 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The evaluation of climate plasticity and content of free amino acids, sugars and fatty acids in Bulgarian soybean cultivars were used as an innovative approach. The field performance, expression and metabolomic profiles of leaves, green seeds and mature seeds of plants grown from low temperature pre-treated and not-treated seeds were assessed by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) and gas chromatography/mass spectrometry (GC/MS). The data from the morphological indicators, expression profiles and content of metabolites confirmed that the plants grown from low temperature pre-treated seeds had better performance. The expression profiles correlated with the content of amino acids and sugars. Inclusion of extruded full-fat soybean from cvs. Srebrina and Richy in the compound feed for pigs and soybean cake obtained after cold pressed soybeans of cv. Srebrina in compound feed for laying hens were investigated. The results confirmed that extruded full-fat soybean cv. Srebrina could be successfully included in the protein component of feed for growing pigs at a rate of 50% of soybean meal in protein equivalent. Extruded full-fat soybean cv. Richy in the amount of 30% of the protein component of the ration, in protein equivalent of the compound feed for the period of fattening of pigs could be included. The results confirmed that feed for laying hens, in which 50% of imported soybean meal is replaced by soybean cake produced from locally grown soybean, could increase the content of free amino and fatty acids of the final product and benefit the quality of eggs.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.1954092 .
Introduction
More than 70% of the human diet is based on seeds mainly from cereals and legumes. Cereal seeds (corn and wheat) are a major source of starch, but in terms of protein stores, their seeds accumulate low amounts of protein (about 16%) [Citation1]. Legumes are widespread throughout the world, providing more than 69% of the protein as well as 30% of the fats/oils needed for the human diet [Citation2]. Pea (Pisum sativum L.), soybean (Glycine max L.), common bean (Phaseolus vulgaris L.) and bean (Vicia faba L.) are the main sources of protein, with a content of 20–40% depending on the genotype and environment. No less important for the widespread use of these crops is the fact that legumes require minimal amounts of soil improvers (fertilizers), because they possess the unique ability to absorb nitrogen from the air through a symbiotic interaction with nitrogen-fixing soil bacteria from genera Rhizobium and Bradyrhizobium. This determines them as one of the global sources of nutrition for the human population, providing sustainable friendly agriculture [Citation3].
Soybean is a crop that is grown to produce cheap and high quality vegetable protein. It contains 3–4 times more protein and essential amino acids than some basic crops such as wheat, corn and barley and 1.5–2 times more protein than other grain legumes. It is the cheapest source of vegetable protein and provides two-thirds of the world’s consumption of protein meals and one-third of the consumption of oils and fats. Therefore, modern soybean production requires new soybean varieties, in addition to the formation of high and stable yields, to have a set of economic qualities, among which the content of crude protein and crude oil in the grain are the two components of greatest economic value. Soybean possesses a high nutritional value and an important role in animal and human nutrition. Soy proteins are rich in essential amino acids, necessary for the construction of the protein in animals and humans. Soybean meal, after oil extraction, is an important animal feed, since in addition to the high percentage of protein (about 43%), it also contains a large amount of essential amino acids lysine, tryptophan and methionine. Moreover, soybean contains vitamin B complex, beta carotene and is rich in minerals, mainly calcium, iron and potassium. In our days, soy has been used as an important source of phytoestrogens (genistein) and isoflavones [Citation4]. Growing soybeans is not very difficult, rather growers are not well informed or do not follow the proper technology. Soybeans are an important part of a crop rotation due to the favourable nitrogen balance and plant residues which it accumulates into the soil. Its proper inclusion in crop rotation with cereals, etc. greatly contributes to long-term sustainability and increased profitability of agricultural production. Soybean seeds and crops do not need to be treated with fungicides, which defines them as more environmentally friendly than other crops. Soybeans are also suitable for organic production, as they are easily included in various organic farming schemes [Citation5–7].
The climatic conditions for soybean cultivation in Europe are not among the most suitable. On the other hand, the imports of soybeans into the European Union (EU) are not subject to customs duties, although it is noteworthy that a significant part of soybeans grown outside the EU is genetically modified (GMO). All these are important reasons to stimulate the soybean production in the EU.
The European Commission (EC) has adopted a report on the development of plant protein crops in the EU. The Commission has decided to use various instruments to stimulate farmers growing protein crops in the new programming period of the Common Agricultural Policy (CAP) and to support farmers growing legumes, as a source of vegetable protein. The integration of legumes in national strategic plans of CAP (promoting the benefits of legumes for agro-ecosystem and climate stability, environmental and climate management commitments under the Rural Development Program; mobilizing support for rural development to stimulate investment and cooperation in the food chain linked to production and income support) is the priority of all Member States [Citation8].
The climate in Bulgaria could be described as moderately continental, different from other parts of Europe. The main factor limiting soybean production in Bulgaria is the summer drought, which could reduce the yield to 40%.
Ongoing research is related to programs for development of cultivars adapted to the specific agro-climatic environment. Bulgarian soybean cultivars are adapted to the soil and climatic conditions of the country and in combination with optimal technology, are a prerequisite for good production results. The imposition of Bulgarian cultivars through the production of high quality certified seeds could be defined as a sustainable approach in agriculture [Citation9]. The current worldwide soybean research aims to study cellular processes, their genetic control and interactions with changes in the environment. Such multiple and focussed research requires large-scale experiments involving whole genetic, structural or functional components. These complex studies are called ‘omics’ and include genomics, transcriptomics, proteomics and metabolomics. These ‘omics’ approaches are routinely used in various research groups working with crop plants, including soybeans. The application of ‘omics’ approaches have moved very fast in the last two decades. The data accumulated from the performed ‘omics’ experiments require extensive computational resources for storage and analysis. As a result, online databases exclusively developed for soybean research data generated from different omics platforms have been developed (Soybean Knowledge Base, http://soykb.org/; Soybean transcription factors database http://casp.rnet.missouri.edu/soydb/; soybean metabolome database http://soymetdb.org) [Citation10]. Worldwide research investigations are aimed at increasing the yield and quality of soybean vegetable protein. The published studies concern soybean varieties developed in other countries according to the specific climatic requirements and needs of agriculture [Citation5,Citation11].
However, the developed Bulgarian soybean cultivars (Avigea, Richy, Rosa and Srebrina) and experimental lines have never been studied at the molecular level, and expression profiles are not available for key genes involved in plant development and synthesis of valuable amino acids. There is also a lack of information on the quality of vegetable protein and the content of essential amino acids determined by modern methods. The presence of essential amino acids in plant protein, such as sufficient lysine in feed, is a problem in Bulgaria due to the use of cheap but low-lysine sources, such as sunflower meal. In this sense, soybean cake and extruded soybeans, products derived from Bulgarian soybean varieties, are essential animal feed, but still unexplored in terms of total protein and the amount of essential amino acids (lysine, tryptophan and methionine, threonine, leucine, valine, isoleucine). The applied approach of pre-treatment of seeds with low temperature before sowing has never been investigated in Bulgarian soybean cvs. Ten years ago Vieira et al. [Citation12] applied cold test for soybean seeds as an oldest and most used seeds vigour test, for a period of 5 days at a temperature of 10 °C. In our study, we presented results for long-lasting 22 days low temperature pre-treatment/2–5 °C/related to plant field vigour, quantitative and qualitative composition of metabolites related to the quality of seeds collected from plants grown from pre-treated seeds.
The present investigation is developed on the basis of soybean cultivars created in Soybean Experimental Station, Pavlikeni, in the last 20 years, as well as technologies for soybean cultivation in soil and climatic conditions of Bulgaria [Citation13,Citation14].
The present study focussed on the evaluation of climate plasticity and qualification of Bulgarian soybean varieties, their inclusion in the composition of feed and assessment of final food product. We evaluated the field performance, expression and metabolomic profiles with seeds pre-treated with low temperature and non-treated of two Bulgarian soybean cultivars, Richy and Avigea, compared to French standard Izidor. A correlation between expression profiles and detected content of amino acids and sugars was determined. Assessments of the quality of feed for pigs and laying hens, after soybean inclusion and amino acids and fatty acids composition in eggs as a final product were done and discussed.
