Abstract
The polyphenol quercetin is associated with numerous beneficial health effects in various pathologies such as oxidative stress, neurodegenerative disorders, inflammation, neoplastic processes, age-related diseases, and so on. However, the molecular mechanisms underlying the effects of quercetin and its implication in the pharmaceutical practice require additional clarification. In this study we analyzed the biochemical mechanisms of quercetin effect on membrane lipids of fibroblasts cultured as three-dimensional (3D) cultures, which resemble living tissues more adequately compared to cellular monolayers. Quercetin treatment of 3D fibroblasts induced alterations of the plasma membrane phospholipid and fatty acid composition as well as in the phospholipid metabolizing enzymes sphingomyelinase and phospholipase A2. The level of sphingomyelin (SM), which acts as endogenous membrane antioxidant, was elevated due to quercetin action. Incubation of 3D fibroblasts with quercetin induced augmentation in the saturated fatty acids and reduction of all polyunsaturated fatty acids in the membrane phospholipid molecules of the plasma membranes, indicating an additional increase of the resistance to oxidative damage. Quercetin treatment reduced cholesterol susceptibility to oxidation, a phenomenon which is probably related to the observed increase in the level of SM. In addition, Western blot analysis showed augmented phosphorylation of Akt and expression of Bcl-2 in quercetin-treated cell, indicating that apoptosis was suppressed by quercetin. In conclusion, the reported results contribute to a better understanding of the beneficial effects of quercetin on the structure and functions of plasma membranes of 3D cell cultures, which are a more adequate model of living tissues.
Introduction
Quercetin (3,3′,4’5,7-pentahydroxyflavone) is a polyphenolic compound that is widely distributed in certain vegetables and fruits. It is associated with antioxidant, anti-inflammatory and even anti-tumor effects [Citation1–3].
The molecular pathogenesis of a wide range of pathological processes is related to accumulation of free radicals, which are highly reactive species due to the existence of one or more unpaired electrons. In some cases free radicals cause irreversible alterations of cellular components as a result of their interactions with biologically active signaling molecules which regulate essential cellular processes [Citation4]. In other cases free radicals interact directly with functional key molecules, thus becoming a direct cause of specific pathophysiological conditions and diseases [Citation5]. Numerous investigations are devoted to the beneficial health effects of quercetin in various pathologies such as oxidative stress, toxicological stress after pesticide intake, neurodegenerative disorders, inflammation, neoplastic processes, age-related diseases, and so on. [Citation6]. In addition, there is evidence indicating that flavonoids take part in regeneration of certain antioxidant molecules [Citation7]. However, the molecular mechanisms underlying the effects of quercetin and the possibilities for its implication in the pharmaceutical practice still require additional clarification.
In this study we used as experimental model fibroblasts cultured as three-dimensional structures, which resemble more adequately living tissues compared to cellular monolayers [Citation8]. In these settings cells interact with a pliable fibrillar meshwork of extracellular proteins, organize specific adhesive contacts and demonstrate changes in their morphology, proliferation and migration [Citation9]. Our previous studies showed well pronounced differences in the lipid composition and metabolism between conventional monolayer and three-dimensional fibroblast cultures [Citation8, Citation10]. This prompted us to study the effect of quercetin on membrane lipids in these in vivo-like conditions.
The present studies showed that quercetin treatment induced changes in the phospholipid and fatty acid composition, phospholipid-metabolizing enzymes, cholesterol content and susceptibility to oxidation, as well as lipid peroxide content in the membranes of 3D cell cultures. It also supported suppressed apoptosis of healthy non-tumor fibroblasts cultured in 3D in vivo-like conditions. Thus, the beneficial effect of quercetin on the functional activity of fibroblast membranes and its potential to affect certain membrane-associated parameters that are related to development of pathological processes, were demonstrated.
Materials and methods
Reagents
Quercetin (3,3′,4’5,7-pentahydroxyflavone) was purchased from Sigma. Egg yolk phosphatidylcholine was obtained from Avanti polar lipids. All other chemicals and solvents were of analytical grade and purchased from Sigma-Aldrich Chemie GmbH.
Cell culture and histochemical staining
Fibroblast cell line GD25β1 was obtained as described elsewhere [Citation11]. The cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) enriched with 10% fetal bovine serum (FBS) with addition of antibiotics and antimycotics: Penicillin (100 U/mL), Streptomycin (100 mg/mL) and Amphotericin B (0.25 mg/mL). Cells were cultured at 37 °C, in a humidified air atmosphere supplemented with 5% CO2. Three-dimensional (3D) cell cultures were prepared as described by Skrobanska et al. [Citation12]. Briefly, the cells were seeded at a concentration of 0.67 × 105 cells per cm2 and allowed to produce their own matrix by culturing them for five consecutive days under the conditions described above.
