Abstract
Glioma is the most prevalent and lethal type of primary tumor of central nervous system. The regulatory role of calcitriol, the active form of vitamin D3, has been determined in various cellular processes including cell survival and apoptosis. To study the impact of AKT-pathway inhibition with or without calcitriol combination on glioma cell viability, the effects of AT7867 (AKT-pathway inhibitor) and calcitriol on cell viability and apoptosis were investigated in glioma C6 cell-line. Optimal doses of calcitriol and AT7867 were determined by MTT- and xCELLigence-assays. Both agents (alone/in combination) effectively suppressed the proliferation of C6 cells. While AT7867 inhibited glioma cell viability more effectively than calcitriol, this inhibitory action of AT7867 was not significantly increased by calcitriol combination. The expression levels of vitamin D receptor(VDR)-triggered molecules, AKT-pathway components and apoptosis factors were compared between calcitriol-, AT7867- and calcitriol + AT7867-treated and non-treated cells. CYP24A1 gene was upregulated and DBP, CYP1A1, CYP27B1, EGFRvIII, AKT-pathway (AKT1, MTOR, CREB, PTEN), apoptosis (BAD, CYC-C, CASP3, APAF-1, BCL2) and MMP3 genes were downregulated by calcitriol treatment.AT7867 treatment induced CYP27B1 upregulation, reduced VDR and AKT-pathway (PI3K, MTOR, CREB, PTEN) gene expression levels but showed a relatively less pronounced effect on apoptosis-related gene levels. The combination of calcitriol and AT7867 treatment generated an effect that was similar to that induced by calcitriol alone. Our results demonstrate that calcitriol and AT7867 have differing actions on AKT- and apoptotic-pathways of C6 glioma cell-line. Calcitriol has a lesser viability reducing impact than AT7867 putatively due to its apoptosis inhibiting action.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.1912641 .
Introduction
Glioma is the most prevalent and one of the most lethal type of primary tumors of the central nervous system (CNS). The median survival rate of patients accounts for <2% [Citation1]. Current treatment for gliomas still remains suboptimal thus, the promise for improved therapies rests largely on a better understanding of the underlying biology and genetics of these tumors. Molecular and genetic heterogeneity of gliomas contributes to the suboptimal response of treatments which are usually based on standard pathologic diagnoses such as cell morphology and common biomarkers of tumor grade [Citation2]. In fact, these surface biomarkers may fail to notice the specific sub-groups of glial tumors [Citation3, Citation4]. Therefore, there is an urgent need to identify relevant predictive and prognostic biomarkers for diagnosis as well as the treatment of primary brain tumors. Besides, radical surgical and chemotherapeutic therapy strategies and additional supplementary treatment policies may offer beneficial effects to patients. However, due to the complexity and multi-factorial nature of cancer pathology these additional treatments must also be well-defined. In recent years, vitamin D has gained great importance as a potential molecule in cancer prognosis, and even in cancer development. However, the biological significance of vitamin D in gliomas is still unclear.
The regulatory role of calcitriol, the active form of vitamin D3, has been determined in various cellular processes including cell proliferation, differentiation, apoptosis, angiogenesis and cancer metastasis. Furthermore, many studies reported calcitriol-mediated anti-proliferative and pro-differentiation effects in various cancer tissues, including gliomas [Citation5–13]. These wide effects of vitamin D on cellular processes occur by way of its specific receptor, vitamin D receptor (VDR), which also acts as a transcription factor [Citation14, Citation15]. In various studies it was shown that the expression rates of VDR affect the anti-neoplastic effects of calcitriol and its analogues [Citation5, Citation16]. In fact, the engagement of calcitriol with VDR creates a complex with retinoid X receptor (RXR). This complex regulates the gene expression of transcription factors that target the inhibitors of cell cycle kinases (or cyclin dependent kinases, CDKs). CDKs which are dependent to cyclins regulate cell-cycle progression by phosphorylation of target molecules. In contrast, the inhibitors of CDKs such as p16, p21 and p27 suppress the activation of cyclin-cyclin dependent kinase (C-CDK) complex, so the lack of activation of C-CDK results in the inhibition of cell cycle progression in the G0/G1 phase. This interaction describes the mechanism by which calcitriol prevents the proliferation of tumor cells [Citation16–18].