Materials and methods
Plant material and growing conditions
Pavlikeni is situated in North-Central Bulgaria. The climate zone belongs to moderate continental climate with maximum rainfall in May–June and severe drought from July to September. The soil type is leached chernozem and possesses good water holding capacity. The humus horizon is about 40–50 cm. The soil (pH) is neutral with humus content of 3–4%; soil porosity is 47% and quench humidity is 15.2% [Citation15]. The experimental work was performed with Bulgarian soybean cultivars with commercial value. Three cultivars belonging to maturity group I – Avigea, Richy and Srebrina and French cultivar Izidor used as a standard, were included in the experimental work. The field experiments were conducted during two consecutive years, 2019 and 2020, through the method of long plots without irrigation and chemical fertilization in the experimental field of Soybean Experimental Station, Pavlikeni. The plots were organized in rows, with row spacing of 70 cm and sowing rate of 40,000 seeds/decare. The seeds were sown after 25th April of each year.
In the experiments biometric measurements were made of the following indicators: plant height (cm), number of branches, number of pods, number seeds per plant, mass of seeds per plant, mass of 100 seeds.
Seeds pre-treatment
The field experiments were performed with pre-treated and non-treated seeds. For the pre-treated seeds the following conditions were applied: seeds of the cultivars Avigea, Ritchy and French standard Isidor were pretreated with low temperature in controlled conditions before sowing. Treatment scheme: For all seeds: 2 days at 2 °C; 10 days at 4 °C; after this period of treatment, the so-called ‘treated control’ were transferred in a controlled chamber with day temperatures of 16 °C and night temperatures of 8 °C with a photoperiod of 16 h day and 8 h night for a period of 10 days. The seeds marked as ‘treated’ were incubated for a further period of 10 days at 5 °C. After pretreatment the seeds and seedlings were transferred to the experimental field in Pavlikeni and they were planted together with the ‘non-treated’ group for the field experiment described above.
Meteorological conditions during the two investigated years
The two experimental years were characterized with different rainfall regimes in the vegetative and reproductive phases of crop development. In the first experimental year, 2019, during the flowering phase the monthly precipitation in June was 149.0 mm, and the distribution of precipitation over 10 days was also even. In the second year of the experiment, 2020, there was drought; the stage of pods development took place in the conditions of severe drought with precipitation amounts of 6.4 mm in July and 36.4 mm in August. The monthly precipitations for the two consecutive years are presented in .
Table 1. Monthly precipitation (mm) and total rainfall amount (mm) during the experimental period.
Expression analysis
For the expression analyses we selected genes highly expressed in the stage of development of soybean R5–R7 (according to published transcriptomics data (www.soybean.org). For the first set of expression analyses, we selected five genes related to plant development, signal transduction and abiotic stress: Glyma05G16960-F-box/RNI-like superfamily protein; Glyma04G138900, which encodes SCL3 protein-GRAS family protein; Glyma05G140400-Della family protein, repressor of gibberellic acid responses and involved in GA mediated signalling. GA inhibitor involved in reducing reactive oxygen species (ROS) accumulation in response to stress by up-regulating the transcription of superoxide dismutases; Glyma05G185500, which encodes alkaline/neutral Invertase involved in sucrose catabolic process; Glyma13G162800, which encodes a H(+)-translocating (pyrophosphate-energized) inorganic pyrophosphatase (H(+)-PPase) located in the vacuolar membrane.
The second group of selected genes for transcriptomic analyses was related to amino acids transporters. According to [Citation16], the amino acids transporters were divided into several groups. For the present study we selected transporters from four groups: ANT group (aromatic and neutral AA transportes): Glyma19G39060, which encodes an amino acid transporter that transports aromatic and neutral amino acids, IAA, and 2,4-D; LHT group (Lysine/Histidine transporters): Glyma13G245000, which encodes transmembrane amino acid transporter, orthologue of AT1G47670, Lys/His transporter 7; ProT group (proline transporters): Glyma18G031300-orthologue AT2G39890, which encodes proline transporter 1 and GAT group: Glyma.13G319700 orthologue AT1G08230, which encodes high affinity gamma-aminobutyric acid (GABA) transporter.
The leaves and green seeds (GS) were collected from the field at stage R5–R7 and mature seeds (MS) were used in further gene expression experiments. Total RNA was extracted from the respective tissues with the RNA Plant Kit (Eurex). The required amount of total RNA was reverse transcribed with the First Strand cDNA Synthesis Kit (Bio-Rad). Relative expression levels were determined with the 7300 Real-Time qPCR System (Applied Biosystems, http://www.appliedbiosystems.com). The qRT-PCR was carried out in a total volume of 20 µL containing 5 µL of cDNA, 0.5 µL gene specific primers (10 µmol/L), 10 µL SYBR Green Mix (Eurex) and 4 µL of RNase free ddH2O. The PCR conditions were as follows: 95 °C for 5 min, followed by 60 cycles of 95 °C for 15 s and 60 °C for 30 s. Two different reference genes (ACTIN and UBIQUITIN10) were used for normalization.
Metabolomic analyses
The polar (amino and organic acids, carbohydrates) and non-polar metabolites (saturated and unsaturated fatty acids, sterols) were determined by gas chromatography–mass spectrometry (GC–MS) analysis in green and mature soybean seeds, extruded full-fat soybean meal, soybean meal, concentrated feeds and eggs.
Briefly, 50 mg lyophilized material from each samples was subjected to the following procedure: 500 µL methanol, 50 µL ribitol and 50 µL n-nonadecanoic acid (internal standards, mg/mL for polar and non-polar metabolites, respectively) were added, then the mixture was heated on a thermoshaker (Analytik Jena AG, Germany) 30 min/70 °C/300 rpm.
After cooling down to room temperature, 300 µL chloroform and 100 µL water were added, then the mixture was centrifuged (5 min/22 °C/13,000 rpm, Beckman Coulter). The lower phase was designed for the analysis of non-polar substances, and the upper phase was for study of the polar metabolites. The two phases obtained were vacuum-dried in a centrifugal vacuum concentrator (Labconco Centrivap) at 40 °C.
To the dried residue of fraction ‘non-polar metabolites’, 1.0 mL 2% H2SO4 in methanol were added and the mixture was heated on a Thermo-Shaker TS-100 (1 h/96 °C/300 rpm). After cooling, the solution was extracted with n-hexane (3 × 10 mL). Combined organic layers were vacuum-dried in a centrifugal vacuum concentrator (Labconco Centrivap) at 40 °C.
Prior to analysis by GC–MS, fractions ‘A’, ‘B’ and ‘C’ were derivatized by the following two procedures:
First, 300 µL solution of methoxyamine hydrochloride (20 mg/mL in pyridine) was added to a fraction ‘polar metabolites’, and the mixture was heated on Thermo-Shaker TS-100 (1 h/70 °C/300 rpm). After cooling, 100 µL N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) were added to the mixture, then heated on Thermoshaker, Analytik Jena AG, Germany (40 min/70 °C/300 rpm). Then, 1 µL from the solution was injected in the GC–MS.
Second, 100 µL pyridine and 100 µL N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) were added to a fraction ‘non-polar metabolites’, then heated on Thermoshaker, Analytik Jena AG, Germany (45 min/70 °C/300 rpm). Then, 1 µL from the solution was injected in the GC–MS.
GC–MS analysis was carried out on a 7890 A gas chromatograph interfaced with a 5975C mass selective detector (Agilent). Separations were performed using a 30 m × 0.25 mm (i.d.) HP-5 ms silica-fused capillary column coated with 0.25 µm film of poly (dimethylsiloxane) as the stationary phase (Agilent). The flow rate of the carrier gas (helium) was maintained at 1.2 mL/min. The injector and the transfer line temperature were kept at 250 °C. The temperature of the MS source was 230 °C. The oven temperature program used was 100 °C for 2 min then 15 °C/min to 180 °C for 2 min then 5 °C/min to 300 °C for 10 min, run time 42 min. The injections were carried out in a splitless mode. The mass spectrometer was scanned from 50 to 550 m/z. The injection volume was 1 µL.
A mixture of aliphatic hydrocarbons (C10–C40) (Sigma) was directly injected under the above temperature program in order to calculate the relative retention indices (as Kovàts index, RI) of each compound. The identification of free amino acids was obtained by comparing the retention times and RI with those of authentic compounds and the spectral data obtained from The Golm Metabolome Database-GMD [Citation17] and National Institute of Standards and Technology (NIST 08) libraries [Citation18].