For histochemical staining 3D cultures were fixed in 4% paraformaldehyde for 20 min, scraped, embedded in paraffin wax and sectioned at 4 µm. Deparafinized and re-hydrated samples were stained with haematoxylin for 15 min, washed in running tap water and counterstained with eosin for 2 min.
Incubation with quercetin
The fibroblasts were incubated in the presence or absence of quercetin (10 μmol/L) delivered from stock solution in dimethylsulfoxide under 95% air and 5% CO2 for1 h. Control cells were incubated only with dimethylsulfoxide. After incubation with quercetin, cell viability was determined by tetrazolum salt measurement (MTT assay) involving assessment of succinate dehydrogenase-induced conversion of (3-[4,5-dimethylthiazol-2-y]-2,5 diphenyl tetrazolium bromide into formazan crystals [Citation13]. Formation of formazan was measured at 570 nm. The viability of the cells after incubations was estimated as percentage of the absorbance of quercetin-treated cells compared to controls.
Isolation of plasma membranes
Plasma membranes were isolated according to the procedure described by Pankov et al. [Citation14] with slight modifications involving differential centrifugation. Briefly, the post-nuclear supernatant was loaded on a discontinued sucrose gradient and centrifuged at 100,000 g for 2.5 h. The plasma membrane fraction was obtained at a density of 8% (w/v), suspended in ice-cold 10 mmol/L Tris HCl buffer pH 7.4 and used immediately for lipid analysis.
Lipid extraction and analysis
Lipids were extracted with chloroform/methanol according to the procedure of Bligh and Dyer [Citation15]. The organic phase was concentrated and analyzed by thin layer chromatography. The phospholipid fractions were separated on silica gel G 60 plates in a solvent system containing chloroform/methanol/2-propanol/triethylamine/0.25% KCl (30:9:25:18:6 v/v) [Citation16]. The spots were scraped and quantified by determination of their inorganic phosphorus content [Citation17]. Cholesterol content was assayed by gas chromatography using a medium polarity RTX-65 capillary column.
Fatty acid analysis
The extracted phospholipids were saponified with 0.5 N methanolic KOH and methylated with boron trifluoride ethanol complex (Merck) [Citation18]. The fatty acid methyl esters were extracted with hexane and separated by gas chromatography on a capillary column Supelcowax 10.
Phospholipase A2 assay
Phospholipase A2 activity was performed by the method described elsewhere [Citation19] with slight modifications. Plasma membranes were incubated with 100 nmol egg yolk phosphatidylcholine as a substrate in 100 mmol/L Tris-HCl pH 8.6 with 5 mmol/L CaCl2 and 0.1% fatty acid free bovine serum albumin. The reaction was stopped with 0.5 mL methanol and the liberated fatty acids were extracted by modified procedure of Dole [Citation20].
Determination of sphingosine-1 phosphate
The method is based on the principle of double-antibody sandwich technique enzyme-linked immunosorbent assay (ELISA) for detecting the amount of human sphingosine-1-phosphate in biological samples (Antibody Research Corporation, USA). The procedure described by the manufacturer was followed and the optical density of the samples was measured at 450 nm on TECAN micro plate reader (Tecan Austria GmbH, Salzburg). The amount of S1P in the samples was calculated using a standard curve.
Western blotting
Control and quercetin-treated 3D cultures were solubilized in RIPA buffer (150 mmol/L NaCl, 2 mmol/L ethylenediaminetetraacetic acid (EDTA), 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 10% glycerol, 50 mmol/L HEPES, pH 7.5) containing Complete Inhibitory Cocktail (Merck). Equal volumes of 5x sample buffer (60 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol and bromophenol blue) were added and samples were heated for 4 min at 95 °C. Proteins were resolved by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane and blocked in 5% non-fat dry milk in TBST (50 mmol/L Tris base, 200 mmol/L NaCl, 0.1% Tween-20, pH 7.4). Membranes were than incubated with appropriate primary and secondary antibodies, including total- and phospho (Ser 473) Akt and Bcl-2, all from Cell Signaling. Anti-actin clone, AC-40 were purchased from Sigma-Aldrich. HRP-conjugated secondary antibodies were from Enzo Life Sciences. Immunoblots were visualized using the ECL system (Santa Cruz). The signals, quantified with Image J software, from p-Akt were normalized to the corresponding signals from total Akt and expressed as percentage from p-Akt expression in control cells. The signals from Bcl-2 were normalized to the corresponding signals from actin and the percentage changes were calculated as for p-Akt.
Determination of lipid peroxides
Lipid peroxidation was determined by the procedure described by Carini et al. [Citation21]. The level of lipid peroxidation was measured by the loss of fluorescence of cis-parinaric acid (PNA) (Molecular Probes, Invitrogen, UK). The plasma membranes were incubated with 10 μmol/L PNA at 37 °C for 30 min in dark. The membranes were dissolved in 10 mmol/L Tris HCl pH 7.4. The emission fluorescence of wavelength 455 nm (slit width 5 nm) was measured using an excitation wavelength of 312 nm (slit width). The level of lipid peroxidation was calculated as fluorescence intensity per mg membrane protein.
