Abstract
Soluble pyridine nucleotide transhydrogenase (STH), catalyzes a reversible hydrogen transfer between NADH and NADPH, is widely utilized in cofactor engineering. In the present study, we expanded the distribution range of STH to more unreported bacteria. Meanwhile, STH was mainly found in α, γ, δ-proteobacteria, acidobacteria, actinobacteria and planctomycetes. The enzymological properties of two novel STHs from Acidobacteria bacterium KBS 146 (AbSTH) and Nocardia jiangxiensis (NjSTH) were characterized. The optimum temperature and pH of AbSTH and NjSTH were pH 6.2 and 35 °C, pH 5.7 and 50 °C, respectively. When using thio-NAD+ as a hydrogen accepter, the 1/Km and kcat/Km for NADH of AbSTH were 1.8 and 1.7-fold greater than NADPH, whereas the 1/Km and kcat/Km of NjSTH for NADPH were 2.0 and 2.2-fold greater than NADH. The physiological hydrogen donor substrate of AbSTH and NjSTH may be NADH and NADPH, respectively. AbSTH activity was inhibited by ATP and strongly stimulated by ADP and AMP. These results may provide new insights into the physiological roles and cofactor engineering application of STH.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.1988708 .
Introduction
Pyridine nucleotides (NADH and NADPH), generally expensive, are crucial for biosynthesis of chemical intermediates, chiral alcohol and amino acid [Citation1]. Although their chemical structures are very similar, the physiological roles of NADH and NADPH are distinct. NADH is an essential component of the respiratory chain to transfer electron to oxygen, thereby driving oxidative phosphorylation of ADP to ATP. On the contrary, NADPH is an essential electron donor and exclusively participates in anabolic reduction reactions [Citation2]. Pyridine nucleotide transhydrogenase is a particular enzyme which can reversibly transfer hydride between NADH and NADPH, with two different isoforms: energy-independent soluble transhydrogenase (STH or UdhA, EC: 1.6.1.1) and energy-dependent, or proton-translocating, membrane-bound transhydrogenase (PntAB or H+-TH, EC: 1.6.1.2) [Citation3]. It was hypothesized that the physiological role of PntAB and STH might be to generate and reoxidate NADPH, respectively [Citation4]. Although subsequent studies further support the hypothesis, the debate is still in the air [Citation5,Citation6].
It is widely accepted that the physiological role of STH is to convert NADPH to NADH. STH is essential for growth of several bacterial species under conditions with excess NADPH formation [Citation3,Citation7–10]. The growth rate is partially restored by overexpressing STH in phosphoglucose isomerase mutant which generates more NADPH via the pentose phosphate (PP) pathway [Citation7,Citation8]. 13C labeling and mutation experiments confirm that STH supplies NADH during growth on acetate [Citation3,Citation9,Citation10]. Studies for transcriptional regulation also link Escherichia coli STH (EcSTH) to respiration [Citation2]. In many cofactor engineering approaches, STH is used to increase the NADH availability and then the productivity and yield of products, such as isobutanol, succinic acid, meso‑2, 3‑butanediol, tryptophan and D-(-)-2, 3-butanediol [Citation11–16]. However, other reports suggest that STH catalyzes the exact opposite reaction namely, from NADH to NADPH. Decorosi et al. [Citation17] have reported that STH mutant leads to a decrease in NADPH pool and fails to sustain an effective defense against the oxidative stress induced by Cr(VI) in the Cr(VI)-sensitive Pseudomonas corrugata 28. Nikel et al. [Citation18] also found that STH more likely reacts to yield NADPH in Pseudomonas putida during biodegradation of aromatic compounds. Increasing application examples also support this claim. For example, STH has successfully been engineered to increase NADPH availability to enhance the ethanol fermentation performance and the productivity of high value-added products, such as (S)-2-chloropropionate, squalene, 3-hydroxypropionate, L-homoserine and thymidine [Citation19–24]. Nevertheless, in other application cases, STH is not efficient to enhance the productivity and yield of target products in a similar fashion [Citation25–27]. Given the above, the physiological role of STH could be driven-by-demand and might be related to the species origin, culture conditions, and genotypes [Citation5,Citation18].