Pig feeding with compound feed with included extruded full-fat soybean
The experiments for fattening of pigs were conducted at the Agricultural Institute – Shumen. In the first experiment, animals of the Danube White breed were used, and in the second, crosses of the Danube White (DB) × Pietren were used. The first experiment was performed with three groups (I control, II and III experimental) of 15 (eight male castrated and seven female) pigs per group, or a total of 45 pigs. The experiment started at 25.714–26.267 kg and ended at 94.571–102.133 kg. In the second experiment, two groups were set: I control and II experimental, 13 pigs (six male castrated and seven female) in a group or a total of 26 pigs. The second experiment started at 32.231 kg and ended at 103.8–105.3 kg live weight. The pigs in the groups were matched by origin, live weight, age and number. Both experiments were performed in two subperiods – the first from the weanling pigs 20 to 60 kg live weight and the second from 60 kg to the end of fattening. The nutrient quality of soybean meal, and extruded full-fat soybean from cvs. Richy and Srebrina was assessed in certified laboratory Alimenti (D & V Consult Ltd.)
The data on the feed composition and the energy and nutrient content in kg of compound feed are shown in . In , the compound feed of group II 50% of the soybean meal protein was replaced by extruded full-fat soybean from cv. Srebrina, and in group III, 75% of soybean meal was replaced by protein equivalent with extruded full-fat soybean. In the second experiment (), extruded full-fat soybean of cv. Richie was used. In the experimental group, part of the soybean meal (30% by protein equivalent) was replaced by extruded soybean. At the end of I subperiod at 60 kg live weight, blood samples were taken from the corner of the eye of animals in both experiments, to determine the values of malondialdehyde in blood plasma. This indicator was studied as a marker of oxidative stress.
Table 2. Accepted, feed consumption and average daily growth (I, II subperiod and whole experimental period) – experiment one with extruded full-fat soybean, cv. Srebrina.
The pigs were grown in individual floor boxes. They were fed ad libitum and received water from nipple drinkers.
Lipid peroxidation (MDA) assay
Lipid peroxidation is the degradation of lipids that occurs as a result of oxidative damage and is a useful marker for oxidative stress. Polyunsaturated lipids are susceptible to oxidative attack, typically by ROS, resulting in the production of end products such as malondialdehyde (MDA). Lipid peroxidation is determined by the reaction of MDA with thiobarbituric acid (TBA) to form a colorimetric (532 nm)/fluorometric product, proportional to the MDA present. For the experiment, a lipid peroxidation MDA assay kit (Sigma-Aldrich) was used and the samples and standards were prepared according to the instruction of the supplier.
Combined feed for laying hens
The recipe of the main component for feed for laying hens of producer 1 is described below, but the components and analytical composition are different in each egg producer. Compound feed samples and egg samples were taken from three egg producers and used further in the performed analyses. The feed samples were assessed in the certified laboratory Alimenti (D & V Consult Ltd.), based on five indicators.
Feed: Corn, wheat, soybean meal, sunflower meal, calcium carbonate, dicalcium phosphate, unrefined oil, vitamin–mineral premix, salt, lysine, methionine, enzyme/physimus/. Vitamin A 12000 U/kg, Vit. D 4000 U/kg; copper in the form of copper sulphate 20 mg/kg, zinc in the form of ‘zinc oxide 30’ 20 mg/kg lysine 0.90%; methionine 0.41%. Analytical composition: crude protein 17.09%, crude fat 0.75%, crude fibre 5.14%, calcium 3.76%, phosphorus 0.59%, crude ash 3.97%, exchange energy 2819.36 kcal/kg.
Statistical analyses
Data represent the mean ± SE or SD and in average were analyzed with n ≥ 3. The one-way analysis of variance (ANOVA) (Holm–Sidak) and Student (t-test) statistical tests were applied to estimate the difference between all the variants (differences were considered statistically significant at the p < .05 level).
Results and discussion
Evaluation of field performance according to six indicators of plants grown from seeds with or without low temperature pre-treatment during a two years’ experiment
The results from the morphological evaluation in the first experimental year are summarized in . The comparison of results based on selected indicators illustrates that the plants grown from the treated seeds ‘treated control’ and ‘treated’ showed higher values of the studied indicators. This difference was significant (p < .05; p < .01; p < .001) in terms of the number of pods, the number of seeds per plant and the mass of seeds per plant, and was most clearly observed for the cultivar Avigea.
Figure 1. Comparative analysis of six indicators of plants grown from seeds pre-treated with low temperature – ‘treated’, ‘treated control’ and ‘non-treated’ – in the first experimental year, 2019. Data represent the mean ± SD. The one-way ANOVA (Holm–Sidak) statistical test was applied to assess the difference between all the variants. Asterisks denote statistically significant differences of treated and treated control compared to ordinary *p < .05; **p < .01; ***p < .001.
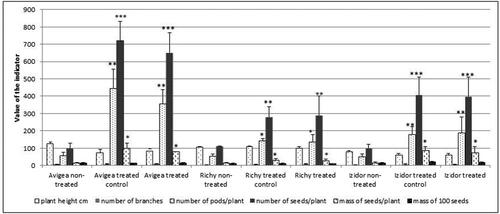
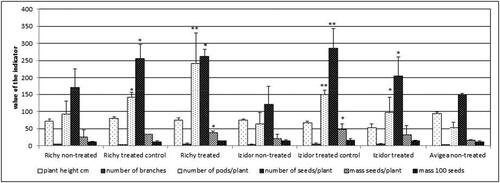
In the second year of experiment, it is important to note that pre-treated seeds ‘treated control’ and ‘treated’ from cv. Avigea were not able to germinate and produce plants. The plants were obtained only from ‘non-treated’ seeds. The second experimental year/2020/was more severe in respect of summer drought and the values of the selected indicators were lower than those in the first experimental year. The comparison of the results based on the selected indicators illustrates that the plants grown from the treated seeds, ‘treated control’ and ‘treated’, showed higher values of the studied indicators in comparison to ‘non-treated’. The difference was significant (p < .05; p < .01) in terms of the number of pods, the number of seeds per plant, the mass of seeds per plant, and was observed for both cultivars ().
Figure 2. Comparative analysis of six indicators of plants grown from seeds pretreated with low temperature ‘treated’, ‘treated control’ and ‘non-treated’ in the second experimental year, 2020. Data represent the mean ± SD. The one-way ANOVA (Holm–Sidak) statistical test was applied to assess the difference between all the variants. Asterisks denote statistically significant differences of treated and treated control compared to ordinary *p < .05; **p < .01.
The pre-treatment of seeds with low temperatures was conducted to test the ability of seeds to germinate in the conditions of abiotic stress/low temperature/and grow strong plants able to produce seeds of the desired quantity and quality. The optimal soil temperature for sowing soybeans on the field is about 10 °C, which determines the period of sowing of soybean in Bulgaria in the last 5–10 days of April. The low temperature pre-treatment of the seeds simulates sowing conditions at the beginning of April. Early planting of soybean at the beginning of April would allow early or mid-early varieties to pass the critical phase of flowering and pods formation in June and to prevent strong summer droughts, which limit the yield of soybean crop in Bulgaria. In the two years of our field experiment, the pre-treated seeds showed lower germination ability than the non-treated ones, but produced plants with higher vigour and performance of the parameters number of pods, number of seeds and mass of seeds per plant in all three tested cultivars. Previously published results of short cold pre-treatment of soybean seeds at 10 °C and for 5 days [Citation12], confirmed seed germination ability of 84–94% of one tested cultivar. In our case, long-lasting low temperature pre-treatment decreased the seed germination ability with 5–10% and this effect varied among the tested cultivars.
