Abstract
Secondary fermentation is generally recommended to improve the aroma, flavour, overall acceptability and stability of the wines. Pediococcus acidilactici BD16 expressing L-alanine dehydrogenase gene is hereby proposed as a starter culture bacterium for the value addition of ginger, kiwi, plum and rose wines through secondary fermentation. Secondary fermentation using Pediococcus acidilactici BD16 (alaD+) imparted a natural sweetness and several health-promoting attributes to the dry wines, thereby improving their sensory and quality attributes. GC-MS-based metabolic fingerprinting further revealed the presence of significantly higher levels of L-alanine and many bioactive secondary metabolites and flavour enhancers such as 3-butynol, acetaldehyde, alaninol, aminopentols, benzene methanol, octanoic acid, ribitol, etc. and therapeutic ingredients such as 8-azanonane, actinobolin, adamantanemethylamine, aminononadecane, amphetamine, benzeneethanamine, guanosine, heptadecanenitrile, isopropyluriedoacetate, octanoic acid, nortriptyline and rimantadine in the secondary fermented wines. Further, a probable metabolic pathway was constructed to indicate major metabolic biotransformations in the wines which accounted for the improved quality attributes and strongly anticipated their therapeutic potential. This study is probably the first of its kind to highlight the application of P. acidilactici BD16 (alaD+) for in situ L-alanine production during secondary wine fermentation for enhancing organoleptic and therapeutic attributes of interest to health-conscious consumers.
Introduction
In the modern era, wine has become one of the most popular starch fermented and fortified alcoholic beverages, being marked as a sign of social status and royalty. Since ancient times, moderate wine consumption has been suggested to offer several health benefits such as reducing the risks associated with ageing, atherosclerosis, cardiac disorders, and other chronic diseases [Citation1–3]. Wine has also been proposed as an aspiring agent for promoting longevity [Citation3]. Usually, wine is a highly enriched decoction of polyphenols and other valuable compounds, even though, secondary fermentation is being recommended after alcoholic fermentation to further improve its quality and desirability. Secondary fermentation mainly involves the conversion of dicarboxylic L-malic acid to monocarboxylic L-lactic acid using suitable lactic acid bacterial strains such as Oenococcus oeni, Lactobacillus sp., and Leuconostoc oenus that primarily aims at deacidification of the wines [Citation2,Citation4,Citation5]. Earlier studies have convincingly indicated the role of lactic acid bacteria (LAB) associated biotransformations in the production of significant classes of compounds such as alcohols, amino acids, amines, aldehydes, and organic compounds which considerably improve quality, acceptability and flavour attributes of the wines during secondary fermentation [Citation6,Citation7]. In a study by Kaur and co-workers, utilisation of Pediococcus sp. has been proposed for the secondary fermentation of wine, which requires just 7 days for the completion after alcoholic fermentation by yeasts. Vanillin producing recombinant P. acidilactici BD16 expressing feruloyl-CoA synthetase and enoyl-CoA hydratase enzymes has been reported to improve flavour and desirability of the wines by in situ production of vanillin and other bioactive phenolics [Citation2].
Dry wines are generally more bitter and astringent due to the complete conversion of fruit sugar during primary alcoholic fermentation. The addition of sugar after primary fermentation is generally not recommended as it again initiates the process of fermentation and also invites many spoilage-causing food pathogens that multiply and deteriorate the final product in due course of time. Thus, in the case of dry wines, secondary fermentation using a natural sweetness imparting LAB strain is highly recommended to reduce astringency and bitterness, and to enhance key quality attributes, desirability and consumer acceptability of the wines.