Another important factor in cellular progression of glioblastoma is the downstream pathways and the gene variations of epidermal growth factor receptor (EGFRvIII), a tyrosine kinase protein [Citation19, Citation20] which is a major survival pathway activated in various types of cancer. Amongst EGFRvIII activated signaling pathways, phosphatidylinositol-3-kinase (PI3K)/serine-threonine protein kinase (AKT) pathway plays a pivotal role on the cell survival. In fact, EGFRvIII preferentially signals through the PI3K/AKT-1 pathway [Citation19, Citation21, Citation22]. Phosphorylation of AKT-1 by PI3K activation regulates various cellular mechanisms including proliferation, cell-survival, cell growth and angiogenesis. It is well-known that one of the inhibitors of PI3K/AKT pathway is phosphatase and tensin homolog protein (PTEN), a tumor suppressor protein which exerts phosphatidylinositol-3,4,5-trisphosphate (PIP3) phosphatase activity. Reduced effect of PTEN has an important role in cancer pathogenesis including glioma as the reduction of PTEN results in the hyperactivation of PI3K/AKTt pathway [Citation10, Citation21–23]. On the other hand, in cell culture studies phosphorylation of mouse double minute 2 homolog protein (MDM2, an E3 ubiquitin-protein ligase) by activated/phosphorilated AKT results in nuclear export of MDM2 and interaction with p53, a transcription factor and major tumor suppressor protein which regulates various cellular pathways [Citation23, Citation24]. In brief, the wide and major important role of AKT pathway in cell survival has prompted to target this pathway as a potential therapeutic approach. The inhibitory impact of vitamin D on AKT pathway has thus gained significant attention. The synergistic inhibition of vitamin D and melatonin through reduction of active-AKT/total-AKT ratio was recently shown [Citation25]. Similarly, enhancement of gemistabine therapy with calcitriol has led to decreasing ratios of active-AKT in in vitro and in vivo pancreatic cancer models [Citation26]. Additionally, several specific AKT pathway inhibitors have been introduced for different cancer types.
Our aim was to investigate whether AKT inhibitors could suppress the viability of glioma cells and if this suppressive effect could be enhanced by combination of calcitriol with an AKT inhibitor. For this purpose, the effects of calcitriol and the AKT pathway inhibitor AT7867 on cell proliferation, apoptosis and survival were investigated using cultured glioma C6 cell line. The expression levels of genes involved in (i) vitamin D-related functions such as VDR, vitamin D binding protein (DBP), cytochrome P450 25-hydroxyvitamin D3-1alpha-hydroxylase (CYP27B1, which catalyzes the synthesis of active form of vitamin D as 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]), cytochrome P450 25-hydroxyvitamin D3-24-hydroxylase (CYP24A1, which catalyzes the conversion of 25OHD3 to 24,25-dihydroxyvitamin D3 [24,25(OH)2D3]), cytochrome P450 1A1 (CYP1A1, which degrades calcitriol to 25OHD3); (ii) AKT-related cell cycle and survival genes such as PI3K, AKT1, mammalian target of rapamycin (MTOR), cyclic AMP (cAMP)-response element binding protein (CREB), phosphatase and tensin homolog (PTEN); (iii) mitochondrial apoptosis such as BAX, BAD, cytochrome-C (CYC-C), caspase-3 (CASP3), APAF1, BCL2; (iv) caspase independent apoptosis pathways such as endonuclease G (ENDOG); (v) epithelial cells apoptosis and invasiveness such as matrix metalloproteinase 3 (MMP3) and (vi) EGFRvIII pathway was analyzed.
Materials and methods
Chemicals
Calcitriol was obtained from Abcam (ab141456, England) and dissolved in ethanol (EtOH, Sigma-Aldrich). AKT pathway inhibitor, AT7867 [Citation27, Citation28], was purchased from Santa Cruz Biotechnology (Dallas, USA) and dissolved in dimethyl sulfoxide (DMSO, Sigma Aldrich, St. Louis, MO, USA) (total concentration used in culture assays <1%) and culture medium. Stocks were prepared and stored at −20 °C until use. Gene expression assays for VDR, DBP, CYP27B1, CYP24A1, CYP1A1, PI3K, AKT1, MTOR, CREB, PTEN, BAX, BAD, CYC-C, CASP3, APAF1, BCL2, EGFRvIII, MMP3, ENDOG, Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, as the housekeeping gene) were obtained from Applied Biosystems (California, USA).
Cell culture assay
This study was designed by using C6 Glioma cell line, which was obtained from American Type Culture Collection ATCC (VA, USA). C6 glioma cells were cultured in F-12K Medium, a base medium defined by ATCC, Catalog No. 30-2004 with 10% (v/v) fetal bovine serum (FBS, Gibco, Paisley, Scotland) and 1% (v/v) penicillin/streptomycin (Gibco, Paisley, Scotland) in a + 37 °C incubator with 5% CO2. Culture medium was exchanged twice a week during cell growth. After 75% confluence the cells were trypsinized, washed with phosphate buffer saline (PBS, Sigma Aldrich, St. Louis, MO, USA) and seeded to proper culture plates. Experiments were performed using 15 × 103 cells per well in 96-well culture plates for determining the concentrations of calcitriol and AKT antagonist AT7867 and for cell viability and index assays, 5 × 105 cells per well in 6-well culture plates for gene expression assays. In each set of treatment tests, the cell lines were treated with the concentrations of 0.1–100 µmol/L AT7867 for 24, 48, 72 h and/or 0.1–100 nmol/L calcitriol for 24 h. Control cell lines without calcitriol and/or AT7867 treatment were also grown with each set of treatments.