Evaluation of gene expression data in leaves, green seeds and mature seeds of plants grown from seeds with or without low temperature pre-treatment during a two years’ experiment
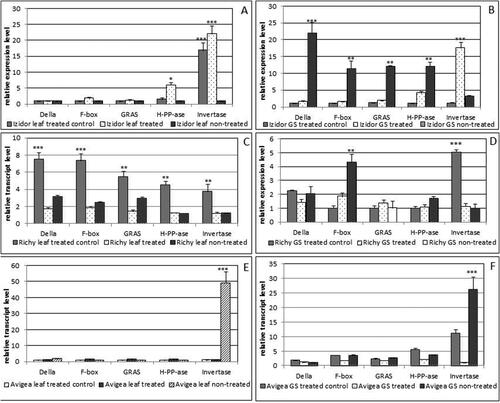
Investigation related to transcriptomics data has never been done with Bulgarian soybean cvs. In this study the results obtained from expression analyses were divided in two parts. The first set of data was related to the evaluation of the expression level of five genes involved in leaf development /F-box/, signal transduction /Della, GRAS/ and response to drought /H-PP-ase/ and catabolism of sucrose Invertase in leaves and GS seeds as a target tissue. The second set of data included four genes related with amino acids transport and the experiments were focussed on green and mature seeds. Transcriptomic data obtained from expression analyses of five selected genes in leaves and green seeds /GS/ collected from plants in two experimental years from the three cultivars, are presented in (year 2019) and Citation4 (year 2020). Five genes were selected as highly expressed during stage (R5–R7) 14–27 days after pollination. The level of expression of Invertase was high in ‘treated’ leaves and GS in cv. Izidor p < .001 () and ‘treated control’ of cv. Richy p < .01; p < .001 () compared to ‘non-treated’ but opposite in cv. Avigea (). The levels of expression of the other four genes varied among ‘treated’ and ‘non-treated’ GS of cv. Richy and were higher in ‘non-treated’ GS of cv. Izidor. In the case of cv. Avigea the expression level of genes was almost equal in ‘treated’ and ‘non-treated’ leaves and GS with the exception of significantly high expression of Invertase in ‘non-treated’ leaves and GS (p < .001).
Figure 3. Relative transcript level of the first set of sleeted genes /Della, GRAS, F-box, H-PP-ase, Invertase/ in leaves and green seeds (GS) of plants grown from ‘treated’, ‘treated control’ and ‘non-treated’ seeds of the three investigated cultivars in 2019. A, B – transcript level in leaves and GS of cv. Izidor; C, D – transcript level in leaves and GS of cv. Richy; E, F – transcript level in leaves and GS of cv. Avigea. Data represent the mean ± SE. The one-way ANOVA (Holm–Sidak) statistical test was applied to assess the difference between all the variants. Asterisks denote statistically significant differences *p < .05; **p < .01; ***p < .001.
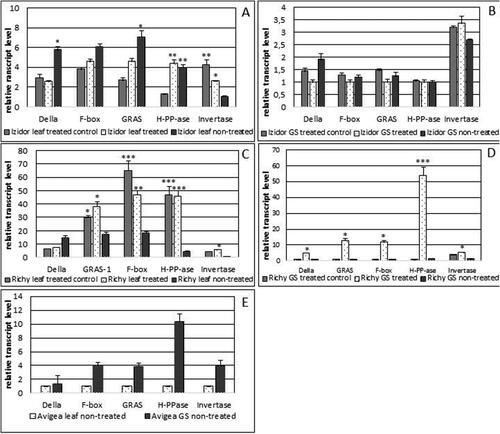
For the second year of experiment, pre-treated seeds ‘treated control’ and ‘treated’ from cv. Avigea were not able to germinate and produce plants. Plants were obtained only from ‘non-treated’ seeds. The weather conditions in the second year of the experiment /2020/ were with severe summer drought and very low precipitation in June–July–August compared to the year 2019. The expression profiles obtained for ‘treated’, ‘treated control’ and ‘non-treated’ leaves and GS for cvs. Richy and Izidor differed from the previous year and are presented in . For cv. Richy the level of Invertase in ‘treated’ and ‘treated control’ GS and leaves again was more pronounced compared to the ‘non-treated’ p < .05 (). The expression of the other four genes was more pronounced in ‘treated’ GS than in ‘treated control’ and ‘non-treated’. The expression profiles of F-box, GRAS, H-PPase in ‘treated’ and ‘treated control’ leaves were significantly higher than in ‘non-treated’ (p < .05; p < .01; p < .001) with the exception of Della gene. Similarly to cv. Richy the transcript level of Invertase in ‘treated’ and ‘treated control’ GS and leaves of cv. Izidor was higher than that in the plants from seeds that were ‘non-treated’ () and almost equal levels of expression of the other four genes were observed for ‘treated’, ‘treated control’ and ‘non-treated’ GS. The transcript level of the other three genes was more pronounced in the leaves of ‘non-treated’ plants compared with the ‘treated’ ones, with the exception of H-PPase. presents the expression profiles of selected genes in ‘non-treated’ leaves and GS of cv. Avigea. In leaves the expression level was low and nearly equal but in GS the transcript level of H-PPase was very high. The function of H-PPase is related to the response to water deprivation, response to salt stress, and establishment and maintenance of transmembrane electrochemical gradient [Citation19,Citation20]. In the condition of severe summer drought in the year 2020, the significantly elevated level of expression of H-PPase in ‘treated’ and ‘treated control’ leaves and ‘treated’ GS of cv. Richy and ‘non-treated’ GS of cv. Avigea could be explained with better adaptability of Bulgarian cultivars to the extreme environmental conditions compared to the French standard.
Figure 4. Relative transcript level of the first set of selected genes /Della, GRAS, F-box, H-PP-ase, Invertase/ in leaves and green seeds (GS) of plants grown from ‘treated’, ‘treated control’ and ‘non-treated’ seeds of the three investigated cultivars in 2020. A, B – transcript level in leaves and GS of cv. Izidor; C, D – transcript level in leaves and GS of cv. Richy; E – transcript level in leaves and GS of cv. Avigea. Data represent the mean ± SE. The one-way ANOVA (Holm–Sidak) statistical test was applied to evaluate the difference between all the variants. Asterisks denote statistically significant differences *p < .05; **p < .01; ***p < .001.
The second set of expression analyses included three selected AAs transporters, /ANT-1/-aromatic and neutral transporter1, LHT-7 transporter-lysine histidine transporter 7 and ProT-proline transporter 1; and one Υ-aminobutyric acid (GABA) GABA transporter. The transcript level of the three AAs transporters in the year 2019 was elevated in GS of ‘treated control’ /ANT-1/ and GS ‘treated’ /LHT-7, ProT-1/ especially for the cv. Richy. The expression of the GABA transporter was highly pronounced in GS ‘treated control’ and ‘treated’ cv. Avigea and cv. Richy and ‘treated control’ of cv. Izidor (, C, E)). In MS of cv. Richy the expression of all investigated transporters was highest in the plants from treated seeds. The level of expression of ANT-1 was elevated in ‘treated’ MS and LHT-7 displayed the highest expression in ‘treated control’ for cv. Izidor. In cv. Avigea the ANT-1 expression was highest in ‘non-treated’ MS, while the expression of LHT-7 was highest in ‘treated control’ (, D, F)).
Figure 5. Relative transcript level of the second set of sleeted genes /ANT-1; LHT-7; Prot-1; GABA-1/ in green seeds (GS) and mature seeds (MS) of plants grown from ‘treated’, ‘treated control’ and ‘non-treated’ seeds of the three investigated cultivars in 2019. A, B – transcript level in GS and MS of cv. Izidor; C, D – transcript level in GS and MS of cv. Richy; E, F – transcript level in GS and MS of cv. Avigea. Data represent the mean ± SE. The one-way ANOVA (Holm–Sidak) statistical test was applied to estimate the difference between all the variants. Asterisk denote statistically significant differences *p < .05; **p < .01; ***p < .001.
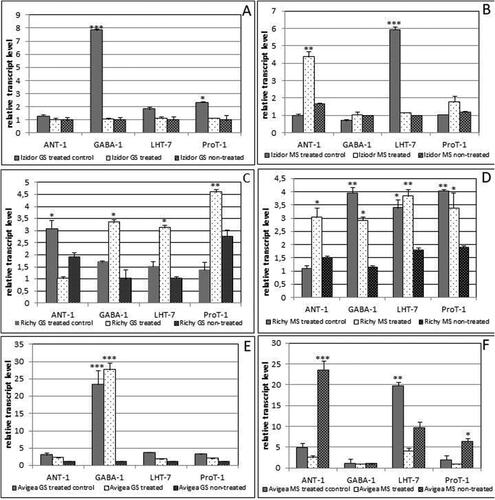
In general, in the leaves of ‘treated control’ and ‘treated’ group of cvs. Richy and Izidor and Avigea, the transcript level of ANT-1, LHT-7 and ProT-1 was significantly higher than that in the ‘non-treated’ leaves, which could be related to the significantly higher values of the morphological indicators in the field trial of 2019 in plants grown from ‘treated’ and ‘treated control’ seeds of cvs. Avigea, Richy and Izidor versus ‘non-treated’ ones (, Supplemental Figures S1, S2 and S3).