Since ancient times, LAB have been used as starter culture organisms to produce functional foods with enhanced nutritive and therapeutic properties. Their well-elucidated genetic and regulatory features and the availability of the robust expression systems make them potential candidates for the production of industrially valuable metabolites for food, feed and pharmaceutical applications [Citation8–10]. Owing to their GRAS (Generally Recognised As Safe) status and probiotics nature, LAB have been extensively harnessed for their food based applications [Citation11,Citation12]. This has aroused a dire need to employ more advanced gene editing tools such as systems metabolic engineering, synthetic biology and enzyme engineering approaches for the development of highly efficient LAB strains to widen their prospects of commercialisation [Citation13,Citation14]. However, the potential use of LAB strains for food based applications have still been limited due to the safety and stability aspects concerned with the gene edited microorganisms [Citation15,Citation16]. The biosafety of the gene edited microorganisms is the only concern which is difficult to address here, but a thoroughly tested and validated strain, along with the spread of awareness among the consumers regarding use of genetically modified microorganisms can change this scenario.
L-alanine is a Food and Drug Administration (FDA) approved food additive and nutritional supplement, mainly used as a low-calorie sweetener and fat substitute [Citation17,Citation18]. It is an ideal ingredient for developing pharmaceutics for the treatment of hypoglycemia, kidney and liver diseases, prostate hypertrophy, and urea cycle disorders [Citation19–21]. Also, it has been widely used as a fragrance ingredient, hair and skin conditioning agent in cosmetics and personal care products [Citation22,Citation23]. In addition, it also provides high energy during intensive workouts, thus is added as a supplement to energy drinks to enhance athletic performance [Citation24]. L-alanine is mainly used in the food industry; thus the functional LAB strains could serve as potential hosts for the in situ L-alanine production during secondary wine fermentation. Therefore, L-alanine producing P. acidilactici BD16 (alaD+) strain carrying pLES003alaD is hereby proposed as a starter culture bacterium for performing secondary fermentation of ginger, kiwi, plum and rose wines. Secondary fermentation of wines was carried out for seven days and variations in the cell biomass and in situ L-alanine production were evaluated. Further, GC-MS-based metabolomic fingerprinting was performed to identify key metabolic biotransformations and bioactive ingredients which are responsible for improving wine attributes. The GC-MS-based metabolomic fingerprinting study has convincingly strengthened the argument of alanine dehydrogenase expressing P. acidilactici BD16 (alaD+) as a starter culture bacterium for performing secondary wine fermentation and intensified the therapeutic claim of wines by identifying novel, valuable and health-promoting metabolic attributes.
Materials and methods
P. acidilactici BD16 (alaD+) strain and culture conditions
A synthetic alaD gene encoding alanine dehydrogenase enzyme, which catalyses the reductive amination of pyruvate to L-alanine, was designed in silico and further cloned into pLES003 vector and expressed using auto-inducible P289 promoter [Citation17,Citation18]. pLES003 vector is a shuttle vector that can replicate in both Gram-negative E. coli as well as in Gram-positive LAB strains [Citation25,Citation26]. The sequence of the synthetic alaD gene construct is available in the Genbank database under the accession number MT108231. Recombinant pLES003alaD vector was further introduced into native P. acidilactici BD16 (MTCC 10973) strain through standard genetic engineering procedures as mentioned earlier by Kaur and co-workers [Citation27]. P. acidilactici BD16 (alaD+) harboring pLES003alaD vector was revived and multiplied in de Man’s Rogosa Sharpe Medium (MRS) broth containing dextrose 20 g/L, beef extract 10 g/L, peptone 10 g/L, sodium acetate 5 g/L, yeast extract 5 g/L, tri-ammonium citrate 2 g/L, di-potassium hydrogen phosphate 2 g/L, magnesium sulfate 0.1 g/L, manganous sulfate 0.05 g/L, erythromycin 20 μg/mL and Tween 80 1 mL/L, pH 6.5 ± 0.2 under microaerophilic and stationary conditions at 37 °C for 24 h. Antibiotic pressure was maintained during the revival and subculturing of the recombinant P. acidilactici (alaD+) strain to maintain its copy number. Keeping in view the food safety aspects, antibiotic addition was avoided during further experiments such as inoculum preparation and secondary fermentation of the wines.