Measurement of the reduced glutathione (GSH)
The content of cell GHS was determined according to Ellman [Citation22] using cell lysate obtained from control and quercetin-treated fibroblasts in 0.25 mol/L Tris-HCl, 20 mmol/L EDTA, pH 8.2). The obtained values were expressed as nmol GSH per milligram protein.
Treatment of cells with cholesterol oxidase
3D fibroblasts were suspended in PBS and incubated with 10 IU/mL of cholesterol oxidase and incubated for 30 min at 37 °C. The reaction was terminated by the procedure of Dole exactly as described by Moore et al. [Citation23]. Dole reagent (2 mL) was added to the reaction mixture (1 mL), followed by 1 mL heptane. Cholest-4-en-3-one, a product of cholesterol oxidation, was determined spectrophotometrically at 235 nm.
Sphingomyelin degradation by exogenous sphingomyelinase
Plasma membrane sphingomyelin was degraded with 2 U/mL sphingomyelinase added to the incubation medium for 30 min at 37 °C. Cell permeability to trypan blue was not altered by the enzyme. The partially delipidated cells were divided into 2 groups: the first was incubated with quercetin, and the second was used as control.
Protein determination
The content of protein was determined according to Bradford [Citation24].
Statistical analysis
Statistical processing of the data was made by one-way analysis of variance (ANOVA) using InStat software. Differences were considered statistically significant at p < 0.05 level.
Results
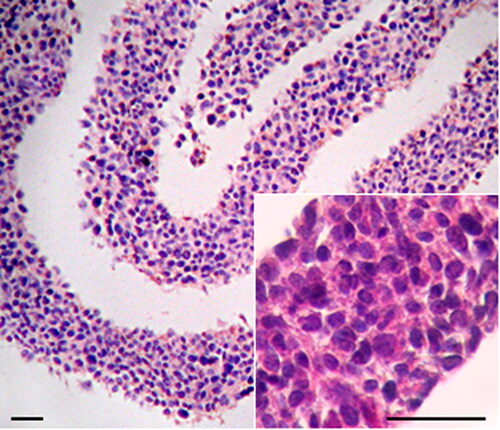
To obtain naturally organized three-dimensional cell cultures we used GD25β1 cells that produce dense extracellular matrix and grow above confluency as multilayered tissue-like structures, where cells demonstrate reduced ageing and proliferation [Citation12]. The general morphology of these 3D fibroblast cultures is illustrated after staining with hematoxylin/eosin in .
Figure 1. Haematoxylin/eosin staining of paraffin cross-sections through 3D culture shown at low and high (inset) magnification. Note the relatively uniform thickness of the cell layer and the uniform distribution of cells throughout. Bar = 200 μm.
Treatment of 3D cultures with quercetin induced significant alterations in the level of some plasma membrane lipids compared to untreated cells (). The choline-containing phospholipids sphingomyelin (SM) and phosphatidylcholine (PC) were increased at a different degree, whereas the aminophospholipids phosphatidylserine (PS) and phosphatidylethanolamine (PE) were decreased.
Table 1. Phospholipid composition of plasma membranes isolated from control and quercetin-treated 3D fibroblasts (mol%).
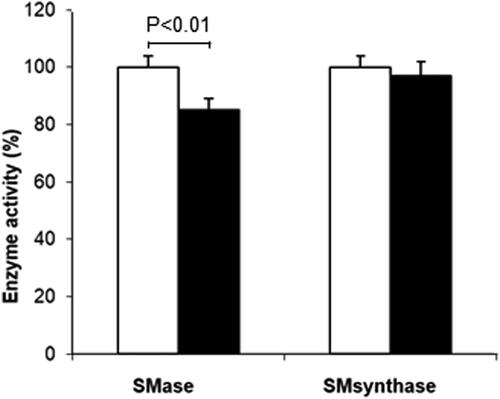
The observed elevation of SM might be due to either a decrease in its hydrolysis through inhibition of neutral sphingomyelinase or activation of its synthesis. To clarify which one of these options was valid, we investigated the activities of neutral sphingomyelinase (nSMase) and sphingomyelin synthase (). Quercetin treatment of 3D fibroblasts induced a statistically significant (p < 0.01) reduction in nSMase activity by 15%, whereas the activity of SM synthase remained almost unchanged.
Figure 2. Changes in sphingomyelinase (SMase) and sphingomyelin synthase (SM synthase) activities after quercetin treatment. Control values are taken for 100% (white bars). Values for quercetin-treated fibroblasts are presented as black bars. Results are means ± SD of three separate experiments. Statistical significance was calculated between the corresponding activity in untreated (control) and treated 3D fibroblasts. Only the differences between SMase in control and quercetin-treated fibroblasts were statistically significant (p < 0.01).