In addition, STH is not a universally present protein, and is only found in some certain bacteria such as α, β, γ, δ-proteobacteria, many actinobacteria and spirochaetales, but not in archaebacteria or eukaryotes [Citation28]. Meanwhile, only Gamma-proteobacteria STHs (E. coli, Azotobacter vinelandii, Pseudomonas fluorescens, Pseudomonas aeruginosa, P. corrugata, P. putida) have been studied in some aspects such as physiological roles, enzymatic properties, catalytic mechanisms and cofactor engineering until now [Citation2,Citation7–9,Citation11,Citation13,Citation17,Citation19–24,Citation28–31]. Especially, the presence of STH in actinobacteria, such as Mycobacterium tuberculosis (MtSTH), has been proposed several times by bioinformatics methods but not unambiguously confirmed [Citation28,Citation32]. In recent years, massive sequencing data are reported, mostly annotated by computational techniques [Citation33]. It also provides a unique opportunity and perspective to reanalyze the distribution range of STH, explore novel STH for industrial application, and further investigate the physiological functions of STH. Here, we identified several putative STHs through bioinformatics analysis, revisited the distribution pattern and constructed a phylogenetic tree of STHs. Then, two novel STHs were expressed and purified, and their enzymatic properties were first thoroughly characterized. These results will provide new insights into the physiological roles and cofactor engineering application of STH.
Materials and methods
Bioinformatics analysis
To find more putative STHs, EcSTH was used as a query sequence to search the protein database by performing NCBI blink search. Sequences with ≥40% similarity to EcSTH were considered as positive hits. To remove redundant information, short protein sequences (<350 Aa) and sequences from symbiont, unclassified and candidatus species were eliminated. For distribution range analysis, only one representative sequence from each genus was selected for further analysis and the taxonomic status of each genus was queried in NCBI Taxonomy Database. In addition, the distribution range was analyzed at the completely sequenced genomes level using Integrated Microbial Genomes (IMG) database. The analysis only included finished genomes and one representative genome from each genus. For phylogenetic analysis, all selected sequences were downloaded from GenBank database. Based on the sequence alignment by ClustalW (ftp://ftp.ebi.ac.uk/pub/software/clustalw2), the phylogenetic tree was constructed using the bootstrapped neighbor joining method of molecular evolutionary genetics analysis software MEGA 7 software (http://www.megasoftware. net/). Dihydrolipoamide dehydrogenase was used as an outgroup to examine phylogenetic prediction.
Gene synthesis
Full-length sth genes from Acidobacteria bacterium KBS 146 (AbSTH, WP_026387261) and Nocardia jiangxiensis (NjSTH, WP_040826097) were codon optimized for expression in E.coli and synthesized by Generay Biotech Co., Ltd. (Shanghai, China). All synthesized genes were cloned into the expression vector pET-28b (+), between Nde I and Xho I sites, resulting in two recombinant plasmids, pET-AbSTH and pET-NjSTH. The entire genes fused to the 6 His-tags at N-terminus were confirmed using Sanger sequencing service by Genscript (Nanjing, China).
Overexpression and purification
All recombinant plasmids were transformed into E. coli Rosetta (DE3). All strains were grown overnight at 37 °C in LB medium supplemented with kanamycin (30 mg/mL) and chloramphenicol (25 mg/mL). The overnight cultures were inoculated (1:100) into 200 mL fresh LB medium and cultured at 37 °C until the density reached an optical density at 600 nm (OD600) of 0.4–0.6. Then the expression conditions, such as temperature and IPTG concentration, were optimized. While expression of AbSTH, and NjSTH was strongly induced by isopropyl-1-thio-β-D-galactopyranoside (IPTG) with a final concentration of 0.25 mmol/L and 0.5 mmol/L, respectively. The expression strains were cultured with agitation (150 rpm) for 24 h at 20 °C. Cells were harvested by centrifugation at 5,000 rpm for 10 min.
Expression strains were re-suspended in Equilibration/Wash buffer (pET-NjSTH: 25 mmol/L Tris-HCl, pH 7.5, 500 mmol/L NaCl, 5% glycerin, 2.5 mmol/L β-ME; pET-AbSTH: 25 mmol/L Tris-HCl, pH 8.0, 500 mmol/L NaCl, 5% glycerin). Cells were lysed by sonication and centrifuged at 15,000 g for 30 min to remove cell debris. Finally, 6 His-tagged STHs were purified using BD TALON Metal Affinity Resin (Clontech, PaloAlto, CA, USA). All purification steps were performed on ice. The expression abundance, purification homogeneity and subunit molecular weight were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Protein bands were stained with 0.25% Coomassie Brilliant Blue R-250.
Enzyme assay
STH activities were determined by measuring thio-NADH formation coupled photometric assay as before with minor modifications [Citation28]. Enzyme assays were performed in 1 mL volume containing 200 μmol/L NADH or NADPH, 200 μmol/L thio-NAD+, 50 μmol/L FAD and 50 μmol/L MES-NaOH at individual optimum pH and temperature (AbSTH, pH 6.2, 52 °C; NjSTH, pH 5.7, 35 °C). Thio-NADH absorbance was monitored at 400 nm by a thermostated Cary 300 UV-Vis spectrophotometer (Agilent Technologies Inc., USA), using molar extinction coefficient of 11 300 M−1 cm−1. Protein concentration was assayed according to the Bio-Rad protein assay kit (Bio-Rad) with bovine serum albumin as the standard. All activity data were represented as mean values with standard deviation (±SD) of at least triplicates.