Wine production
Dry wines including Ginger, Kiwi, Plum, and Rose were prepared according to the following methods:
Ginger wine: To prepare ginger wine, 0.666 kg fresh ginger was procured from the vegetable market. After peeling and washing with potassium metabisulfite solution (KMS; 200ppm), it was further crushed using a mechanical blender and 10 L wine must was prepared by the addition of Reverse Osmosis (RO) purified water. Sugar content in the must was adjusted to 22°Brix using table sugar and pH was adjusted to 4.2 by the addition of fresh lime juice.
Kiwi wine: 2.5 kg Kiwi fruit pulp was procured from the Himachal Pradesh Horticultural Produce Marketing and Processing Corporation Limited (HPMC), Solan, Himachal Pradesh, India. Further, 10 L fruit must was prepared by the addition of RO purified water. Sugar content in the must was adjusted to 22°Brix using table sugar and pH was adjusted to 4.2 by the addition of fresh lime juice.
Plum wine: 2.5 kg plum fruit pulp was procured from the HPMC, Solan, Himachal Pradesh, India. Further, 10 L fruit must was prepared by the addition of RO purified water. Sugar content in the must was adjusted to 22°Brix using table sugar and pH was adjusted to 4.2 by the addition of fresh lime juice.
Rose wine: For the preparation of rose wine, about 0.666 kg freshly harvested petals of the wild rose were procured from the local market. After washing with potassium metabisulfite solution (KMS; 200 ppm), petals were crushed using a mechanical blender and 10 L wine must was prepared by the addition of RO purified water. Sugar content in the must was adjusted to 22°Brix using table sugar and pH was adjusted to 4.2 by the addition of fresh lime juice.
After preparation of the musts, primary fermentation was initiated by the addition of 200 ppm of the Lalwin yeast EC1118 at 25 °C for 15–20 days. After completion of the primary fermentation, wine yeast was separated by settling and wines were clarified by rac-king. The sugar content of the prepared wines was estimated by determining the true Degree Brix of the solution using a handheld refractometer [Citation28]. The alcoholic content of the wines was estimated using a standard distillation procedure as proposed by Park and co-workers [Citation29].
Secondary fermentation of the dry wines
The clarified wines were further subjected to the secondary fermentation using P. acidilactici (alaD+) culture (@ 2% v/v freshly prepared inoculum containing 106 cfu/mL) and kept for incubation at 37 °C for 7 days under stationary conditions [Citation2]. Wines which were not subjected to the secondary fermentation process using recombinant LAB strain (but incubated under similar conditions) were used as controls for the comparative biochemical analysis. During secondary fermentation, the cellular biomass and in situ L-alanine production were estimated periodically for seven days. Secondary fermentation of the prepared wines was also performed using wild-type P. acidilactici BD16 for seven consecutive days for the comparative analysis.
Analysis of the bacterial growth
During secondary fermentation, the growth pattern of P. acidilactici BD16 (alaD+) in different dry wine samples was observed for seven consecutive days. For estimation, 1 mL of each wine sample was withdrawn after proper mixing and centrifuged at 12000 × g for 5 min to harvest the pellet. During fermentation, the growth profile of P. acidilactici BD16 (alaD+) was studied by determining cell density in terms of colony-forming units (cfu/mL).
In-situ L-alanine production
In situ L-alanine production during secondary wine fermentation was estimated for seven consecutive days on daily basis using the method reported by Citation30. For this, 1 mL wine sample was withdrawn and 5 mL Dichlone reagent (prepared by dissolving and diluting 62.5 mg Dichlone in dimethyl sulfoxide to make a final concentration of 2.5 mg/mL) was added and kept for incubation in a boiling water bath for 10 min to allow development of the orange colour. Further after cooling, the reaction mixture was diluted in 94 mL of 0.5 M HCl, and absorbance was measured at 470 nm against blank to quantify the L-alanine contents present in different wine samples [Citation17,Citation18]. The concentration of L-alanine in the control wine samples was also estimated using the same procedure to compare its titer with the secondary fermented wines. The enantiomeric purity of the in situ produced L-alanine was estimated by analysing its specific optical rotation using a polarimeter [Citation31].