The other augmented phospholipid fraction was PC. One possible reason for the observed elevation of membrane PC could be a decrease in membrane-bound phospholipase A2 (PLA2) activity, which hydrolyzes phospholipids to produce lysophospholipid and unsaturated fatty acid. This possibility was confirmed by the observed decrease in the amount of lysophosphatydylcholine (LPC), which is a product of PLA2 (). If quercetin indeed inhibits PLA2 activity, it would induce a reduction of the unsaturated fatty acids such as arachidonic acid (C20:4), which is a major PLA2 product. To verify this presumption we analyzed the fatty acid composition of the plasma membrane phospholipids in quercetin treated fibroblasts (). The amount of almost all unsaturated fatty acids, including C20:4, was decreased after quercetin treatment, except the monounsaturated C18:1. However, the saturated C14:0, C16:0 and C18:0 were elevated as a result of quercetin treatment.
Table 2. Fatty acid composition of phospholipids in plasma membranes from control and quercetin-treated 3D fibroblasts (mol%).
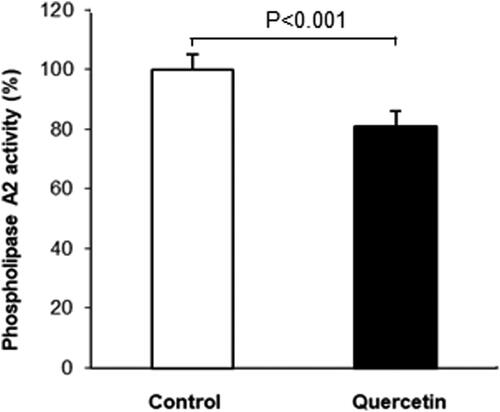
To analyze the molecular mechanisms underlying the observed changes in the phospholipid fatty acids we determined the activity of membrane-bound PLA2. This enzyme regulates the phospholipid deacylation/reacylation cycle, the latter modulating the phospholipid acyl chain composition. The obtained results demonstrated a decrease in PLA2 activity after quercetin treatment of 3D fibroblasts ().
Figure 3. Changes in phospholipase A2 activity (% of control- untreated 3D fibroblasts) after quercetin treatment. Results are means ± SD of three separate experiments. The observed differences were statistically significant (p < 0.001).
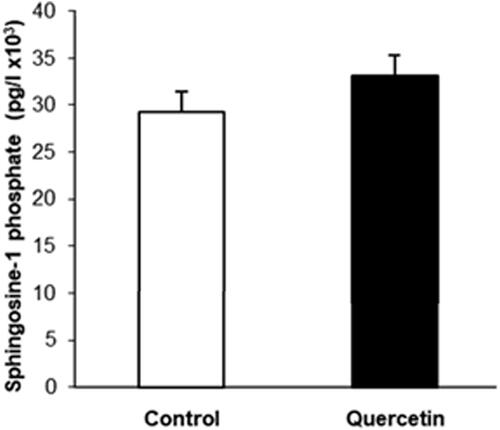
Since alterations in the level and membrane localization of PS are related to apoptosis, we used Western blot analysis to study the changes in the cellular apoptotic state. It is well established that activation of Akt pathway promotes survival by inhibiting apoptotic pathways. Activated Akt phosphorylates and inhibits the pro-apoptotic Bcl-2 family members (e.g. Bad, Bax) and up-regulates the expression of anti-apoptotic Bcl2 family proteins (e.g. Bcl2, BclXL) [Citation25]. The results showed a statistically significant elevation of the phosphorylation of AKT and Bcl-2 expression (), indicating reduction in the apoptotic processes. In addition, the alterations in the sphingolipid metabolism induced by quercetin could also be related to apoptosis, because changes in the sphingosine-1-phosphate/ceramide balance are responsible for prevalence of either proliferation or apoptotic processes. To elucidate if the balance between these sphingolipids was shifted, we determined the level of sphingosine 1-phosphate, which is basically responsible for proliferative processes. As evident from , the level of sphingosine 1-phosphate showed an increasing trend but the observed change was not statistically significant. This sphingolipid is an indicator of the proliferative potential of cells and its balance with ceramide suggests that the apoptotic processes are suppressed.
Figure 4. Changes in Akt activity and Bcl-2 expression in 3D fibroblast culture after quercetin treatment. (A) Representative images from Western blotting, performed with total lysates from non-treated (Control) and quercetin-treated (Quercitin) fibroblasts and antibodies against phosphorylated (pAkt) and total (tAkt) forms of Akt and B cell lymphoma 2 protein (Bcl-2). Reaction with actin antibody (actin) was used as internal control for loading. (B) Quantification of the results from Western blotting, presented as percentage change in quercetin-treated cells (black bars) compared to control non-treated cells (white bars) taken for 100%. Total Akt and actin were used as internal controls for determination of the percentage changes in pAkt and Bcl-2 correspondingly. Error bars represent ± SD. The results are from at least three independent experiments.