Kinetic characterization
Kinetic characterization was performed with naturally occurring pyridine nucleotides (NADH or NADPH) as a hydrogen donor and thioanalogues (thio-NAD+) as an acceptor for the NADH/thio-NAD+ and NADPH/thio-NAD+ transhydrogenase activity, and determined by varying substrate concentrations. The kinetic parameters were calculated by nonlinear regression analysis of Michaelis-Menten or Substrate Inhibition program using GraphPad Prism 7 software (GraphPad Software, La Jolla, CA, USA).
pH, temperature effects and thermostability
The effects of pH and temperature on STH activity were studied using the method described above. For optimum pH, the transhydrogenase activity of purified AbSTH and NjSTH were assayed in 50 mmol/L MES-NaOH buffer with pH varying from 4.9 to 8.0 and 4.9 to 6.4, respectively. The optimum temperature values of AbSTH and NjSTH were determined at temperatures that ranged from 30 to 62 °C and from 20 to 50 °C, respectively. To determine the thermostability, purified AbSTH and NjSTH were preincubated at 0–54 °C and 0–44 °C for 30 min, respectively. Then, the samples were immediately cooled on ice for 5 min and assayed for residual activity by using the standard enzyme assay.
Chemicals effects
The effects of metal ions (NaCl, RbCl, KCl, LiCl, MgCl2, CaCl2, MnCl2, CoCl2, ZnCl2, NiCl2 and CuCl2), adenine nucleotide (2 mmol/L ATP, ADP and AMP) and reducer (2 mmol/L DTT and β-ME) on STH activity were measured by the method described previously. In the calculation of relative activity, the transhydrogenase activity determined without chemicals addition was considered as 100%.
Results
Distribution range analysis
Herein, more putative STH sequences from NCBI database were analyzed. After removing redundant sequences, about 331 potential STHs were obtained from different genera. In the taxonomical status, apart from the above-mentioned bacteria, acidobacteria, planctomycetes, chlamydiae, deinococcus-thermus, nitrospirae and a few lower eukaryotes (oomycetes and Acanthamoeba castellanii) all contained STH (). A similar result was obtained from the IMG database, suggesting that STH was distributed in 165 genera of bacteria (actinobacteria, α, β, γ, δ-proteobacteria, acidobacteria, planctomycetes, Spirochaetales, chlamydiae, chrysiogenetes, gemmatimonadetes and nitrospirae) and 2 lower eukaryotes (alveolata) (). Collectively we found that STH is mainly present in α, γ, δ-proteobacteria, acidobacteria, actinobacteria and planctomycetes, and a few other organisms.
Table 1. Overview for the distribution of the number of STH in NCBI and IMG database.
Phylogenetic and sequence analysis
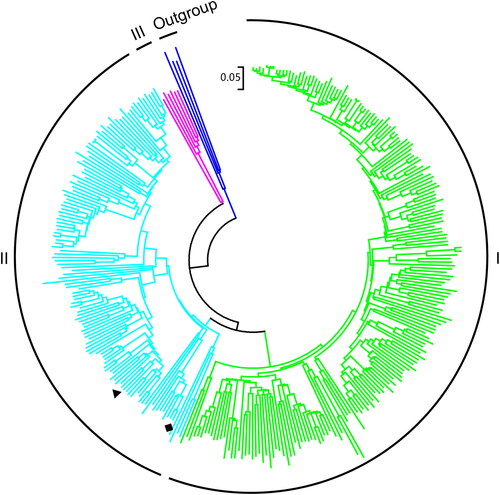
STHs belong to a large and heterogeneous protein family of flavoprotein disulfide reductases. Although the phylogenetic relationship of the flavoprotein disulfide reductase protein family has been analyzed using different methods, the phylogenetic relationship of STHs has not been reported. Herein, we constructed the phylogenetic trees of STH by incorporating all new members. The results reveal that STH protein sequences clustered into three subgroups (). The subgroup I STHs was mostly form γ, δ-proteobacteria, planctomycetes and Spirochaetales. These STHs showed >52% protein sequence identity with EcSTH. The subgroup II mainly consisted of α, β-proteobacteria, acidobacteria, actinobacteria and eukaryote STHs. They exhibited relatively low identity about 40–48% with EcSTH. AbSTH and NjSTH belonged to this novel subgroup. Meanwhile, A. bacterium KBS 146 and N. jiangxiensis belong to acidobacteria and actinobacteria respectively. The subgroup III STHs mainly derived from chlamydiae, which shared about 41% identity with EcSTH.