Sensory evaluation of wines
Sensory evaluation of the respective wine samples was carried out to test their consumer liking, sensory attributes, and overall acceptability. Sensory analysis of the fermented drinks was performed among a panel of 10 healthy volunteers of both the genders (6 females and 4 males; including authors, research scholars and faculty from the Department of Biotechnology, Punjabi University, Patiala, Punjab, India) within the age group of 20 and 45 years. The participation of all the members was voluntary and no monetary compensation was given. The volunteers were first briefed about the experiment and were randomly served with about 20 mL of each wine sample. They were asked to drink water every time before testing the next sample and were then asked to record the variations in the wine attributes in terms of colour, taste, mouth-feel, odor and overall acceptability. The variations were recorded in terms of Hedonic scale parameters and radar graphs. The 9-point Hedonic scale was used to evaluate where the degree of acceptance (between extremely liked to extremely disliked) [Citation2,Citation32,Citation33].
Comparative wine metabolomics and identification of key metabolic biotransformations during the secondary wine fermentation process
For the comparison of wine metabolites, Gas Chromatography–Mass Spectrometry (GC-MS) based analysis was performed using unfermented control and the wines which were subjected to the secondary fermentation using P. acidilactici BD16 (alaD+). For this, 1 mL of each wine sample was drawn and was extracted using ethyl acetate and centrifuged at 12000 × g for 5 min. After centrifugation, the upper layer was separated and transferred to a fresh Eppendorf for its derivatisation. The derivatisation was carried out by adding mixture of N,O-bis (trimethylsilyl) trifluroacetamide (BSTFA): ethyl acetate: pyridine in the ratio of 3:1:1 v/v/v) in equal amount and was kept for incubation at 37 °C for 24 h. Extraction of wine metabolites in ethyl acetate and their further derivatisation with BSTFA facilitates the chromatographic separation by reducing polarities of the functional groups present in different analytes [Citation34]. The prepared samples were then injected manually in the GS-MS system (Perkin Elmer Clarus 500). The GC-MS protocol for the comparative wine metabolomics standardised by the authors includes the following conditions: DB-5MS capillary column (25 × 0.2 nm ID, 0.25 um film thickness) operating in an electron impact mode at 70 eV; using helium as a carrier gas at a constant flow of 1 mL/min and an injection volume of 1 μL, employed at an injector temperature of 250 °C. The selected temperature range was 130–210 °C, with an incremental increase of 10 °C/min. Mass spectra were captured at 70 eV; at a scan interval of 0.5 sec and fragments from 40 to 550 Da were recorded. The mass spectra of the separated components were compared with those stored in the National Institute of Standards and Technology (NIST) database for compound identification [Citation35]. The fold changes in wine metabolites were calculated using the following standard formula [Citation36]:
Fold change = PH after secondary fermentation − PH before secondary fermentation
PH before secondary fermentation
where PH = Peak height.
Statistical analysis
All the estimations were performed in triplicates and the observed values were averaged to calculate the mean, standard deviation and standard error. p-value was calculated using Chi-square test to substantiate the statistical significance of the data.
Results
Effect of P. acidilactici BD16 (alaD+) on key wine attributes
The present study aims at the application of P. acidilactici BD16 (alaD+) for the in situ production of L-alanine during secondary wine fermentation. The sugar content of the selected wines was estimated in terms of true degree Brix, which ranged from 5 to 6 degrees for all the four tested dry wines. While the alcohol contents of the wines varied from 10% to 12%, the alcohol content of ginger wine was estimated to be 10%, and in Kiwi, Plum and Rose wines it was found to be 12%, 11% and 10.5%, respectively. All the four selected wines were further subjected to a secondary fermentation process, which was carried out for seven consecutive days under ambient conditions to facilitate modulation of the key wine attributes and for the production of therapeutic metabolites of bacterial origin. In situ L-alanine production in the wild type P. acidilactici BD16 strain was almost negligible to report here in comparison to the recombinant strain when estimated spectrophotometrically. Therefore, only the recombinant P. acidilactici BD16 (alaD+) was taken further for application-based studies and metabolomic fingerprinting of the dry wines.