xCELLigence cell index assay
xCELLigence real-time cell analyzer system (Acea Biotechnology) was used to determine the optimal doses of the antagonist agent AT7867 in the concentration of 0.1, 1, 10, 100 µmol/L and vitamin D in the concentration of 0.1, 1, 10, 25, 50, 100, 200, 500 and 1000 nmol/L. The instrument measures cell index (CI) values by electronic readout of cell-sensor-detecting impedance for every 15 min.The assay begun with the seeding of C6 glioma cells (15 × 103 cells/well) into an xCELLigence 96-well plate. After 20–24 h incubation with initial CI values measured by the xCELLingence instrument, six replicates of 0.1–100 µmol/L AT7867 were applied for 24, 48 and 72 h to identify the optimal dose and time in regard to the effects on cell proliferation. For the negative controls, cells were supplemented with the medium after 24 h. In case of the solvent controls, cells were treated only with the corresponding solvent. The doses of calcitriol were determined with the same approach. The cell index assay was also controlled with both MTT and trypan blue viability assays.
MTT cell viability assay
MTT assay, based upon 3-(4 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma Aldrich, St. Louis, MO, USA) uptake, was used to determine the effects of 0.1, 1, 10, 100 µmol/L AT7867 and 0.1, 1, 10, 25, 50 and 100 nmol/L calcitriol on cell viability. C6 glioma cells were seeded in 96-well plates and after 24 h, the medium was changed to test the individual and combined effects of calcitriol and AT7867 for 24 h. Before the measurement of absorbance at 540 nm, 0.5 mg/mL MTT agent dissolved in dimethyl sulfoxide (DMSO, Sigma Aldrich, St. Louis, MO, USA) was added and incubated for 2 h at 37 °C. All the experiments were performed in triplicate wells. GraphPad (Prism 6) Software was used to determine dose–response curves and IC50 values.
Trypan blue viability assay
Vi-Cell XR Cell Viability Analyzer was used for automated Trypan Blue Dye Exclusion method. All measurements were done according to the manufacturer’s (Beckmann Coulter, Indiana, USA) instructions. Similar to MTT assay treatment after the incubation period of proper doses the cell culture plate was trypsinized and 1 mL of cell suspension directly applied to the instrument.
Gene expression analysis
The expression levels of the selected genes were evaluated following a 24-hour incubation period with the optimal calcitriol (100 nmol/L) and AT7867 (10 µmol/L) dosages obtained in preliminary experiments. Total RNA was extracted from treated or non-treated cell cultures by using Invitrogen-Ambion PureLink RNA extraction kit (Life Technologies, Carlsbad, California) according to the manufacturer’s protocol. RNA was quantified using a NanoDrop ND-2000 spectrophotometer (Thermo Fischer Scientific, Wilmington, USA). RNA was reverse-transcribed with Applied Biosystems High-Capacity cDNA Reverse Transcription kit (Thermo Fischer Scientific, Carlsbad, USA). The newly synthesized cDNA was subsequently amplified by Stratagene Mx-3005P Real-Time PCR System (Agilent Technologies, USA) using TaqMan Gene Expression Assays () according to the manufacturer’s recommendations. For each condition—non-treated control cells, only calcitriol treated, only AT7867 treated and calcitriol + AT7867 treated cells with identified concentrations—two technical and three biological replicas were analyzed. The normalized expression for each sample was obtained by the ΔCT method by subtracting the CT values of the GAPDH housekeeping gene from the CT values of each gene of interest. The relative expression levels were determined by using the 2−ΔΔCT (Livac) method by subtracting the ΔCT of non-treated control samples from the ΔCT values of the treated samples [Citation29].
Table 1. Analyzed gene expressions.
Statistical analysis
Statistical analysis was performed with SPSS for windows 21.0 package and GraphPad Prism 6. Data were expressed as mean values with standard deviation (x ± SD). Student’s t test, one-way analysis of variance (ANOVA) and post-hoc Tukey analyses were used to reveal the differences in mean values of cell viability among different groups. Differences were considered statistically significant at a p value of less than 0.05 (p < 0.05).