Figure 1. Neighbor-joining phylogenetic tree of STHs. The tree using 331 STH from different representative organisms from each genus with 1000 bootstrap replicates. Phylogenetic analyses were conducted in MEGA 7. The STH sequences used are listed in . AbSTH and NjSTH are marked by “♦”, and “▼” respectively.
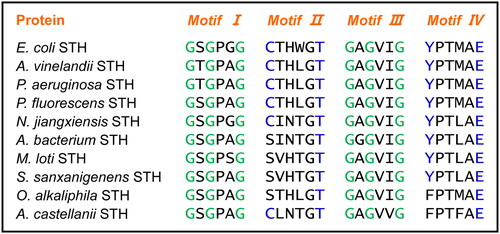
Multiple sequence alignment displayed that sequence identities of AbSTH and NjSTH with EcSTH was 44% and 43%, respectively. AbSTH showed 57% identity with NjSTH. Like all reported STHs, NjSTH possess four signature motifs: two Rossman fold fingerprint motifs (GXGXXG, Motif I and Motif III), a CXXXXT motif (Motif II) and a Tyr-Glu pair (YXXXXE, motif IV) (). However, the cysteine of the CXXXXT motif was not conserved and was replaced by serine in AbSTH ().
Overexpression and purification of STHs
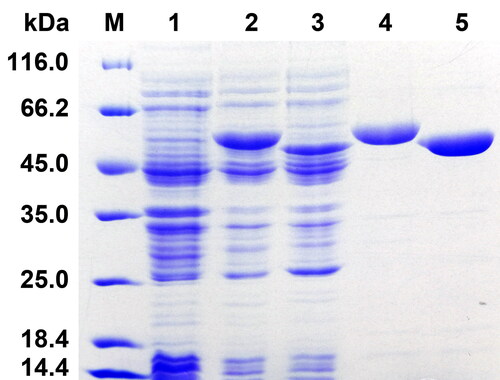
Two STHs, AbSTH and NjSTH that belong to a newly identified STH subgroup were selected for further study. They were heterologously expressed as soluble proteins in E. coli and then were purified by Co2+ affinity chromatography (). The molecular weight of 6 His-tagged AbSTH and NjSTH were approximately 51 kDa and 50 kDa, respectively, which is consonant with the theoretical molecular weight (AbSTH, 50.8 kDa; NjSTH, 50.8 kDa).
Figure 3. Representative SDS-PAGE of the two recombinant STHs. M, protein marker; lane 1, crude extracts of cells harboring pET-28b(+) with IPTG induction; lane 2, 3, crude extracts of cells harboring recombinant plasmid pET-AbSTH and pET-NjSTH with IPTG induction; lane 4, 5, purified recombinant AbSTH and NjSTH.
Kinetic characterization and substrate inhibition
Enzyme activity assay showed that both recombinant STHs are able to reduce the thio-analogues of NAD+ (thio-NAD+) with NADH and NADPH. The transhydrogenase kinetic studies of the reduction of thio-NAD+ by NADH or NADPH were performed in this study. The kinetic parameters were calculated using a nonlinear fitting method ().
Table 2. Kinetic parameters for AbSTH and NjSTH.
When NADH and thio-NAD+ were used as substrates, the Km value for NADH of AbSTH slightly exceeded that of NjSTH. The maximum turnover rate (kcat) of NjSTH for NADH was 4.4-fold greater than that of AbSTH. The specificity constant (kcat/Km) of NjSTH for NADH was 7.3-fold greater than that of AbSTH. The Km value for thio-NAD+ of AbSTH and NjSTH was 199.1 μmol/L and 205.2 μmol/L, respectively. The kcat of NjSTH toward thio-NAD+ was 5.4-fold higher than that of the AbSTH. Meanwhile, NjSTH showed 5.3-fold preference for thio-NAD+ relative to AbSTH. Moreover, substrate inhibition for both STHs was observed at high concentrations of NADH, but not of thio-NAD+ (; ).
Figure 4. Substrate inhibition profiles for hydrogen donor. (a) Profile of NADH for AbSTH. (b) Profile of NADPH for AbSTH. (c) Profile of NADH for NjSTH. (d) Profile of NADPH for NjSTH.