Analysis of bacterial growth during secondary wine fermentation
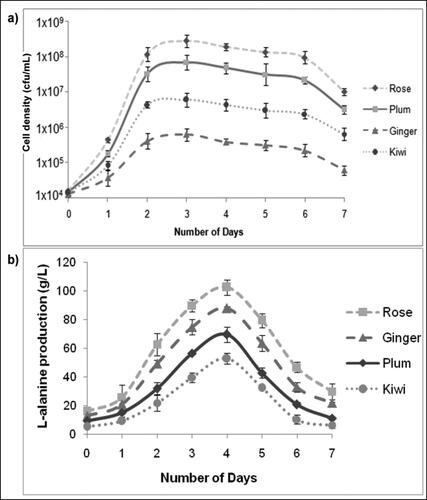
The growth pattern of P. acidiclactici BD16 (alaD+) in different dry wines was studied for seven consecutive days on the daily basis. From the observed values, growth plots depicting variations in the cellular biomass (in terms of cfu/mL) were constructed for ginger, kiwi, plum and rose wines which revealed a logarithmic growth phase which initiated at 24 h post-inoculation due to rapid bacterial multiplication (as depicted in ). Maximum growth was observed on the third day and then the cell density remained in a plateau for the next 3 days, which was followed by initiation of the decline phase after 6 days of inoculation. The maximum cell density of the P. acidiclactici BD16 (alaD+) was observed in the case of rose wine, followed by plum, kiwi and ginger wine. After achieving maximum growth, the cell counts started declining subsequently due to the scarcity of nutrients and enhanced production of L-alanine and other secondary metabolites.
In-situ L-alanine production during secondary wine fermentation
L-alanine production levels in the selected dry wines, i.e. ginger, kiwi, plum and rose were enhanced significantly (p < 0.005) after the secondary fermentation process. Before the secondary fermentation process, very low L-alanine levels were observed in the control (unfermented) wines, with a maximum value of 13.25 g/L in the case of rose wine, followed by 11.75, 9.25 and 5.25 g/L L-alanine reported in the case of ginger, plum and kiwi wines, respectively. To enhance L-alanine levels further and to impart sweetness to the selected dry wines, a secondary fermentation process was initiated by inoculating wines with P. acidilactici BD16 (alaD+) which lasted for approximately 7 days, and the in situ L-alanine production and its enantiomeric purity was estimated at regular intervals. It was evident from the results that the content of L-alanine (>97% enantiomeric pure) in dry wines enhanced drastically during the secondary fermentation process due to the expression of alanine dehydrogenase enzyme encoded by alaD gene in P. acidilactici BD16 (alaD+). A concomitant increase in the cellular biomass and L-alanine concentration was observed up to the third day, thereafter, a little downward trend in the cellular biomass was observed (). However, maximum in situ L-alanine production was reported on the 4th-day post-inoculation, which subsequently declined and marked the end of the secondary fermentation phase. Therefore, the optimal secondary fermentation period recommended for P. acidilactici BD16 (alaD+) is only four days. The process has resulted in the significantly higher L-alanine levels in the case of rose wine (102.5 g/L) followed by ginger (86.25 g/L), plum (69.25 g/L) and kiwi wines (52.75 g/L). The only soluble form of L-alanine was quantified in the process as the losses observed due to co-precipitation of L-alanine with wine phenolics are difficult to quantify. To date, in situ production of L-alanine in any of the dry wine using GRAS LAB strain has not been reported in the literature. Thus, this study is probably the first of its kind to envisage the role of P. acidilactici BD16 (alaD+) in a secondary fermentation process for the in situ production of a sugar substitute and bitterness masking agent L-alanine for imparting natural sweetness to the dry wines and improving their desirability.