Transcription profiles of AAs transporters in the year 2020 were characterized with almost equal expression in ‘treated control’, ‘treated’ and ‘non-treated’ GS of cv. Izidor with the exception of GABA in ‘treated’ GS. The expression was significantly pronounced in MS of ‘treated control’ and ‘treated’ compared to ‘non-treated’ for all four transporters (). In cv. Richy, the expression levels of the four transporters were significantly higher in ‘treated’ GS compared to ‘treated control’ and ‘non-treated’ ones but in MS the expression level of ANT-1 was elevated 2–5 fold compared to the other three transporters where moderate expression was observed compared to ‘treated control’ and ‘non-treated’ ()).
Figure 6. Relative transcript level of the second set of sleeted genes /ANT-1; LHT-7; Prot-1; GABA-1/ in green seeds (GS) and mature seeds(MS) of plants grown from ‘treated’, ‘treated control’ and ‘non-treated’ seeds of two investigated cultivars in 2020. A, B – transcript level in GS and MS of cv. Izidor; C, D – transcript level in GS and MS of cv. Richy. Data represent the mean ± SE. The one-way ANOVA (Holm–Sidak) statistical test was applied to assess the difference between all the variants. Asterisks denote statistically significant differences *p < .05; **p < .01; ***p < .001.
The observed expression level in the leaves of ‘treated control’ and ‘treated’ of cvs. Richy and Izidor of all four investigated transporters was higher than in the ‘non-treated’ leaves, which again correspond with significantly higher values of morphological indicators in the field trial of 2020 in plants grown from ‘treated’ and ‘treated control’ seeds of cvs. Richy and Izidor compared to ‘non-treated’ ones (, Supplemental Figures S4 and S5).
Evaluation of the level of free amino acids, fatty acids and sugars in green and mature seeds of plants grown from seeds with or without low temperature pre-treatment during a two years’ experiment
The content of metabolites such as free amino acids, fatty acids and sugars has not been investigated in Bulgarian soybean cultivars. These metabolites are key to promoting the high environmental plasticity and quality of protein of Bulgarian soybean cultivars compared to the European standard. Another task in the present study was to find out if there is a correlation between the transcript profiles and the content of metabolites.
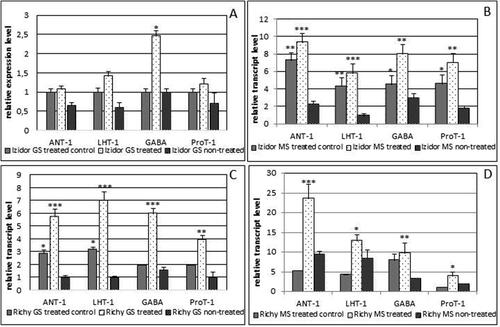
In the year 2019 from the obtained metabolite profile of ‘treated’ GS of cv. Izidor, we observed the highest content of free sucrose (10.323 mg/g dw) compared to ‘treated control’ (6.818 mg/g dw) p < .01 and ‘non-treated’ (8.571 mg/g dw) p < .05. In the expression analyses of GS in the year 2019 we found that the level of expression of Invertase gene was highly elevated in ‘treated’ GS of cv. Izidor. In cv. Richy, we observed the same correlation between gene expression and metabolite content but ‘treated control’ GS possessed high content of free sugar (9.886 mg/g dw) compared to ‘treated’ (6,427 mg/g dw) p < .01 and ‘non-treated’ (8.156 mg/g dw) p < .05. High expression level was observed in ‘treated control’ GS (, Supplemental Figure S6).
In plants, Invertases irreversibly catalyze the hydrolysis of sucrose into hexoses (glucose and fructose). Sucrose is the major end product of photosynthesis and glucose and fructose are known to be used as nutrients and energy sources, and as signalling molecules for plant growth, yield formation and stress responses [Citation21,Citation22]. The content of the assayed free fatty acids /two saturated and four unsaturated/ in GS of the three tested cultivars is presented in Supplemental Figure S7. The content of linoleic acid of ‘treated control’ of cv. Richy was significantly higher than ‘treated’ and higher than ‘non-treated’. In cv. Izidor, the content of linoleic acid in ‘treated control’ was significantly higher than ‘treated’ and ‘non-treated’.
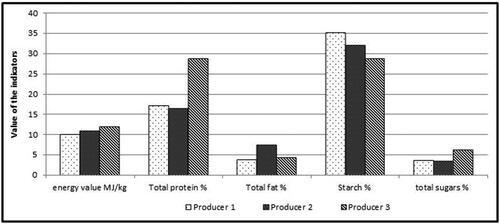
Figure 7. Comparison of feed from three egg producers based on five indicators measured in a certified laboratory.
In respect to the AA transporters, there was correlation between the observed transcript level and the content of AAs. In the GS ‘treated control’ from cv. Izidor the elevated transcript level of Prot-1 correlated to higher proline content in GS ‘treated control’ (0.647 mg/g dw)compared to ‘treated’ and ‘non-treated’, which were (0.456 mg/g dw) and (0.552 mg/g dw), respectively.
The expression of GABA transporter in GS ‘treated control’ was significantly higher /p < .001/ than in GS ‘treated’ () and the content of free GABA in GS ‘treated control’ was 2.554 mg/g dw, or 3.5 times more than this of GS ‘treated’ (0.748 mg/g dw) and ∼1.5 times more than this of ‘non-treated’ GS (1.651 mg/g dw) (Supplemental Figure S8). In plants, GABA is non-protein amino acids and it accumulates in plant tissue during biotic and abiotic stress. The role of GABA in plants is still discussed but recent studies propose its signalling role in plant development and stress condition [Citation23]. A recent report suggests that GABA could be an important intermediate of nitrogen metabolism and amino acid biosynthesis [Citation24]. This suggestion could explain the high content of free GABA in GS during the stage of seed filling and active synthesis of amino acids.
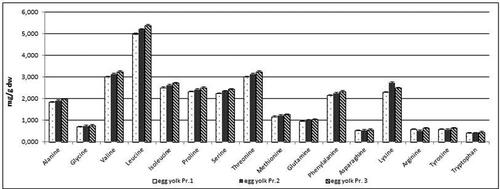
Figure 8. Free amino acids content in mg/g dw in egg yolk samples from three egg producers. Data represent the mean ± SD.
The content of free histidine in GS ‘treated control’ (2.781 mg/g dw) was significantly higher /p < 0.05/ compared to the histidine content in GS ‘treated’ (0.261 mg/g dw), and in GS ‘non-treated’ (1.521 mg/g dw), which was in accordance with the higher level of expression of LHT-7 in GS ‘treated control’ than those in GS ‘treated’ and ‘non-treated’(Supplemental Figure S8).
In cv. Richy, the content of some free neutral and aromatic AAs, alanine, leucine, serine, phenylalanine and tyrosine, in GS ‘treated control’ was higher than in ‘treated’ and ‘non-treated’ but not significantly and correlated with the transcript level of ANT-1, which was significantly higher in GS ‘treated control’. The level of free GABA was three times higher in the GS ‘treated’ (2.407 mg/g dw) compared to ‘treated control’ (0.716 mg/g dw) and 1 time more (1.562 mg/g dw) compared to GS ‘non-treated’ and the level of expression of GABA transporter was higher in ‘treated’ (but not significantly) than that observed in ‘treated control’ and significantly higher than in ‘non-treated’. The observed content of free histidine was ∼1.5 times higher in GS ‘treated’ (2.621 mg/g dw) than in GS ‘non-treated’ (1.436 mg/g dw), which is in accordance with the expression level of LHT-7 detected in GS ‘treated’. The content of free proline also was double in GS ‘treated’ than in ‘treated control’ and slightly higher than in ‘non-treated’, which was in accordance with significantly higher expression level of ProT-1 in GS ‘treated’ (, Supplemental Figure S8).