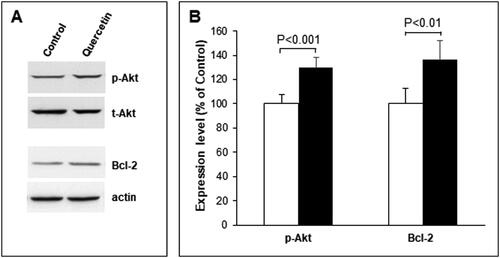
Figure 5. Sphingosine-1-phosphate (S1P) level in control (white bars) and quercetin-treated (black bars) 3D fibroblasts, determined by ELISA test. The data are means of three separate experiments performed in triplicate. The difference between the measured values was not statistically significant.
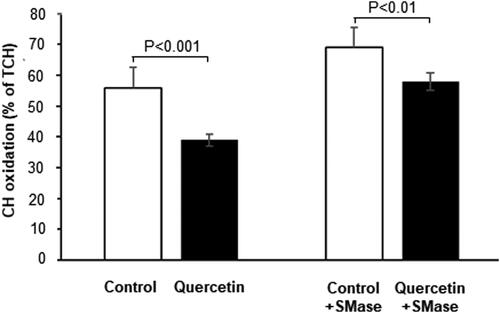
As the level of membrane sphingomyelin was altered by quercetin, and sphingomyelin is in dynamic equilibrium with cholesterol, especially in the membrane raft domains, we investigated the effect of quercetin on the level of membrane cholesterol. Quercetin treatment induced a reduction in the cholesterol/phospholipid ratio in plasma membranes of 3D fibroblasts (). To study the antioxidant effect of quercetin in more detail we analyzed its effect on the susceptibility of membrane cholesterol to oxidative damage (Figure7). Apparently, quercetin treatment reduced the level of cholesterol molecules in the membrane monolayer that are susceptible to oxidation. Considering the opposite effects of quercetin on the level of the two major lipid components of raft domains, SM and cholesterol ( and ), we manipulated the SM content in fibroblast membranes using exogenous SMase, and analyzed the effect of SM reduction on cholesterol susceptibility to oxidation (). Interestingly, partial membrane depletion of SM (by 28%) resulted in elevation of cholesterol oxidation in quercetin-treated cells, implying that SM played an additional protective antioxidant role toward cholesterol molecules.
Figure 6. Alterations in the cholesterol/total phospholipids molar ratio (mmol/mol) in plasma membranes from control (white bars) and quercetin-treated (black bars) 3D fibroblasts. Results are means ± SD of three separate experiments. The observed differences were statistically significant (p < 0.001).
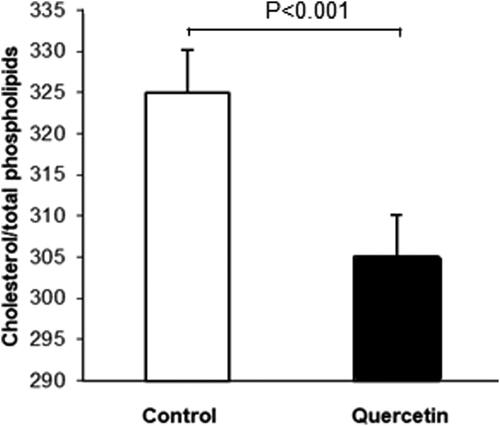
Figure 7. Susceptibility to oxidation of cholesterol (CH) in control (white bars) and quercetin-treated (black bars) 3D fibroblasts before and after treatment with exogenous sphingomyelinase (SMase). The differences between the corresponding control and quercetin-treated cells before (p < 0.001) and after (p < 0.01) incubation with SMase were statistically significant. TCH-total cholesterol.
Finally, since most information about the antioxidant effect of quercetin is obtained using conventional 2D cell cultures, we tried to confirm the antioxidant role of quercetin in 3D cells. For this purpose we measured the level of lipid peroxides and GSH before and after quercetin treatment. As expected, we observed a decrease in lipid peroxides determined by fluorescence of parinaric acid, and a significant increase in GSH in comparison with untreated cells ().
Table 3. Levels of LPO and GSH in control and quercetin-treated 3D fibroblasts.
Discussion
Quercetin is an effective free radical scavenging antioxidant that protects the lipid molecules against oxidative attack. Despite the numerous investigations devoted to the various effects of quercetin, the fine molecular mechanisms underlying its action on cells and membranes still need to be clarified. In this work we used as experimental model quercetin-treated three-dimensional tissue-like fibroblast cultures, which mimic closely the structure and functions of native tissues [Citation12]. On this model we tried to reveal the biochemical basis of quercetin impact on cellular membranes, presuming that the obtained results would be more relevant to in vivo-like conditions than ones obtained on monolayer cells.