Results
Determination of optimal calcitriol and AT7867 doses
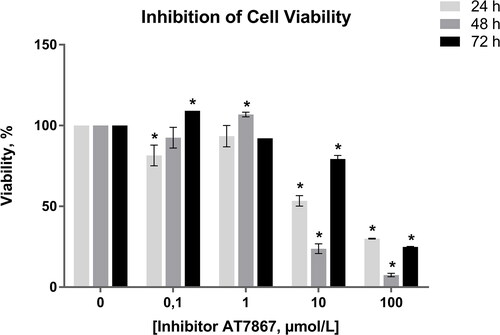
For the determination of the optimal dose of AT7867, C6 glioma cell line was treated with 0.1, 1, 10, 100 µmol/L of the AKT inhibitor for 24, 48 and 72 h. According to the results of the MTT cell viability assay shown in , the IC50 value of the inhibitor, AT7867, was determined within a wide range of ˜8–38 µmol/L with GraphPad Prism 6 and ˜12 µmol/L (upplementary material) with xCELLigence software after treatment for 24 h. As seen in , higher concentrations of AT7867 had a higher inhibitory effect evaluated on the basis of cell viability. Since 10 and 100 µmol/L showed similar marked inhibition on C6 cell survival, 10 µmol/L of concentration was selected for further analysis as the inhibition of cell viability was ˜50.0% (p = 0.02 as compared to non-treated cells). In addition, based on the dose–response curve, the incubation period of 24 h was selected as optimal due to breakdown of AT7867 in longer periods of incubation.
Figure 1. Inhibition of C6 glioma cell viability across different concentrations of AT7867 and time periods. GraphPad Prism 6 was used to analyze the inhibition of cell viability and statistical analysis was performed using Student’s t-test among treated and untreated cells. Boldface values of a, b, c show statistical significance at p < 0.05.
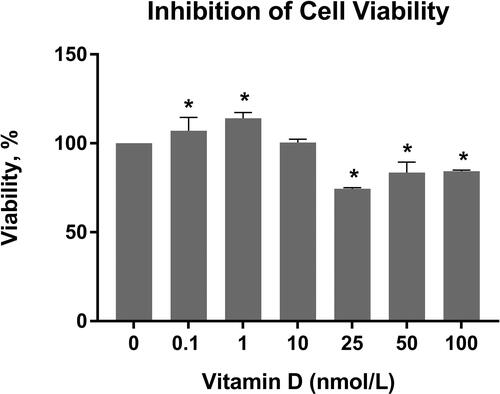
Previous cell culture studies suggested the optimal dose of calcitriol as 100 nmol/L for 24 h [Citation9]. However, there are still some conflicting results about the effective dose of calcitriol. Thus, in the present study the trial doses of calcitriol were selected as 0.1, 1, 10, 25, 50 and 100 nmol/L for the determination of the effective dose. The cell growth inhibition across different calcitriol treatment concentrations for 24 h is shown in . As seen in this figure, while there was statistically significant difference between the viability of calcitriol treated and non-treated cells, the IC50 value could not be obtained. Notably, a mild proliferation enhancing effect was observed in lower doses of calcitriol, while doses ≥25 nmol/L significantly decreased the cell viability. Thus, in consistency with the literature, 100 nmol/L of calcitriol was used in further experiments [Citation9].
Figure 2. Inhibition of C6 glioma cell viability across different concentrations of calcitriol for 24 h. GraphPad Prism 6 was used to analyze the inhibition of cell viability, and statistical analysis was performed using Student’s t-test among treated and untreated cells. Boldface values of * show statistical significance at p < 0.05.
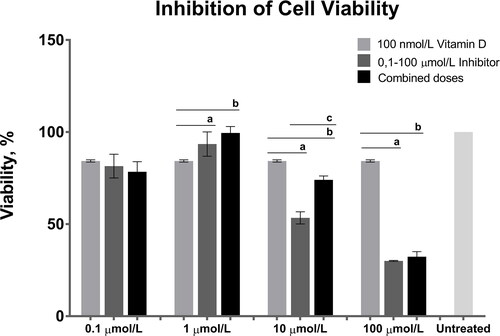
The combined effect of 100 nmol/L calcitriol and all the doses of AT7867 for 24 h is shown in . At doses of 0.1 and 1 µmol/L, AT7867 treatment did not induce a more marked viability inhibition than calcitriol treatment. Treatment with 10 µmol/L of AT7867 induced more significant viability suppression than calcitriol treatment alone. This effect was diminished by combined calcitriol and AT7867 treatment. Treatment with 100 µmol/L of AT7867 (individually or in combination with calcitriol) induced a significant reduction in cell viability as compared to wells individually treated with calcitriol.
Figure 3. Combined effects of 100 nmol/L calcitriol (vitamin D) and the 0.1, 1, 10 and 100 dose interval of AT7867 (inhibitor) for 24 h. GraphPad Prism 6 was used to analyze the inhibition of cell viability and statistical analysis was performed using Student’s t-test among treated and untreated cells, p values were shown in . The significances among groups were analyzed by one-way ANOVA test and boldface values of a, b and c show statistical significance at p < 0.05 by Tukey’s post-hoc test, a is between calcitriol and inhibitor, b is between calcitriol and combined dose and c is between inhibitor and combined dose.
shows the relative proliferation index scale against various individual and combined doses of calcitriol and AT786 for 24 h as compared to untreated cells.