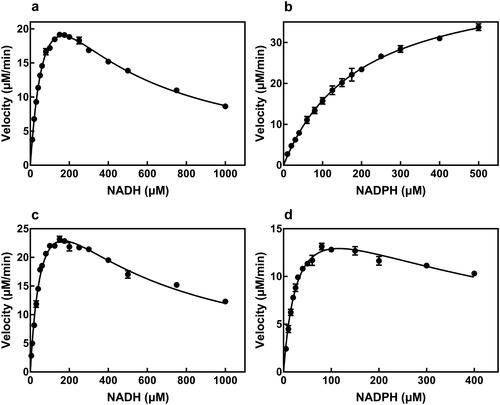
When NADPH and thio-NAD+ were used as substrates, Km for NADPH of NjSTH and AbSTH was 33.2 μmol/L and 188.1 μmol/L, respectively. The kcat of NjSTH for NADPH was 4.7-fold that of AbSTH. So, the kcat/Km value of NjSTH for NADPH was about 26.8-fold higher than that of AbSTH. The Km value of thio-NAD+ by AbSTH was 71.7 μmol/L and 285.1 μmol/L, respectively. The kcat of NjSTH toward thio-NAD+ was 10-fold higher than that of AbSTH. Consequently, NjSTH displayed about 2.6-fold preference for thio-NAD+ relative to AbSTH. Moreover, high concentrations of NADPH reduced NjSTH activity, but not affect AbSTH activity (; ).
Km of AbSTH for NADPH was over 1.8-fold greater than Km for NADH. The kcat/Km of AbSTH was 1.7-fold greater for NADH than NADPH (). On the contrary, the Km of NjSTH for NADH was over 2-fold greater than the Km for NADPH. The kcat/Km of NjSTH was 2.2-fold greater for NADPH than NADH (). Hence, thio-NAD+ as a hydrogen accepter, recombinant NjSTH and AbSTH showed a higher affinity and conversion efficiency for NADPH and NADH, respectively.
Optimum pH and temperature of the recombinant STHs
To determine the effect of pH on the activity of the two purified recombinant STHs, an activity analysis was performed using standard reaction conditions at various pH, between 4.9 and 8.0. The optimum pH for AbSTH and NjSTH was pH 6.2 and 5.7, respectively (). Interestingly, NjSTH exhibited activity profiles in a narrow pH range (4.9–6.4), but AbSTH exhibited a relatively high activity in a broad range of pH (4.9–8.0) ().
Figure 5. Effects of pH and temperature on the activity of the recombinant STHs. AbSTH and NjSTH were marked by “■”, and “●” respectively. (a) Effects of pH on the activity of two STHs. (b) Effects of temperature on the activity of STHs. (c) Thermal profiles for two STHs. (d) Effect of metal ions on the activity of AbSTH and NjSTH.
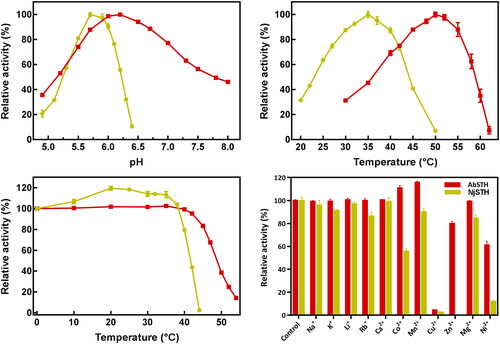
The optimal temperature for catalysis by NjSTH was around 35 °C (). The suitable temperature for AbSTH was 50 °C. The activity of AbSTH rapidly declined at temperatures above 55 °C (). The results from the heat inactivation study demonstrated that NjSTH and AbSTH was stable below 40 °C and 45 °C, respectively (). NjSTH and AbSTH were retaining 79.3% and 83.4% activity after incubation for 30 min at 40 °C and 45 °C, respectively. Then, STHs were rapidly inactivated at high temperatures. In particular, the NjSTH activity was increased by 7%–20% after incubation for 30 min at 10–38 °C, possibly due to alteration of NjSTH structure in this temperature range.
Effects of metal ions and adenine nucleotides on the recombinant STHs activity
The effects of several metal ions on STH activity were also examined (). The activity of STHs was not affected or was only slightly inhibited by monovalent metal ions in this study (). The effects of divalent metal ions on AbSTH were pleiotropic. Ca2+ and Mg2+ did not affect AbSTH at all; Zn2+, Ni2+ and Cu2+ had inhibitory effects, of which Cu2+ in particular caused 95% loss of activity; whereas Mn2+ and Co2+ slightly increased the activity to 111.0% and 115.8%, respectively. However, the NjSTH activity was inhibited at varying degrees by divalent cations. Mg2+ and Mn2+ slightly inhibited the activity, Co2+ caused 45% loss of activity, Ni2+, Zn2+ and Cu2+ were strong inhibitors.
The effects of adenine nucleotides (ATP, ADP and AMP) on the activity of the two recombinant STHs were different (). The AbSTH activity was inhibited by ATP, but strongly activated by 5.7-fold and 7.7-fold by ADP and AMP, respectively. However, NjSTH was inhibited by ATP, ADP and AMP.