First of all we investigated the effect of quercetin on the lipid composition of the analyzed 3D cells. The btained results demonstrated an increase in the choline-containing phospholipids, PC and SM, and a decrease in LPC, PS and PE in plasma membranes of 3D fibroblasts after quercetin treatment (). To shed light on the biochemical mechanisms that induce SM increase, we investigated the activity of membrane-bound sphingomyelinase, which is responsible for maintenance of the membrane level of SM, and also for accumulation of ceramide, the latter acting as a pro-apoptotic agent in cells. As evident from , SMase activity was decreased in quercetin-treated 3D fibroblasts, whereas the observed change in SM synthase was not statistically significant. These findings imply that quercetin-induced elevation of SM content was most probably due to its reduced degradation by SMase. In addition, the membrane level of sphingosine-1-phosphate showed a trend for augmentation. Sphingosine-1-phosphate elevation is basically associated with lower pro-apoptotic potential of cells, which results in higher survival ability, and this can be one significant beneficial effect of quercetin treatment. This finding could be related to the reported protective effect of quercetin in combined treatment of tumor cells with anti-cancer agents, since quercetin is presumed to reduce the cytotoxicity of antitumor therapy toward healthy cells [Citation26]. What is more, immunoblot analysis confirmed the presumption that quercetin treatment was accompanied by elevated phosphorylation of AKT and Bcl-2 expression, thus supporting the presumption for suppressed apoptosis ().
Phospholipase A2 activity, which is responsible for phospholipid deacylation and accumulation of lysophospholipids, was measured, in order to reveal the mechanisms underlying the observed changes in the level of the major membrane phospholipid, PC. PLA2 is closely related to regulation of inflammatory processes in cells, since the main fatty acid product of its activity, arachidonic acid, is known to act as precursor of pro-inflammatory intermediates such as thromboxanes, leukotrienes and eicosanoids [Citation27]. We observed a decrease in PLA2 activity after quercetin treatment of 3D fibroblasts (). There are reports providing evidence for an increase in cellular reactive oxygen species (ROS) after activation of PLA2, and treatment with antioxidants represses PLA2 [Citation28]. Augmentation of ROS is presumed to be due to stimulated hydrolysis of membrane phospholipids and higher oxidative destruction of arachidonic acid [Citation29]. In addition, the metabolism of the other product of PLA2, LPC, induces oxidative stress in senescence via the 5-lipooxigenase pathway [Citation30]. Thus, PLA2 seems to play a significant functional role in the regulation of oxidative stress. It could be supposed that one of the possible mechanisms of quercetin effect that contributes for improvement of cellular physiology is by inhibiting the activity of membrane-bound PLA2. Suppression of this enzyme would result in reduction of the lysophospholipid level which is known to act as detergent that destroys the bilayer structure. There are different explanations of the inhibitory effect of quercetin on PLA2 activity: via modulatory effects on membrane structural organization [Citation31] or by modification of the enzyme secondary structure [Citation32], but the exact mechanisms underlying these interactions are still unclear.
Quercetin treatment of 3D fibroblasts induced alterations of almost all fatty acid species and especially of the saturated acyl chains of the phospholipid molecules in the cellular plasma membranes (). Our results do not confirm the results of Brusselmans et al. [Citation33] who reported an inhibitory effect of flavonoids on fatty acid synthase activity. In the present studies we observed elevation of the saturated C:14, C16:0 and C18:0, and reduction of all unsaturated acyl chains except the monounsaturated C18:1. It is possible that quercetin treatment leads to an increase in the synthesis of saturated fatty acids, whereas the elongation and desaturation of the saturated fatty acids could be slowed down or inhibited. These processes can be considered as protective quercetin-induced mechanisms that are aimed at reduction of the relative content of unsaturated chains, the latter being highly susceptible to oxidative damage. Since we observed a decrease in the content of almost all unsaturated fatty acids except C18:1, it may be suggested that this is a compensatory mechanism for maintenance of the balance between saturated and unsaturated acyl chains. Moreover, the relative increase in monounsaturated fatty acids is more favorable in view of antioxidant protection, because monounsaturated fatty acids are far less susceptible to oxidative damage compared to polyunsaturated acyl chains.