In the case of cv. Avigea, the level of all detected AAs in GS ‘treated’, ‘treated control’ and ‘non-treated’ was almost equal. The expression levels obtained for AA transporters ANT-1, LHT-7 and ProT-1 were almost equal for GS ‘treated’ and ‘treated control’ and slightly lower in GS ‘non-treated’. The only exception was the highly elevated level of the GABA transporter in both GS ‘treated’ and ‘treated control’, which did not correspond to the almost equal level of detected free GABA in GS ‘treated’, ‘treated control’ and ‘non-treated’ (0.676, 0.639, 0.657 mg/g dw, respectively).
The content of all detected free AAs, free sugars and fatty acid in MS ‘treated’ of the three investigated cultivars, was higher than in MS ‘treated control’ but the difference was not significant. Significant difference was found in the content of all detected free metabolites in MS ‘non-treated’, which was 2–3 times less than in ‘treated’ and ‘treated control’ (p < .05, p < .01, p < .001) (Supplemental Figures S9, S10 and S11). In cv. Izidor, the observed transcript level of ANT-1 in MS ‘treated’ was three times higher than in MS ‘treated control’ in accordance with the higher content of aromatic and neutral AAs in MS ‘treated’. The expression of ProT-1 was almost double in MS ‘treated’ and the content of proline was higher in MS ‘treated’. In spite of elevated expression of LHT-1 in MS ‘treated control’ the content of lysine and histidine were slightly less than that of MS ‘treated’. Similarly to cv. Izidor, in cv. Richy, the content of all free AAs detected in MS ‘treated’ was higher than in MS ‘treated control’ but not significantly and the content of AAs in MS ‘non-treated’ was two times lower compared to both MS ‘treated’ and ‘treated control’, which was in correlation with the transcript level of ANT-1 and LHT-7 transporters with the exception of the transcript level of ProT-1 detected in MS ‘treated’. In cv. Avigea, MS ‘treated’ and ‘treated control’, the content of free AAs, sugar and fatty acid was significantly higher than those in MS ‘non-treated’, as was observed for cv. Izidor and Richy (Supplemental Figure S10 and S11).
Figure 9. Free amino acids content in mg/g dw in egg white samples from three egg producers. Data represent the mean ± SD.
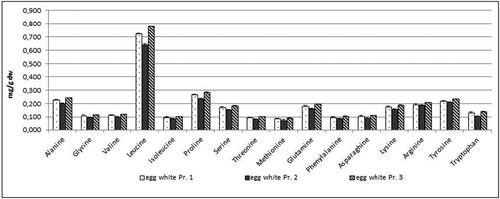
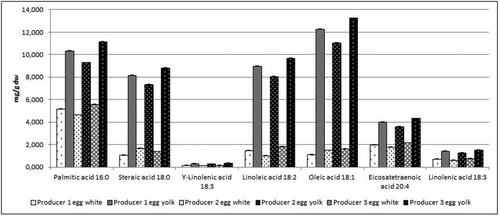
Figure 10. Free fatty acids content in mg/g/dw in egg white and yolk from three egg producers. Data represent the mean ± SD.
For cv. Avigea in experimental year 2020 ‘treated’ and ‘treated control’ seeds were not able to germinate and produce plants. The obtained results from metabolic analyses were done only with GS and MS ‘treated’, ‘treated control’ and ‘non-treated’ from cvs. Izidor and Richy.
The assessed contents of free AAs, sugars and fatty saturated and unsaturated acids of cv. Izidor were higher but not significantly in GS ‘treated’ compared to ‘treated control’ and significantly higher than GS ‘non-treated’ (p < .05; p < .01; p < .001) (Supplemental Figures S12, S13 and S14). In the year 2020, for cv. Izidor the content of sucrose (13.397/mg/g dw) in the GS treated was higher than that in ‘treated control’ (11.961 mg/g dw) and significantly lower in GS ‘non-treated’ (6.896 mg/g dw) (Supplemental Figure S13). This result correlated with the expression level of Invertase, which was higher in GS ‘treated’ than in ‘treated control’ and ‘non-treated’ but not significantly (). The content of all detected free AAs was higher in GS ‘treated’ compared to ‘treated control’ but not significantly and significantly higher than the content detected in GS ‘non-treated’ for most of the AAs (p < .05), which corresponds to the elevated level of expression of ANT-1, LHT-7, GABA and ProT-1 transporters in GS ‘treated’. The contents of all free AAs in ‘treated’ MS were higher compared to ‘treated control’ and there was a higher content of proline, serine, methionine, threonine, glutamine, asparagine, lysine, arginine compared to ‘non-treated’ MS. The expression profiles of ANT-1, LHT-7, GABA and ProT-1 corresponding to the content of AAs (Supplemental Figure S15). The content of detected free sugars and fatty acids also was higher, but not significantly in MS ‘treated’ than in ‘treated control’ and ‘non-treated’ (Supplemental Figure S16 and S17).
In cv. Richy, the contents of free AAs, sugars and fatty saturated and unsaturated acids were higher but not significant in GS ‘treated’ compared to ‘treated control’ and significantly higher than GS ‘non-treated’ (p < .05). The obtained expression profiles for ANT-1, LHT-7, GABA and ProT-1 transporters corresponding to the higher content of respective AAs in ‘treated’ GS. The expression level of Invertase in GS ‘treated’ also was in accordance to the higher level of free sugar in GS ‘treated’ (12.082 mg/g dw) compared to GS ‘non-treated’ (9.610 mg/g dw) (, Supplemental Figures S12, S13 and S14). Detected contents of free AAs, sugars and fatty saturated and unsaturated acids were higher in MS ‘treated’ compared to ‘treated control’ and ‘non-treated’ but not significantly (Supplemental Figures S15, S16 and S17). The expression profiles of ANT-1, LHT-7 and ProT-1 were significantly higher in MS ‘treated’ compared to MS ‘treated control’ and ‘non-treated’ ().
The obtained results from metabolomic analyses clearly indicated that low temperature pre-treatment on seeds before sowing was associated with higher content of free AAs, sugars and fatty acids in the MS of plants grown from those seeds. This observation was clearer in the first year of the experiment /2019/, which was more favourable in climatic conditions. The data obtained from morphological indicators also confirmed our observation. The plants grown from low temperature pre-treated seeds were with better field performance and significantly higher value of some of the selected morphological indicators. The data from expression analyses indicated that transcript level of some of the selected genes encoding Invertase, ANT-1, LHT-7, GABA and ProT-1, correlated with the respective content of metabolites and could be used for a fast screen of breeding materials related to seeds quality.
Altogether, the obtained results confirm the benefits of low temperature pre-treatment of seeds as a cheap and easy method to improve plants vigour on the field and the quality of accumulated protein, even if pre-treatment was related with reduced germination rate. Our future investigations will be aimed at optimizing the correct duration of low temperature pre-treatment of seeds in order to preserve the germination capacity and enhance the field performance and quality of protein.
Use of extruded full-fat soybean from Bulgarian cultivars as a protein source in the composition of feed for pigs
In our days, applying of circular economy in agriculture is a goal of the most farmers and local producers. It could be achieved by establishment of balanced agro-ecological approach, for agricultural production, which combines different scientific approaches and solutions. Applying of circular economy requires its own production of protein for livestock. Local production of plant protein will have a positive economic effect in animal husbandry.
Soybean meal is the world’s most common source of protein used in pig diets. It is widely available and often leads to the most economical gains compared to other sources of protein. In Europe and Bulgaria, soybean meal is imported mainly from South America. For various reasons, feeding of pigs with extruded full-fat soybean as a supplement or instead of soybean meal could be an attractive alternative in a country where the facilities for production of soybean meal is not available. It is also more effective for the farms where soybean is included in a cropping system.