Table 2. MTT results of different individual and combined doses of Vitamin D and AT7867 at 24 h.
Gene expression studies
After the detection of optimal doses for the determination of the effects of calcitriol and AT7867, the expressional pattern of several survival-related genes including VDR, DBP, CYP1A1, CYP27B1, CYP24A1, PI3K, AKT1, MTOR, CREB, PTEN, BAX, BAD, CYC-C, CASP3, APAF1, BCL2, MMP3, ENDOG and EGFRvIII were analyzed and relative fold changes (2−ΔΔCT) and log2 (fold change) graphs were shown in and , respectively.
Figure 4. Relative expression levels expressed as Log22(−ΔΔCt) of related genes across 100 nmol/L calcitriol (vitamin D), 10 µmol/L AT7867 (inhibitor), and for both calcitriol and AT7867 for 24 h. Positive log2 fold change (FC) of expressions show the upregulated genes, and negative of those show downregulation of genes.
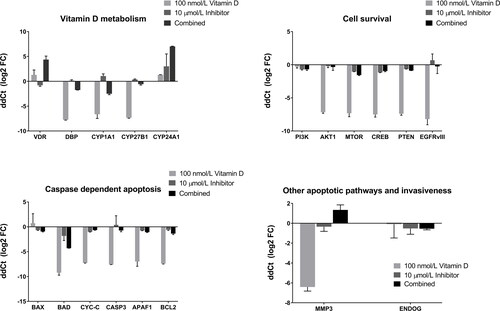
Table 3. Relative fold changes and p values of gene expressions.
The VDR gene was ˜2.5 fold upregulated with 100 nmol/L calcitriol treatment, however the induction was not statistically significant (p > 0.05). It was seen that CYP24A1 gene expression was induced by 100 nmol/L calcitriol treatment (p < 0.001). On the other hand, DBP (p < 0.001), CYP1A1 (p = 0.007), CYP27B1 (p < 0.001), AKT1 (p = 0.001), MTOR (p = 0.003), CREB (p = 0.001), PTEN (p < 0.001), BAD (p = 0.002), CYC-C (p < 0.001), CASP3 (p < 0.001), APAF-1 (p = 0.006), BCL2 (p < 0.001), EGFRvIII (p = 0.003) and MMP3 (p = 0.001) genes were downregulated by calcitriol treatment. The expressional differences for the application of 10 µmol/L AT7867 were as follows: CYP27B1 was upregulated (p = 0.004), and the downregulated genes were VDR (p = 0.037), PI3K (p = 0.016), MTOR (p = 0.002), CREB (p < 0.001), PTEN (p < 0.001), BAX (p = 0.013), CYC-C (p < 0.001), APAF1 (p = 0.017), and BCL2 (p < 0.001). Consequently, VDR (p = 0.007), CYP24A1 (p < 0.001) and MMP3 (p = 0.040) genes were highly overexpressed across the combined application of 100 nmol/L calcitriol and 10 µmol/L AT7867. However, the expressions of DBP (p = 0.003), CYP1A1 (p = 0.004), CYP27B1 (p = 0.026), PI3K (p = 0.027), MTOR (p = 0.004), CREB (p = 0.005), PTEN (p < 0.001), BAX (p = 0.005), BAD (p = 0.001), CYC-C (p = 0.002), CASP3 (p = 0.011), APAF1 (p = 0.001), BCL2 (p = 0.005) and MMP3 (p = 0.040) genes were downregulated with the combination of calcitriol and AT7867 treatment. Individual calcitriol treatment had a more marked inhibitory effect on cell survival pathways, caspase-dependent apoptosis pathway and MMP3 gene expression levels than individual and combined administration of AT7867. None of the treatment arms showed a significant impact on the caspase independent apoptosis factor ENDOG ().
Discussion
Astrocyte originated glioma is one of the most aggressive brain tumors with a higher mortality rate [Citation1]. The contribution of vitamin D, as a regulatory molecule, on the prevention of cell proliferation, the increment of cell differentiation and/or the regulation of immune system is well-known and many studies have reported the association of vitamin D and its metabolic pathways with the risk of cancer development and progression [Citation5–8, Citation13, Citation16, Citation30]. These wide effects of vitamin D are driven through its receptor VDR, which also acts as a transcription factor [Citation5, Citation13]. Recent clinical studies revealed the association of differential expressional patterns of vitamin D with various cancer types [Citation16, Citation17, Citation19, Citation20, Citation31–33]. Indeed, preclinical studies have shown that calcitriol or its analogues might have potential therapeutic effect as an anticancer agent [Citation5–8, Citation13, Citation15, Citation34]. However, the underlying mechanism is not well defined yet.