The present studies showed that quercetin treatment reduced the cholesterol/phospholipid molar ratio in plasma membranes of 3D fibroblasts (). This result is in accordance with the reports of other authors that quercetin stimulates cholesterol efflux from cells [Citation34], and inhibits cholesterol uptake [Citation35]. Based on the data showing the alterations of the membrane level of cholesterol, we analyzed the changes in its susceptibility to oxidation induced by the antioxidant quercetin. The observation that quercetin reduced the susceptibility to oxidation of membrane cholesterol () was not unexpected. However, the interesting finding was that reduction of membrane SM made cholesterol more susceptible to oxidative damage despite the quercetin treatment, implying that SM was an essential factor in the protection of cholesterol molecules from oxidation. This observation can be explained with the function of SM as endogenous membrane antioxidant [Citation36]. In a previous paper we reported a hypothesis that flavonoids interact with SM at membrane level [Citation37]. There is evidence suggesting that quercetin enters the plasma membrane via the raft domains, which contain predominantly SM and cholesterol. It is possible that SM reduction through degradation by exogenous SMase leads to accumulation of free cholesterol molecules that are not tightly associated with SM. The high affinity between SM and cholesterol underlies the formation and stability of raft domains in cellular membranes. Thus, the results showed that a decrease in membrane SM leads to accumulation of higher content of oxidatively damaged cholesterol molecules despite the treatment with a potent antioxidant like quercetin.
In order to assess the antioxidant effect of quercetin in 3D cells, we measured the level of membrane lipid peroxides as a parameter indicative of the degree of oxidative stress in cells. This was done because there is also evidence that quercetin can act as a pro-oxidant under definite circumstances as well [Citation38]. As expected, we observed a decrease in the amount of lipid peroxides after quercetin treatment (), which illustrates the protective role of this flavonoid on the membrane structure and functions in 3D cells. It is well known that one mechanism of quercetin action is via the non-enzymatic antioxidant GSH, which is in accordance with our observation that GSH level was augmented after quercetin treatment (). These results are in agreement with other reports showing elevation of GSH as a result of quercetin treatment [Citation39]. The increased GSH level protects the cellular components against oxidative damage by providing reducing equivalents to the enzymes of the antioxidant system of cells by scavenging hydroxyl radicals. Accordingly, there is evidence that quercetin stimulates the formation of GSH in rabbit red blood cells [Citation40].
Conclusions
The presented results showed that quercetin treatment of 3D fibroblasts induced elevation of the membrane level of SM, which acts as endogenous antioxidant and decreases the susceptibility of membrane cholesterol to oxidative damage. Quercetin also suppressed apoptotic processes in healthy, non-tumor cells, cultured in in vivo-like conditions. In addition, quercetin induced reduction of the polyunsaturated fatty acids and augmentation of the saturated ones, which can be considered as a protective mechanism against oxidative damage of the membrane lipids. The observed beneficial effects of quercetin imply that it could be involved in complex therapies for reduction of cellular oxidative stress. We also suggest that quercetin can be applied in combined anti-tumor treatment as an agent which protects predominantly healthy cells that are generally affected by the therapy side effects. In addition, the experimental model used in the present studies is closer to native tissues than the conventional monolayer cell cultures, which makes the obtained results more relevant to in vivo conditions.
Authors’ contribution
AM and RP conceptualized and designed the study; SP, GS and AP developed methodology; RS, GG, TM and DP acquired data and performed analysis; AM, RP and AP wrote and revised the manuscript; AM and RP acquired funding
| Abbreviations | ||
| 3D cells | = | cells cultured in three-dimensional conditions |
| SM | = | sphingomyelin |
| PC | = | phosphatidylcholine; |
| PE | = | phosphatidylethanolamine |
| PS | = | phosphatidylserine |
| LPC | = | lysophosphatidylcholine; SMase: sphingomyelinase |
| PLA2 | = | phospholipase A2 |
| PNA | = | parinaric acid |
| LPO | = | : lipid peroxides |
| GSH | = | reduced glutathione |
Disclosure statement
The authors declare no competing interests.
Data availability
All data that support the findings reported in this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Saito K. Possible site of flavonoid synthesis in the photosynthetic apparatus. Biochem J. 1974;144(2):431–432.
- Filip R, López P, Giberti G, et al. Phenolic compounds in seven South American Ilex species. Fitoterapia. 2001;72(7):774–778.
- Gugliucci A, Stahl AJ. Low density lipoprotein oxidation is inhibited by extracts of Ilex paraguariensis. Biochem Mol Biol Int. 1995;35(1):47–56.
- Viña J, Borras C, Abdelaziz KM, et al. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal. 2013;19(8):779–787.
- Packer L, Cadenas E. Oxidants and antioxidants revisited. New concepts of oxidative stress. Free Radic Res. 2007;41(9):951–952.
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585(2-3):325–337.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933–956.
- Lupanova T, Stefanova N, Petkova D, et al. Alterations in the content and physiological role of sphingomyelin in plasma membranes of cells cultured in three-dimensional matrix. Mol Cell Biochem. 2010;340(1-2):215–222.
- Cukierman E, Pankov R, Stevens DR, et al. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712.
- Stefanova N, Staneva G, Petkova D, et al. Cell culturing in a three-dimensional matrix affects the localization and properties of plasma membrane cholesterol. Cell Biol Int. 2009;33(10):1079–1086.
- Pankov R, Cukierman E, Clark K, et al. Specific β1 integrin site selectively regulates Akt/protein kinase B signaling via local activation of protein phosphatase 2A. J Biol. Chem. 2003; 278(20):18671–18681.