As a next step in this study, we investigated the possibility of inclusion of extruded full-fat soybean in a diet of pigs in order to offer the studied feed composition to the mixed farms with plant and animal production. We conducted two sets of experiments. The feed composition of the two experiments is presented in and the experimental design is described in MM section. The results obtained for accepted feed, feed intake, nutrients, growth and feed consumption, feed conversion, for the first experiment are presented in . In the first experiment during the first subperiod there was a trend for lower feed intake by 3.31% in the animals of group III, compared to the control group. The pigs of group II received more fat by 3.69 g/head/day (p ≤ .001), and those of III, with 5.18 g/head/day (p ≤ .001) fat, compared to the control group. The pigs from groups II and III received more metabolizable energy: by 0.88 MJ/head/day and 0.16 MJ/head/day, respectively. The higher levels of ingested fats were in line with the extruded full-fat soybean used, which possesses a significantly higher fat content than soybean meal. The value of the main indicators for nutrient quality of soybean meal and extruded soybean from Richy and Srebrina cvs. was assessed in a certified laboratory and is presented in Supplemental Figure S18. The average daily gain in the first subperiod was practically the same in group II, but lower by 12.73% in group III pigs compared to the control group. The difference was not proven and could be considered as a trend. The consumption of feed consumed per kilogram of growth was in line with the growth intensity, being 11.18% higher in pigs of group III. In animals of group II, the feed conversion was the same as that of the control group. Fat conversion was significantly higher in the experimental groups: by 5.16 g/head/day (p ≤ .001) in group II and by 9.43 g/head/day (p ≤ .001) in pigs from group III, compared with group I. The results obtained for subperiod II () showed that the pigs from the different groups received practically the same daily ration. The differences between the animals from the different groups were insignificant. Group II pigs received more fat with 2.72 g/head/day (p ≤ .001) and group III pigs received 3.9 g/head/day (p ≤ .001) compared to animals from the control group. The inclusion of extruded soybean in compound feed (4.6% in group II and 7.00% in group III) reduced the average daily gain by 61 g (7.43%) in group II pigs and by 70 g (8.39%) in those of group III, compared with the control. The feed conversion ratio per kilogram gain is in accordance with the intensity of growth: at lower gain the feed conversion is higher. The inclusion of extruded soybean, which increases the fat content, influenced negatively the feed utilization. In groups II and III, compared to group I, the costs of the mixture were higher by 7.31% and 8.39%, respectively. However, the differences were not significant and can only be considered as a trend. The pigs in the experimental groups II and III consumed higher amounts of fats due to the high fat content of the extruded soybean. Throughout the experimental period, the pigs from the individual groups received practically the same amount of combined feed.
The higher fat intake of 3.19 g/head/day (p ≤ .001) in group II and 4.53 g/head/day (p ≤ .001) in group III pigs compared to group I was in compliance with the scheme of the experiment. The inclusion of extruded soybean in the compound feed in the amount of 50% and 75% of the soybean meal protein adversely affected the growth intensity. The average daily gain showed a trend towards lower values in the animals of group II by 3.13% and in those of group III by 9.14%, as the differences are not statistically significant. The feed conversion per kilogram gain was higher by 3.95% and 7.90% in pigs from the experimental groups compared to the control group.
From the analysis of the results of the first experiment, we can conclude that extruded full-fat soybean cv. Srebrina could be successfully included in the protein component of feed for growing pigs with a live weight of 30–60 kg at a rate of 50% of soybean meal in protein equivalent.
The results from the second experiment with included extruded full-fat soybean from cv. Richy are presented in (). During the first subperiod, no significant differences were found in the intake of feed and nutrients, except for the intake of fats, which was 3.22 g (p ≤ .001) higher in group II pigs, in whose diet extruded soybean was included according to the experimental scheme. The average daily gain was lower by 5.76%, but the difference was not significant and could be considered as a trend. In our opinion, the difference was not due of the tested factor, but because of a disease of one of the experimental animals. The feed conversion ratio and nutrients was higher by 6.22–6.98%, due to the slightly lower growth, but the differences were non-significant. The fats costs per kilogram of gain were significantly higher by 4.83 g (p ≤ .001). During the second subperiod, the intake of compound feed and nutrients, excluding the intake of fats, was practically equal in the two groups of animals. The average daily gain is higher by 8.95% in animals fed with compound feed with included extruded soybean. The consumed amounts of compound feed and nutrients were lower by 7.12–6.67% in the animals of experimental group II. The analysis of the results for the whole experimental period showed practically the same intake of feed and nutrients and there were no significant differences in the average daily gain and feed conversion per kilogram gain. The obtained higher fats intake by 3.1 g/kg was due to the higher fat content of extruded soybean.
Тable 3. Accepted feed consumption and average daily growth (I. II subperiod and whole experimental period) - experiment with extruded full-fat soybean cv. Richy
In conclusion, we could recommend the inclusion of extruded full-fat soybean cv. Richy in the amount of 30% of the protein component of the ration, in protein equivalent of the combined feed for the period of fattening of pigs.
Along with the measurements made on the pigs and monitoring their development, we analyzed the blood plasma of the pigs. The influence of the diet of the animals from the three experimental groups was reported in relation to the amount of malondialdehyde in the blood plasma of the experimental animals. Malondialdehyde (MDA) is an end product of lipid peroxidation and is widely used as an indicator for the determination of oxidative stress and the formation of ROS. Lower MDA blood plasma values indicate lower oxidative stress. The obtained data from the first experiment showed that animals from experimental group III with a higher percentage of extruded soy included in the feed composition were found to have the lowest level of detected MDA in the blood plasma (0.0277 nmol/µL) followed by group II (0.0310 nmol/µL) and group I control (0.0337 nmol/µL). There was a significant difference between group I control and group III and between groups II and III (p < .05, t-test). The results of the second experiment also detected differences in the amount of MDA in blood plasma of animals from two experimental groups. The level of MDA in experimental group II was 0.0438 nmol/µL while that in control group I was 0.0594 nmol/µL. The performed t-test detected a significant difference between group I (control) and group II at p < .01. The addition of extruded full-fat soybean had a positive effect on animal nutrition and caused less oxidative stress. The lowest levels of MDA in the blood plasma were detected for the group receiving more full-fat soybean. This indicates that less lipid peroxidation occurred within the animals, with less amount of MDA in blood plasma as an end product. The parameters clearly indicate a reduction in the oxidative stress when more full-fat soybean was added to the feed. What is more, the study of [Citation25] found that the addition of oil seeds or full-fat soybean to pig diet resulted in pork meat and back fat with higher content of polyunsaturated fatty acids.
Use of soybean meal, soybean cake as a protein source in feed for laying hens and content of amino acids and fatty acids in eggs
Next, we traced the quality of eggs in different egg producers depending on the feed used for laying hens. Three different egg producers were visited for conversation and discussion on the used feed and egg production. The egg producers were concentrated in North West and North-Central Bulgaria. All three producers are specialized in eggs and poultry production with their own feed factories.
During the visits, feed samples were taken from all three producers. In two of the producers imported soybean meal was included as a protein source in the feed composition. In the composition of the feed of the third producer, 50% of the protein source was imported soybean meal (50%) and the other 50%, soybean cake produced from cold pressed soybeans, own production from Bulgarian cv. Srebrina.
The precise composition of the compound feed of each of the three producers is not available, but the samples taken from each of them were analyzed in a certified laboratory based on five indicators (energy value MJ/kg; total protein %; total fat %; starch %; total sugars). The comparison of the feed from the three producers based on the five indicators is presented in . The inclusion of 50% protein source in the form of soybean cake in the feed of producer 3 significantly enhanced the total protein value and slightly the energy value. The soybean cake is produced from cold pressed soybean grain from cv. Srebrina with separation of soybean oil, and the cake possess high protein value of more than 37%. This is the reason for the higher total protein content in the feed of producer 3. Defatted and untreated soybean cake obtained by cold pressing was firstly evaluated by Woyengo et al. [Citation26]. It is an excellent source of crude protein in animal nutrition. The most important feature of the soybean cake is a significant amount of leucine. Leucine a is branched chain amino acid, which is synthesized only by plants and is not only a substrate for protein synthesis but also acts as a signalling molecule for activation of the gene expression of many developmental genes [Citation27]. The study of Yin et al. [Citation28] found that leucine promotes protein synthesis and the growth of skeletal muscle, liver and intestinal tract in weanling pigs.