Table 3. Effects of adenine nucleotides on the activity of some reported STHs.
Discussion
STH is not a universally present protein [Citation36]; our previous studies have shown that STH is only found in certain bacteria such as α, β, γ, δ-proteobacteria, many actinobacteria and spirochaetales [Citation28]. Although the distribution range was expanded to more unreported bacteria and even to a few lower eukaryotes in this study, it is still very narrow. First, to improve the reliability of the distribution range, we only selected suspected sequences which show ≥40% identity with EcSTH from NCBI. Second, there are other biochemical mechanisms or many unknown genes that encode proteins with similar functions to STH in some species which do not contain STH. Two transhydrogenase activities were identified in potato tubers and pea leaves. Diphenyleneiodonium (DPI) insensitive activity has the same catalytic characteristics as STH [Citation37]. Methanobacterium thermoautotrophicum, an archaea which does not have STH, was found to contain two malate dehydrogenases that function in NADPH: NAD+ transhydrogenation [Citation38]. Moreover, except for STH, there are other biochemical mechanisms that maintain the balance of NAD(P)H pools, such as NAD+ and NADH kinases, NADP+-dependent hydrogenases, and so on [Citation18].
The retrieval result showed that more putative STH sequences have serine-substituted CXXXXT (SXXXXT), such as Mesorhizobium loti STH (WP_059184891.1), Sphingomonas sanxanigenens STH (WP_025292554.1), Oblitimonas alkaliphila STH (WP_053101563) and so on. On the contrary, the threonine in this motif is exclusively the same among all STHs (). Since AbSTH have soluble transhydrogenase activity, the conserved threonine might be more important for STH activity than the cysteine in the CXXXXT motif. We also noticed that the tyrosine of the YXXXXE motif was replaced by phenylalanine only in two suspected STH (O. alkaliphila; Acanthamoeba castellanii, XP_004344653) (). Due to the lack of structural information for STH so far, we cannot know what the function of these amino acid residues in substance binding and catalytic activity might be. It will be interesting to investigate the crystal structure, catalytic mechanism and molecular evolution of STH in further studies.
In the literature, very few studies have been focused on kinetic characterization of STH. When NADH and thio-NAD+ were used as substrates, the Km value for NADH of AbSTH (107.4 μmol/L) slightly exceeded that of NjSTH (65.2 μM) and AvSTH (60–77 μmol/L) [Citation28,Citation39]. The Km value for thio-NAD+ of AbSTH (199.1 μmol/L), and NjSTH (205.2 μmol/L) was about 3–5 fold that of AvSTH (40–50 μmol/L) [Citation28,Citation39]. When NADPH and thio-NAD+ were used as substrates, NjSTH (33.2 μmol/L) showed higher affinity to NADPH than other STH, such as AvSTH (40 μmol/L) [Citation39], EcSTH (68.29 μmol/L) [Citation28] and AbSTH (188.1 μmol/L). The Km value of thio-NAD+ by AbSTH (71.7 μmol/L) was similar to AvSTH (75 μmol/L) [Citation39], but lower than that of NjSTH (285.1 μmol/L), EcSTH (133.2 μmol/L) and AvSTH (250 μmol/L) [Citation28]. Moreover, the kcat and kcat/Km of both STH for thio-NAD+ and NADPH were lower than that of EcSTH [Citation28]. Significantly, the preference and conversion efficiency of two STHs for the hydrogen donor were dramatically different. NjSTH showed a higher affinity and conversion efficiency for NADPH. However, AbSTH preferred NADH. This demonstrated that the physiological hydrogen donor substrate of AbSTH and NjSTH may be NADH and NADPH, respectively.
AbSTH and NjSTH, with slightly acidic optimum pH, were different from previously reported STH, such as EcSTH (7.5), A.vinelandii STH (AvSTH, 7.0–8.0) and Vibrio natriegens STH (VnSTH, 9.6) [Citation28,Citation30,Citation34,Citation40]. They may provide new choices to enhance the overall catalytic activity in a multicatalytic system under weak acidity conditions. The thermal stability of AbSTH and NjSTH was lower than that of EcSTH, which retained 80% and 10% activity after incubation for 30 min at 50 and 62 °C [Citation28]. It suggested that STH could be a thermolabile protein.
STH showed various effects of adenine nucleotides among organisms (). NjSTH and VnSTH were also strongly inhibited by adenine nucleotides [Citation34]. However, EcSTH was activated by adenine nucleotides [Citation28]. In the present study, the AbSTH activity was inhibited by ATP, but strongly activated by ADP and AMP. Moreover, the activity of AvSTH and PaSTH were also activated by AMP at a different level in earlier studies () [Citation28,Citation35].