- Skrobanska R, Evangelatov A, Stefanova N, et al. Cell proliferation in in vivo‐like three‐dimensional cell culture is regulated by sequestration of ERK 1/2 to lipid rafts. Cell Prolif. 2014; 47(4):336–346.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1):55–63.
- Pankov R, Markovska T, Hazarosova R, et al. Cholesterol distribution in plasma membranes of beta1integrin-expressing and beta 1 integrin deficient fibroblasts. Arch Biochem Biophys. 2005;442(2):160–168.
- Bligh E, Dyer W. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917.
- Touchstone J. Thin-layer chromatographic procedures for lipid separation. J Chromatogr. 1995;671(1-2):169–195.
- Kahovcov J, Odavi R. A simple method for analysis of phospholipids separated on thin layer chromatography. J Chromatogr. 1969;40(1):90–96.
- Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res. 1964;5(4):600–608.
- Koumanov K, Momchilova A, Quinn P, et al. Ceramides increase the activity of secretory phospholipase A2 and alter its fatty acid specificity. Biochem J. 2002;363(1):45–51.
- Tsujishita Y, Asaoka Y, Nishizuka Y. Regulation of phospholipase A2 in human leukemia cell lines: its implication for intracellular signaling. Proc Natl Acad Sci USA. 1994;91(14):6274–6278.
- Carini M, Aldini G, Piccone M, et al. Fluorescent probes as markers of oxidative stress in keratinocyte cell lines following UVB exposure. Farmaco. 2000;55(8):526–534.
- Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77.
- Moore N, Patzer E, Barenholz Y, et al. Effect of phospholipase C and cholesterol oxidase on membrane integrity, microviscosity and infectivity of vesicular stomatitis virus. Biochemistry. 1977;16(21):4708–4715.
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Pugazhenthi S, Nesterova A, Sable C, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol. Chem. 2000;275(15):10761–10766.
- Brito AF, Ribeiro M, Abrantes AM, et al. Quercetin in cancer treatment, alone or in combination with conventional therapeutics?Curr Med Chem. 2015;22(26):3025–3039. 10.2174/0929867322666150812145435
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488(1-2):1–19.
- Kim HJ, Kim KS, Kim SH, et al. Induction of cellular senescence by secretory phospholipase A2 in human dermal fibroblasts through an ROS-mediated p53 pathway. J Gerontol A Biol Sci Med Sci. 2009;64(3):351–362.
- Muralikrishna AR, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40(3):376–387.
- Zou Y, Kim DH, Jung KJ, et al. Lysophosphatidylcholine enhances oxidative stress via the 5-lipoxygenase pathway in rat aorta during aging. Rejuvenation Res. 2009; 12(1):15–24.
- Chiou YL, Lin SR, Hu WP, et al. Quercetin modulates activities of Taiwan cobra phospholipase A2 via its effects on membrane structure and membrane-bound mode of phospholipase A2. J Biosci. 2012;37(2):277–287.
- Cotrim CA, de Oliveira SC, Diz Filho EB, et al. Quercetin as an inhibitor of snake venom secretory phospholipase A2. Chem Biol Interact. 2011;189(1-2):9–16.
- Brusselmans K, Vrolix R, Verhoeven G, et al. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280(7):5636–5645.
- Chang YC, Lee TS, Chiang AN. Quercetin enhances ABCA1 expression and cholesterol efflux through a p38-dependent pathway in macrophages. J Lipid Res. 2012; 53(9):1840–1850.
- Nekohashi M, Ogawa M, Ogihara T, et al. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter Niemann-pick c1-like 1 in caco-2 cells and rats. PLoS One. 2014; 9(5):e97901.
- Sargis RM, Subbaiah PV. Protection of membrane cholesterol by sphingomyelin against free radical-mediated oxidation. Free Radic Biol Med. 2006; 40(12):2092–2102.
- Momchilova A, Petkova D, Staneva G, et al. Resveratrol alters the lipid composition, metabolism and peroxide level in senescent rat hepatocytes. Chem Biol Interact. 2014;207:74–80.
- Prabu SM, Shagirtha K, Renugadevi J. Amelioration of cadmium-induced oxidative stress, impairment in lipids and plasma lipoproteins by the combined treatment with quercetin and α-tocopherol in rats. J Food Sci. 2010;75(7):132–140.
- Surapaneni KM, Jainu M. Comparative effect of pioglitazone, quercetin and hydroxy citric acid on the status of lipid peroxidation and antioxidants in experimental non-alcoholic steatohepatitis. J Physiol Pharmacol. PMID:24622831. 2014;65(1):67–74.
- Jarosiewicz M, Krokosz A, Marczak A, et al. Changes in the activities of antioxidant enzymes and reduced glutathione level in human erythrocytes exposed to selected brominated flame retardants. Chemosphere. 2019;227:93–99.