Samples from eggs from all three egg producers were taken also for detailed analysis of the content of free amino acids and fatty acid. The analyses of eggs were performed separately on egg yolk and egg white. The content of free amino acids of egg yolk and egg white is presented in and . The results obtained in the content of free AAs in egg yolk indicated higher level of each of the detected AAs in the egg yolk from producer 3 with the exception of lysine. Similar results were obtained for the content of free AAs in egg white. The contents of all detected AAs were higher in the egg samples from producer 3 ().
The content of the free fatty acids also was evaluated in the egg samples (yolk and white) collected from the three eggs producers. shows the content of two saturated and five unsaturated fatty acids in egg white and egg yolk. The highest content of unsaturated fatty acids was detected in egg white and egg yolk samples from producer 3. The same trend was observed for saturated palmitic acid, and the only exception was the content of stearic acid in egg white where the value of stearic acid was higher in the egg white samples from producer 2.
The soybean cake obtained by cold pressing of soybean grain is an easy process which does not require very expensive equipment [Citation29]. The inclusion of soybean cake in the animal feed on the other hand could benefit animal health and the quality of the final product. In our study, we found that feed for laying hens in which 50% of imported soybean meal is replaced by soybean cake produced from locally grown soybean, could increase the content of free AAs and fatty acids in the final product and benefit the quality of eggs.
Based on the data obtained in our study we could recommend to Bulgarian farmers to include soybean cake or full-fat soybean and partially replace the imported soybean meal. The enhanced field production of soybean in the region of North West and Central Bulgaria will benefit sustainable agriculture and enhance circular economy in the region.
Conclusions
The investigated low temperature pre-treatment of seeds before sowing indicated better field performance and increased climate plasticity of plants grown from treated seeds. The detected content of free amino acids, sugars and fatty acids in soybean grains (green and mature) was strongly affected by pre-treatment and was elevated in ‘treated’ seeds compared to ‘non-treated’. Clear correlation was found between the obtained expression profiles of selected AAs transporters and the content of AAs. The expression profile of the selected gene Invertase could be a marker for the level of free sucrose. The expression profile of H-PPase could serve as a marker for the adaptability of plants grown in unfavourable climate conditions during the period of seeds filling. Altogether, the obtained results in the present study could benefit the experimental work of plant breeders and farmers growing soybean and its further processing for inclusion in animal feed. The findings from this study contribute towards relating the production of legumes supported cropping system to value-chain by linking the sources and users and final food products.
Consent to participate
All the authors have approved their participation in the final manuscript.
Consent for publication
All the authors have read and approved the final manuscript and its submission for publication.
Availability of data and material (data transparency)
All data generated or analyzed during this study are included in this manuscript [and its electronic supplementary material].
Supplemental Material
Download PDF (1.4 MB)Acknowledgements
The authors are grateful to the three egg producers for their willingness to provide samples of used feed and eggs, as well as for their support during the visits.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Verdier J, Kakar K, Gallardo K, et al. Gene expression profiling of M. truncatula transcription factors identifies putative regulators of grain legume seed filling. Plant Mol Biol. 2008;67(6):567–580.
- Ge L, Yu J, Wang H, et al. Increasing seed size and quality by manipulating BIG SEEDS1 in legume species. Proc Natl Acad Sci USA. 2016;113(44):12414–12419.
- Iantcheva A, Naydenova G. Biological nitrogen fixation in legumes, understanding the process. Practical notes. Legumes Translated Project. 2020.
- Sakthivelu G, Devi MK, Giridhar P, et al . Isoflavone composition, phenol content, and antioxidant activity of soybean seeds from India and Bulgaria. J Agric Food Chem. 2008;56(6):2090–2095.
- Mandić V, Đorđević S, Đorđević N, et al. Genotype and sowing time e_ects on soybean yield and quality. Agriculture. 2020;10(11):502.
- Carrera CS, Reynoso CM, Gustavo Javier Funes GJ, et al. Amino acid composition of soybean seeds as affected by climatic variables. Pesq Agropec Bras. 2011; 46(12):1579–1587.
- Grieshop CM, Fahey GCJr. Comparison of quality characteristics of soybeans from Brazil, China, and the United States. J Agric Food Chem. 2001;49(5):2669–2673.
- Vision of Republic of Bulgaria for CAP 2021-2027. https://www.mzh.government.bg/en/cap-2021-2027/vision-republic-bulgaria-cap-2021-2027/.
- Georgiev G. Analysis of the vegetation rainfall and its relation to soybean yield under non-irrigation growing conditions. Rastenievadni nauki/Bulgarian J Crop Sci. 2017;54(4):14–19. (Bg).
- Deshmukh R, Sonah H, Patil G, et al. Integrating omic approaches for abiotic stress tolerance in soybean. Front Plant Sci. 2014; 5:244.
- Zając T, Oleksy A, Ślizowska A, et al. Aboveground dry biomass partitioning and nitrogen accumulation in early maturing soybean ‘Merlin’. Acta Agrobot. 2017;70(4):1728.
- Vieira BGTL, Vieira RD, Krzyzanowski FC, et al. Alternative procedure for the cold test for soybean seeds. Sci Agric (Piracicaba, Braz). 2010; 67(5):540–545.
- Georgiev G. Influence of sowing date and inter-row spacing on the structural yield elements in two soybean varieties. Field Crop Studies. 2019;XII(3):93–104.
- Georgiev G, Tododrova R. Results of demonstration trial with our and foreign soybean varieties under non-irrigation growing conditions. Field Crops Studies. 2018; XI(1):49–60.
- Naydenova G, Vasileva V. Comparative evaluation of diploid and tetraploid red clover genotypes in a flat area of Northern Bulgaria. J Cent Eur Agric. 2019;20(3):919–927.
- Cheng L, Yuan H-Y, Ren R, et al . Genome-wide identification, classification, and expression analysis of amino acid transporter gene family in glycine max. Front Plant Sci. 2016;7:515.
- Hummel J, Strehmel N, Selbig J, et al. Decision tree supported substructure prediction of metabolites from GC–MS profiles. Metabolomics. 2010;6(2):322–333.
- Manion RE, Huie RD, Levin DR, et al. NIST Chemical Kinetics Database, NIST Standard Reference Database 17, Version 7.0 (Web Version), Release 1.6.8, Data version 2015.09, National Institute of Standards and Technology, Gaithersburg, Maryland, 20899–208320. http://kinetics.nist.gov/.
- Schilling RK, Tester M, Marschner P, et al. AVP1: one protein, many roles. Trends Plant Sci. 2017;22(2):154–162.
- Bhaskaran S, Savithramma DL. Co-expression of Pennisetum glaucum vacuolar Na+/H + antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J Exp Bot. 2011;62(15):5561–5570.
- Wan H, Wu L, Yang Y, et al. Evolution of sucrose metabolism: the dichotomy of invertases and beyond. Trends Plant Sci. 2018; 23(2):163–177.
- Vargas WA, Pontis HG, Salerno GL. New insights on sucrose metabolism: evidence for an active A/N-Inv in chloroplasts uncovers a novel component of the intracellular carbon trafficking. Planta. 2008;227(4):795–807.
- Roberts MR. Does GABA act as a signal in plants?: hints from molecular studiesPlant Signal Behav. 2007;2(5):408–409.
- Ramos-Ruiz R, Martinez F, Knauf-Beiter G. The effects of GABA in plants. Cogent Food Agric. 2019;5(1):1670553.
- Đorđević V, Đorđević J, Baltić Ž M, et al. Effect of sunflower, linseed and soybean meal in pig diet on chemical composition, fatty acid profile of meat and backfat, and its oxidative stability. Acta Veterinaria-Beograd. 2016;66(3):359–372.
- Woyengo TA, Patterson R, Levesque CL. Nutritive value of cold-pressed soybean cake with or without extrusion or supplementation of multi-enzyme for pigs. J Anim Sci. 2016;94(12):5230–5238.
- Li F, Yin Y, Tan B, et al. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41(5):1185–1193.
- Yin Y, Yao K, Liu Z, et al. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids. 2010;39(5):1477–1486.
- Özbek ZA, Ergönül PG. Cold pressed soybean oil. In: Ramadan MF, editor. Green technology, bioactive compounds, functionality, and applications. Cambridge, Massachusetts: Academic press, Elsevier; 2020. p. 575–585.