Conclusions
Overall, the present study reanalyzed the distribution range of soluble pyridine nucleotide transhydrogenase and verified two novel STHs from A. bacterium KBS 146 and N. jiangxiensis by assaying transhydrogenase activity for the first time. The two novel STHs displayed some drastically different enzymatic properties. In subsequent research, these results will provide new choices for designing more efficient enzymatic systems used for cofactor engineering and some basic information for the studies of physiological roles of STH in vivo.
Supplemental Material
Download MS Word (46.1 KB)Disclosure statement
All authors declare there is no conflict of interest for this study.
Data availability statement
The datasets used or analyzed in the present study are available from the corresponding author on reasonable request.
Supplemental data
Supplemental data can be accessed at: https://doi.org/10.6084/m9.figshare.16644754.v3
Additional information
Funding
References
- Liu J, Li H, Zhao G, et al. Redox cofactor engineering in industrial microorganisms: strategies, recent applications and future directions. J Ind Microbiol Biotechnol. 2018;45(5):313–327.
- Haverkorn van Rijsewijk BR, Kochanowski K, Heinemann M, et al. Distinct transcriptional regulation of the two Escherichia coli transhydrogenases PntAB and UdhA. Microbiology (Reading). 2016;162(9):1672–1679.
- Sauer U, Canonaco F, Heri S, et al. The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem. 2004;279(8):6613–6619.
- Voordouw G, van der Vies SM, Themmen APN. Why are two different types of pyridine nucleotide transhydrogenase found in living organisms? Eur J Biochem. 1983;131(3):527–533.
- Spaans SK, Weusthuis RA, van der Oost J, et al. NADPH-generating systems in bacteria and archaea. Front Microbiol. 2015;29:742.
- Alina S, Meike B, Michael B. NADPH-related processes studied with a SoxR-based biosensor in Escherichia coli. MicrobiologyOpen. 2018;8(7):e785.
- Canonaco F, Hess TA, Heri S, et al. Metabolic flux response to phosphoglucose isomerase knock-out in Escherichia coli and impact of overexpression of the soluble transhydrogenase UdhA. FEMS Microbiol Lett. 2001;204(2):247–252.
- Hua Q, Yang C, Baba T, et al. Responses of the central metabolism in Escherichia coli to phosphoglucose isomerase and glucose-6-phosphate dehydrogenase knockouts. J Bacteriol. 2003;185(24):7053–7067.
- Zhu GP, Golding GB, Dean AM. The selective cause of an ancient adaptation. Science. 2005;307(5713):1279–1282.
- Zhao HJ, Wang P, Huang EQ, et al. Physiologic roles of soluble pyridine nucleotide transhydrogenase in Escherichia coli as determined by homologous recombination. Ann Microbiol. 2008;58(2):275–280.
- Meng J, Wang B, Liu D, et al. High-yield anaerobic succinate production by strategically regulating multiple metabolic pathways based on stoichiometric maximum in Escherichia coli. Microb Cell Fact. 2016;15(1):141.
- Fu J, Wang Z, Chen T, et al. NADH plays the vital role for chiral pure D-(-)-2, 3-butanediol production in Bacillus subtilis under limited oxygen conditions. Biotechnol Bioeng. 2014;111(10):2126–2131.
- Fu J, Huo G, Feng L, et al. Metabolic engineering of Bacillus subtilis for chiral pure meso-2,3-butanediol production. Biotechnol Biofuels. 2016;9:90.
- Olajuyin AM, Yang M, Mu T, et al. Enhanced production of succinic acid from methanol-organosolv pretreated Strophanthus preussii by recombinant Escherichia coli. Bioprocess Biosyst Eng. 2018;41(10):1497–1508.
- Robert N, Andreas A, Ilona W, et al. Engineering Pseudomonas putida KT2440 for the production of isobutanol. Eng Life Sci. 2020;20(5–6):148–159.
- Li Z, Ding D, Wang H, et al. Engineering Escherichia coli to improve tryptophan production via genetic manipulation of precursor and cofactor pathways. Synth Syst Biotechnol. 2020;5(3):200–205.
- Decorosi F, Lori L, Santopolo L, et al. Characterization of a Cr(VI)-sensitive Pseudomonas corrugata 28 mutant impaired in a pyridine nucleotide transhydrogenase gene. Res Microbiol. 2011;162(8):747–755.
- Nikel PI, Pérez-Pantoja D, de Lorenzo V. Pyridine nucleotide transhydrogenases enable redox balance of Pseudomonas putida during biodegradation of aromatic compounds. Environ Microbiol. 2016;18(10):3565–3582.