In the present study, the effect of AKT pathway inhibition on glioma cell survival and proliferation was investigated using calcitriol and an AKT inhibiting molecule. The dose evaluation treatments in the present study demonstrated that the IC50 value of calcitriol cannot be obtained as it maybe has a biphasic effect as mildly inducing cell proliferation in lower doses (<10 nmol/L), and anti-proliferation in higher doses (≥25 nmol/L). Hence, in consistence with several reports, 100 nmol/L of calcitriol was determined as the optimal dose and used for all experiments. The phosphorylation of AKT1 by activation of PI3K, regulates many cellular processes such as proliferation, cellular survival, growth and angiogenesis. The loss of activity of a tumor suppressor phosphatase or loss of PTEN protein causes AKT activation [Citation21, Citation22, Citation32, Citation33]. AT7867, a potential inhibitor of ribosomal protein S6 kinase (p70S6K), which is activated by AKT and mTOR, was shown to inhibit the proliferation of various human tumor cell lines by suppressing the phosphorylation of GSK3β, the substrate of AKT, and ribosomal protein S6 (S6RP), the substrate of p70S6K [Citation34–37]. In the present study, survival of C6 cells was reduced and AKT and AKT pathway related genes, including PI3K, MTOR, CREB and PTEN, were downregulated by AT7867 treatment, demonstrating the anti-proliferative effect of AT7867 on glioma cells for the first time, to our knowledge.
An important question of our study was whether the anti-glioma action of the AKT inhibitor could be enhanced with the combination of calcitriol, which is also known to inhibit the AKT pathway. Our findings suggest that individual administration of calcitriol has a less pronounced effect on glioma cell viability than AT7867. Moreover, combination of calcitriol with AT7867 failed to increase the impact of AT7867 on cell viability. Overall, our results argue against combined calcitriol-AKT inhibitor administration in future glioma treatment trials. Based on our findings, at least one of the factors underlying the reduced anti-glioma effect of calcitriol might be its anti-apoptotic impact on glioma cells.
While the treatment of calcitriol significantly affected the cell survival rates, a ˜2-fold upregulation of the VDR gene was detected as compared to untreated glioma cells. The increase in the VDR expression level was found as a physiological response against the inducer calcitriol treatment. Additionally, our finding of cell survival inhibition was in consistence with the prior studies [Citation10, Citation16, Citation17, Citation19, Citation20]. On the other hand, ˜2-fold decrease in VDR gene expression as a response to 10 µmol/L AT7867, a potential inhibitor of AKT, suggests that there may be a relationship between vitamin D and AKT pathway. Indeed, several studies reported the down-regulatory effect of vitamin D on AKT pathway [Citation19, Citation20, Citation23, Citation25]. On the other hand, interestingly, the combined dose treatment induced a ˜20-fold VDR expression which suggests the individual effect of calcitriol was straightened with AT7867.
Our finding of reduction in the expression profile of DBP gene was a result of physiological response against the treatment of cells with an active form of vitamin D, calcitriol. Besides, increased expression of DBP against AT7867 treatment indicates the potential role of AKT pathway inhibition in downregulation of the cellular effects of calcitriol since DBP is a carrier and/or neutralizer of vitamin D. Likewise, a recent study suggested that the suppression of calcitriol-related genes may be caused by elevated levels of DBP [Citation38]. Additionally, the CYP1A1 gene, which is involved in degradation of the active form of vitamin D [Citation16, Citation39, Citation40], was upregulated by AT7867 treatment. Thus co-treatment by AKT pathway inhibitors and vitamin D may dampen the impact of calcitriol by increasing its neutralization.
On the other hand, AT7867 treatment upregulated CYP27B1, which encodes 1-alpha hydroxylase enzyme function in the activation of vitamin D [Citation16, Citation40], and downregulated CYP24A1, an inactivating enzyme of vitamin D. Inhibition of the CYP24A1 gene has resulted in the preservation of the vitamin D driven anti-proliferative effect [Citation30, Citation41]. Indeed, Zhang et al. [Citation31] suggested that the mutations of EGFR and K-RAS genes indirectly affected the metabolism of vitamin D through dysregulation of the CYP24A1 gene. These results suggest that AKT inhibitors may also promote the actions of vitamin D.
ENDOG, known as the main modulator of the caspase independent pathway, plays a very active role in cancer cells. ENDOG is known to be cytotoxic and its presence in the nucleus results in the death of the host cell [Citation42–44]. None of the treatment arms in our study had a significant impact on the ENDOG expression levels, suggesting that the anti-apoptotic effect of calcitriol is mainly mediated by the caspase dependent apoptotic pathway. MMP3 plays an important role in high grade gliomas and especially invasive phenotypes of astrocytomas, and is involved in apoptosis, as well [Citation45, Citation46]. Indeed, some new studies have explored the inhibition of MMP-3 activity for the treatment of glioma [Citation47]. In the present study, the MMP3 expression level of glioma cells was suppressed by individual calcitriol treatment (but not by AT7867 alone or in combination with calcitriol), suggesting that calcitriol might have a restrictive impact on the invasiveness of high-grade glioma cells.