Sensory evaluation of the wines before and after the secondary fermentation process
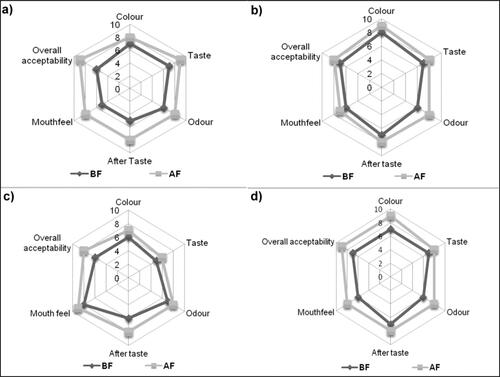
Various wine attributes like taste, odor, colour, texture, mouthfeel and overall acceptability were improved after secondary fermentation using P. acidilactici BD16 (alaD+). Wines before secondary fermentation were more astringent and bitter in taste, as well as comparatively lighter in colour. However, after secondary fermentation, the specific wine attributes changed significantly due to the enhanced levels of in situ produced L-alanine and other flavour-enhancing compounds, which provided a creamy mouthfeel, improved bulk properties, reduced astringency, reduced acidity and also improved the aromatic characteristics of the finished product. The highest acceptability was observed for the rose wine followed by ginger, plum and kiwi wine. It is evident from the radar graphs that the overall acceptability of the wines increased considerably after performing their secondary fermentation (). Hence, the present study strengthens the claim of P. (alaD+) strain as a starter culture candidate for imparting natural sweetness and masking off-flavours in the secondary fermented wines.
Figure 2. Radar plots showing sensory evaluation of dry wines before and after the secondary fermentation where (a) Rose wine (b) Ginger wine (c) Kiwi wine and (d) Plum wine; where BF-Before secondary fermentation; AF-After secondary fermentation. Hedonic scale parameters are graded as, 9-Like extremely; 8-Like very much; 7-Like moderately; 6-Like slightly; 5-Neither like nor dislike; 4-Dislike slightly; 3-Dislike moderately; 2-Dislike much; 1-Dislike extremely.
Comparative wine metabolomics and identification of key metabolic biotransformations during the secondary wine fermentation process
This GC–MS-based study has revealed many significant biochemical variations in the secondary fermented wines. Decisively, the quantity and the nature of bioactive compounds present in each wine sample increased considerably after secondary fermentation using P. acidilactici BD16 (alaD+). The study has revealed the presence of 21 compounds before and 41 after secondary fermentation of rose wine, while in the case of ginger wine, 43 compounds before and 54 after, in the case of plum wine, 24 compounds before and 59 after, and in the case of kiwi wine, 16 compounds before and 36 after secondary fermentation were identified. Manual integration with the available literature helped to predict their biological activities and potential implications as summarised in .
Table 1. GC-MS based metabolomics fingerprint data of Ginger wine (manually integrated with previously reported biological activities).
Table 2. GC-MS based metabolomics fingerprint data of Kiwi wine (manually integrated with previously reported biological activities).
Table 3. GC-MS based metabolomics fingerprint data of Plum wine (manually integrated with previously reported biological activities).
Table 4. GC-MS based metabolomics fingerprint data of Rose wine (manually integrated with previously reported biological activities).
The identified compounds belong to diverse classes like alcohols, aldehydes, alkanes, amines, amides, carbohydrates, carboxylic acids, ethers, esters, fatty acids, ketones and organic compounds, which contribute in the improvement of wine characteristics as well as enhance the anticipated therapeutic potential of the wines due to their anti-ageing, anti-bacterial, anti-cancer, anti-depressant, anti-fungal, anti-oxidant, anti-protozoan and anti-viral attributes [Citation45,Citation57,Citation61,Citation63,Citation66,Citation68], (Drugbank; PubChem Database (Db)). Analysis of the metabolomic fingerprinting data has convincingly revealed the accumulation of important classes of bioactive and flavour-enhancing metabolites while depletion of others due to differential activation or suppression of the respective biosynthetic pathways in P. acidilactici BD16 (alaD+) strain ( and ).
