobalakrishnan R, Bhuvaneswari R, M R. Natural antimicrobial and bioactive compounds from Ludwigia parviflora Roxb. JAPLR. 2020; 9(1):37–42.
- Calzada F, Alanis AD, Meckes M, et al. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to some medicinal plants used by the people of Southern Mexico. Phytother Res. 1998; 12(1):70–72.
- Amaral FM, Ribeiro MNS, Barbosa-Filho JM, et al. Plants and chemical constituents with giardicidal activity. Rev Bras Farmacogn. 2006; 16:696–720.
- Andriamanantena M, Danthu P, Cardon D, et al. Malagasy dye plant species: a promising source of novel natural colorants with potential applications – a review. Chem Biodivers. 2019; 16(12):e1900442.