In brief, our results indicate that calcitriol targets and inhibits both cell survival and apoptotic pathways of C6 glioma cells, whereas AT7867 primarily affects the AKT pathway with little or no influence on apoptosis pathways. This difference makes AT7867 a more potent antagonist of glioma cells and thus utilization of this agent or other AKT inhibitors is recommended in future glioma treatment trials.
Acknowledgements
The present work was supported by a grant from the Scientific Research Projects Coordination Unit of Istanbul University (Project No: 46482).
Data availability
All data that support the findings reported in this paper are available from the corresponding author upon reasonable request.
Disclosure statement
The authors report no conflict of interest.
References
- Tan AC, Ashley DM, Lopez GY, et al. Management of glioblastoma: state of the art and future directions. CA A Cancer J Clin. 2020;70(4):299–312.
- Chen R, Smith-Cohn M, Cohen AL, et al. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14(2):284–297.
- Delgado-Lopez PD, Saiz-Lopez P, Gargini R, et al. A comprehensive overview on the molecular biology of human glioma: what the clinician needs to know. Clin Transl Oncol. 2020;22(11):1909–1922.
- Zhou YH, Hess KR, Liu L, et al. Modeling prognosis for patients with malignant astrocytic gliomas: quantifying the expression of multiple genetic markers and clinical variables. Neuro Oncol. 2005;7(4):485–494.
- Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50(4):1–14.
- Gallagher JC, Bikle DD. Vitamin D: mechanisms of action and clinical applications. Endocrinol Metab Clin North Am. 2017;46(4):xvii–xviii.
- Campbell FC, Xu H, El-Tanani M, et al. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010;79(1):1–9.
- Duffy MJ, Murray A, Synnott NC, et al. Vitamin D analogues: potential use in cancer treatment. Crit Rev Oncol Hematol. 2017;112:190–197.
- Cataldi S, Arcuri C, Lazzarini A, et al. Effect of 1α,25(OH)2 Vitamin D3 in mutant P53 glioblastoma cells: involvement of neutral sphingomyelinase1. Cancers (Basel). 2020;12(11):3163.
- Bak DH, Kang SH, Choi DR, et al. Autophagy enhancement contributes to the synergistic effect of vitamin D in temozolomide-based glioblastoma chemotherapy. Exp Ther Med. 2016;11(6):2153–2162.
- Naveilhan P, Berger F, Haddad K, et al. Induction of glioma cell death by 1,25(OH)2 vitamin D3: towards an endocrine therapy of brain tumors?J Neurosci Res. 1994;37(2):271–277.
- Hu P, Li S, Tian N, et al. Acidosis enhances the self-renewal and mitochondrial respiration of stem cell-like glioma cells through CYP24A1-mediated reduction of vitamin D. Cell Death Dis. 2019;10(1):25.
- Bikle DD. Vitamin D: newer concepts of its metabolism and function at the basic and clinical level. J Endocr Soc. 2020;4(2):bvz038.
- Xu Y, He B, Pan Y, et al. Systematic review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Tumour Biol. 2014;35(5):4153–4169.
- Elmaci I, Ozpinar A, Ozpinar A, et al. From epidemiology and neurometabolism to treatment: Vitamin D in pathogenesis of glioblastoma Multiforme (GBM) and a proposal for Vitamin D + all-trans retinoic acid + Temozolomide combination in treatment of GBM. Metab Brain Dis. 2019;34(3):687–704.
- Norlin M. Effects of vitamin D in the nervous system: Special focus on interaction with steroid hormone signalling and a possible role in the treatment of brain cancer. J Neuroendocrinol. 2020;32(1):e12799.
- Sui A, Xu Y, Pan B, et al. Histone demethylase KDM6B regulates 1,25-dihydroxyvitamin D3-induced senescence in glioma cells. J Cell Physiol. 2019;234(10):17990–17998.
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710.
- Maj E, Trynda J, Maj B, et al. Differential response of lung cancer cell lines to vitamin D derivatives depending on EGFR, KRAS, p53 mutation status and VDR polymorphism. J Steroid Biochem Mol Biol. 2019;193:105431.
- Vanoirbeek E, Krishnan A, Eelen G, et al. The anti-cancer and anti-inflammatory actions of 1,25(OH)2D3. Best Pract Res Clin Endocrinol Metab. 2011;25(4):593–604.
- Li X, Wu C, Chen N, et al. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7(22):33440–33450.
- Zhao HF, Wang J, Shao W, et al. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: current preclinical and clinical development. Mol Cancer. 2017;16(1):100.