Figure 3. Probable metabolic pathway indicating significant biotransformations by P. acidilactici BD16 (alaD+) during secondary fermentation of the dry wines (Red highlighted metabolites depict major flux diversion of glycolytic flux from pyruvate into L-alanine pathway; Red block arrows show upregulation and downregulation of the major metabolites as analyzed from the GC-MS data). Enzymes involved are 1: Hexokinase; 2: Phosphoglucomutase; 3: Phosphose glucose isomerase; 4: Phosphofructokinase; 5: Pyruvate kinase; 6: Pyruvate dehydrogenase; 7: L-alanine dehydrogenase; 8: Alanine racemase; 9: Lactate dehydrogenase; 10: Lactate CoA transferase; 11: Phosphotransacetylase; 12: Acetyl-CoA hydrolase; 13: Acetyl Co-A thiolase; 14: Aldehyde dehydrogenase; 15: Alcohol dehydrogenase; 16: Threonine dehydratase; 17: Isoleucine transaminase; 18: β-Alanyl Co-A ammonia lyase; 19: β-Alanine CoA transferase; 20: Propionate CoA transferase; 21: Acetyl-CoA C-acetyl transferase; 22: Propionyl Co-A carboxylase; 23: Methyl malonyl Co-A mutase; 24: L-glutamate dependant transaminase; 25: Acetyl-CoA carboxylase; 26: Ribulose-5-phosphate isomerase; 27: Phosphoserine phosphatase; 28: Serine hydroxymethyl transferase; 29: Aconitase; 30: Aconitase; 31: Isocitrate dehydrogenase; 32: α-ketoglutarate dehydrogenase; 33: Succinyl CoA synthetase; 34: Succinate dehydrogenase; 35: Fumarase; 36: Malate dehydrogenase; 37: Citrate synthase; 38: Glutamate dehydrogenase; 39: Pyruvate decarboxylase; 40: Malic enzyme.
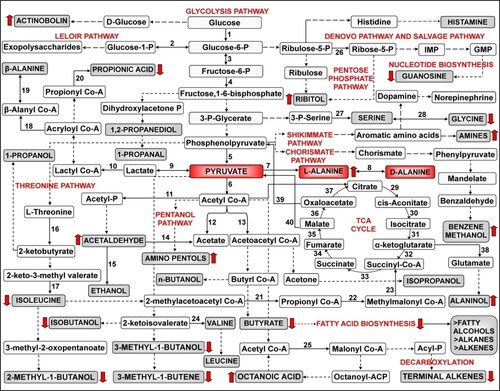
Levels of acetaldehyde, alaninol, aminopentols, benzene methanol, octanoic acid and ribitol showed an upward trend due to expression of the alanine dehydrogenase enzyme and reversal of the pyruvate flux. Reduction in the levels of certain wine metabolites, imparting bitterness and astringency to the wines, such as 2-butanamine, butyric acid, 2-methyl-1-butanol, 3-methyl-1-butanol, 1-methyldodecylamine, 1-propanol, 2-propanamine, 1,3-propanediamine and propionic acid was also observed. These compounds majorly belong to diverse classes including biogenic amines, fusel alcohols, hydroxyl acids and fatty acids, and are believed to be responsible for imparting unpleasant odour, unhealthy attributes and spoiled flavour to the alcoholic beverages. Biogenic amines such as 2-butanamine, 2-propanamine and 1,3-propanediamine usually provide a fishy, offensive and pungent odor, while alcohols such as 2-methyl-1-butanol, 3-methyl-1-butanol and 1-propanol contribute to the fusel odor and roasted flavour to the alcoholic beverages. Organic acids such as butyric and propionic acids are responsible for the fatty flavour and rancid or spoiled odor in the fermented alcoholic beverages. Thus, their reduction in fermented wines signifies the role of P. acidilactici BD16 (alaD+) in improving wine quality and sensory attributes.