- Lee HC, Kim JS, Jang W, et al. High NADPH/NADP+ ratio improves thymidine production by a metabolically engineered Escherichia coli strain. J Biotechnol. 2010;149(1–2):24–32.
- Jan J, Martinez I, Wang Y, et al. Metabolic engineering and transhydrogenase effects on NADPH availability in Escherichia coli. Biotechnol Prog. 2013;29(5):1124–1130.
- Cai D, He P, Lu X, et al. A novel approach to improve poly-γ-glutamic acid production by NADPH regeneration in Bacillus licheniformis WX-02. Sci Rep. 2017;7:43404.
- Liu B, Xiang S, Zhao G, et al. Efficient production of 3-hydroxypropionate from fatty acids feedstock in Escherichia coli. Metab Eng. 2019;51:121–130.
- Mu Q, Zhang S, Mao X, et al. Highly efficient production of L-homoserine in Escherichia coli by engineering a redox balance route. Metab Eng. 2021;67:321–329.
- Xu W, Yao J, L. Ma, X L, et al. Improving squalene production by enhancing the NADPH/NADP+ ratio, modifying the isoprenoid-feeding module and blocking the menaquinone pathway in Escherichia coli. Biotechnol Biofuels. 2019;12:68.
- Chin JW, Khankal R, Monroe CA, et al. Analysis of NADPH supply during xylitol production by engineered Escherichia coli. Biotechnol Bioeng. 2009;102(1):209–220.
- Luo ZW, Kim WJ, Lee SY. Metabolic engineering of Escherichia coli for efficient production of 2-pyrone-4, 6-dicarboxylic acid from glucose. ACS Synth Biol. 2018;7(9):2296–2307.
- Yang Z, Zhang Z. Production of (2R, 3R)-2, 3-butanediol using engineered Pichia pastoris: strain construction, characterization and fermentation. Biotechnol Biofuels. 2018;11:35.
- Cao Z, Song P, Xu Q, et al. Overexpression and biochemical characterization of a soluble pyridine nucleotide transhydrogenase from Escherichia coli. FEMS Microbiol Lett. 2011;320(1):9–14.
- French CE, Boonstra B, Bufton KAJ, et al. Cloning, sequence, and properties of the soluble pyridine nucleotide transhydrogenase of Pseudomonas fluorescens. J Bacteriol. 1997;179(8):2761–2765.
- Voordouw G, van der Vies S, Scholten JW, et al. Pyridine nucleotide transhydrogenase from Azotobacter vinelandii: differences in properties between the purified and the cell-free extract enzyme. Eur J Biochem. 1980;107(2):337–344.
- van den Broek HWJ, Santema JS, Veeger C. Pyridine nucleotide transhydrogenase 3. Effect of NADP+ on the spectral properties of transhydrogenase from Azotobacter vinelandii. Eur J Biochem. 1971;24(1):55–62.
- Boonstra B, Björklund L, French CE, et al. Cloning of the sth gene from Azotobacter vinelandii and construction of chimeric soluble pyridine nucleotide transhydrogenases. FEMS Microbiol Lett. 2000;191(1):87–93.
- Wang P, Lv C, Zhu G. Novel type II and monomeric NAD+ specific isocitrate dehydrogenases: phylogenetic affinity, enzymatic characterization, and evolutionary implication. Sci Rep. 2015;16:9150.
- Collins PA, Knowles CJ. Transhydrogenase activity in the marine bacterium Beneckea natriegens. Biochim Biophys Acta. 1977;480(1):77–82.
- Widmer F, Kaplan NO. Regulatory properties of the pyridine nucleotide transhydrogenase from Pseudomonas aeruginosa. Kinetic studies and fluorescence titration. Biochemistry. 1976;15(21):4693–4699.
- Argyrou A, Blanchard JS. Flavoprotein disulfide reductases: advances in chemistry and function. Prog Nucleic Acid Res Mol Biol. 2004;78:89–142.
- Bykova NV, Rasmusson AG, Igamberdiev AU, et al. Two separate transhydrogenase activities are present in plant mitochondria. Biochem Biophys Res Commun. 1999;265(1):106–111.
- Thompson H, Tersteegen A, Thauer RK, et al. Two malate dehydrogenases in Methanobacterium thermoautotrophicum. Arch Microbiol. 1998;170(1):38–42.
- van den Broek WJ, Veeger C. Pyridine-nucleotide transhydrogenase. 5. Kinetic studies on transhydrogenase from Azotobacter vinelandii. Eur J Biochem. 1971;24(1):72–82.
- Mouri T, Shimizu T, Kamiya N, et al. Design of a cytochrome P450BM3 reaction system linked by two-step cofactor regeneration catalyzed by a soluble transhydrogenase and glycerol dehydrogenase. Biotechnol Prog. 2009;25(5):1372–1378.