- Ryskalin Ryskalin L, Gaglione A, Limanaqi F, et al. The autophagy status of cancer stem cells in glioblastoma multiforme: from cancer promotion to therapeutic strategies. IJMS. 2019;20(15):3824.
- Proietti S, Cucina A, D’Anselmi F, et al. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J Pineal Res. 2011;50(2):150–158.
- Yu WD, Ma Y, Flynn G, et al. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle. 2010;9(15):3022–3029.
- Bai L, McEachern D, Yang CY, et al. LRIG1 LRIG1 modulates cancer cell sensitivity to Smac mimetics by regulating TNFα expression and receptor tyrosine kinase signaling. Cancer Res. 2012;72(5):1229–1238.
- Fabian AK, Marz A, Neimanis S, et al. InterAKTions with FKBPs-mutational and pharmacological exploration. PLoS One. 2013;8(2):e57508.
- Peng X, Tiwari N, Roy S, et al. Regulation of CYP24 splicing by 1,25-dihydroxyvitamin D3 in human colon cancer cells. J Endocrinol. 2012;212(2):207–215.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408.
- Zhang Q, Kanterewicz B, Buch S, et al. CYP24 inhibition preserves 1α,25-dihydroxyvitamin D(3) anti-proliferative signaling in lung cancer cells. Mol Cell Endocrinol. 2012;355(1):153–161.
- Zhang Q, Kanterewicz B, Shoemaker S, et al. Differential response to 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) in non-small cell lung cancer cells with distinct oncogene mutations. J Steroid Biochem Mol Biol. 2013;136:264–270.
- Axanova LS, Chen YQ, McCoy T, et al. 1,25-dihydroxyvitamin D(3) and PI3K/AKT inhibitors synergistically inhibit growth and induce senescence in prostate cancer cells. Prostate. 2010;70(15):1658–1671.
- Kure S, Nosho K, Baba Y, et al. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2765–2772.
- Grimshaw KM, Hunter LJ, Yap TA, et al. AT7867 is a potent and oral inhibitor of AKT and p70 S6 kinase that induces pharmacodynamic changes and inhibits human tumor xenograft growth. Mol Cancer Ther. 2010;9(5):1100–1110.
- Zhang Q, Yan HB, Wang J, et al. Chromatin remodeling gene AT-rich interactive domain-containing protein 1A suppresses gastric cancer cell proliferation by targeting PIK3CA and PDK1. Oncotarget. 2016;7(29):46127–46141.
- Zhang S, Deng Z, Yao C, et al. AT7867 inhibits human colorectal cancer cells via AKT-dependent and AKT-independent mechanisms. PLoS One. 2017;12(1):e0169585.
- Bjune K, Wierod L, Naderi S. Inhibitors of AKT kinase increase LDL receptor mRNA expression by two different mechanisms. PLoS One. 2019;14(6):e0218537.
- Huang YF, Wu YH, Cheng WF, et al. Vitamin D-binding protein enhances epithelial ovarian cancer progression by regulating the insulin-like growth factor-1/Akt pathway and vitamin D receptor transcription. Clin Cancer Res. 2018;24(13):3217–3228.
- Matsunawa M, Akagi D, Uno S, et al. Vitamin D receptor activation enhances benzo[a]pyrene metabolism via CYP1A1 expression in macrophages. Drug Metab Dispos. 2012;40(11):2059–2066.
- Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55(1):13–31.
- Hu N, Zhang H. CYP24A1 depletion facilitates the antitumor effect of vitamin D3 on thyroid cancer cells. Exp Ther Med. 2018;16(4):2821–2830.
- Zhdanov DD, Fahmi T, Wang X, et al. Regulation of apoptotic endonucleases by EndoG. DNA Cell Biol. 2015;34(5):316–326.
- Minchenko OH, Tsymbal DO, Minchenko DO, et al. Hypoxic regulation of the expression of cell proliferation related genes in U87 glioma cells upon inhibition of ire1 signaling enzyme. Ukr Biochem J. 2016;88(1):11–21.
- Waye S, Naeem A, Choudhry MU, et al. The p53 tumor suppressor protein protects against chemotherapeutic stress and apoptosis in human medulloblastoma cells. Aging (Albany, NY). 2015;7(10):854–868.
- Kunapuli P, Kasyapa CS, Hawthorn L, et al. LGI1, a putative tumor metastasis suppressor gene, controls in vitro invasiveness and expression of matrix metalloproteinases in glioma cells through the ERK1/2 pathway. J Biol Chem. 2004;279(22):23151–23157.
- Choi DH, Kim EM, Son HJ, et al. A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J Neurochem. 2008;106(1):405–415.
- Poole AT, Sitko CA, Le C, et al. Examination of sulfonamide-based inhibitors of MMP3 using the conditioned media of invasive glioma cells. J Enzyme Inhib Med Chem. 2020;35(1):672–681.