L-alanine titers were enhanced significantly in all the wine samples which were subjected to the secondary fermentation process. In the case of ginger wine, L-alanine intensity enhanced by 7.16-fold, while in the case of kiwi, plum and rose wines, the values were elevated by 6.29, 5.53 and 7.51 folds, respectively. The increased intensities of 2-aminononadecane, 4-amino-1-pentanol, 8-azanonane, actinobolin, adamantane methylamine, benzene thanamine, benzene methanol, 3-butynol, heptadecanenitrile, octanoic acid, etc. are hereby suggested for the improvement in key wine attribute likes aroma, flavour, creamy mouthfeel, taste, overall acceptability and therapeutic attributes. Presence of acetylpyridine, butan-2-one, 4-octen-3-one, heptaldehyde, pyridine and ribitol has imparted a distinct flavour to the alcoholic beverages. In ginger wine, significantly higher levels of benzene methanol (9.44 folds), octanoic acid (6.52 folds), 2-aminononadecane (5.45 folds), actinobolin (4.48 folds) and 4-amino-1-pentanol (4.45 folds) were reported after secondary wine fermentation. In kiwi wine, a remarkable increase of 14.10 folds was observed in the case of 8-azanonane levels. Heptadecane nitrile also displayed a notable increase of 11.94 folds. Propanamine, an alkylamine, showed 4.44 folds higher intensity after secondary fermentation. In plum wine, heptadecanenitrile showed an increase of 10.67 folds, while the intensities of 8-azanonane and benzene methanol enhanced by 5.33 and 3.60 folds, respectively.
In the case of rose wine, maximum modulation in the wine’s metabolic profile was observed. With 20.59 folds enhanced intensity, adamantane methylamine became the most differentially expressed metabolite. An aromatic compound, 3-butynol also showed a remarkable increase of 17.16 folds. Benzene thanamine level was 14.99 folds higher after secondary fermentation. Heptanol, which is being primarily used as a flavoring enhancer in a variety of food and beverages, showed a significant increase of 2.29 folds.
Discussion
Pediococcus acidilactici strains are naturally resistant to the gastric acids and bile salts and possess an ability to produce many broad-spectrum and heat resistant antimicrobial peptides known as pediocins, which are active against many food spoilage bacteria and opportunistic human gut pathogens. These properties make them suitable candidates for several food-based applications [Citation17,Citation18,Citation73,Citation74]. P. acidilactici has already been declared a GRAS organism by FDA (GRN no. 000171); thus, it could be utilised as a food additive for imparting health-promoting attributes to the food products [Citation27]. Earlier, recombinant P. acidilactici BD16 (fcs+/ech+) was also explored to enhance flavour and aroma characteristics of the wines during secondary fermentation due to the increased concentration of cinnamic acid derivatives. Mass spectral analysis has revealed the development of many bioactive phenolic compounds such as apigenin, catechin, coniferyl aldehyde, cyanidin, hydroxybenzoic acids, laricitrin, luteolin, malvidin 3-glucoside, myricetin, naringenin, pelargonin, piceatannol, quercetin and vanillin during fermentation, which could be attributed for improving organoleptic and nutritional properties of the fermented wines. This study has convincingly highlighted the role of recombinant P. acidilactici BD16 (fcs+/ech+) in the production of several phenolic derivatives including vanillin during fermentation, which plays an important role in determining the organoleptic properties contributing to the aroma, colour and flavour of the finished product [Citation2].
Recently, an L-alanine producing recombinant P. acidilactici BD16 (alaD+) strain was used as a starter culture candidate for the production of functional buttermilk and soymilk. Fermentation using P. acidilactici BD16 (alaD+) strain has enhanced the intensities of many health-promoting and flavour enhancing compounds, while reduced the intensities of several compounds imparting bitterness, unpleasant flavour, rancidity and unhealthy attributes to the fermented drinks [Citation18].
Keeping in view the safety aspects regarding utilisation of the recombinant strain for food-based applications, biosafety assessment and evaluation of the probiotic attributes of recombinant P. acidilactici BD16 (alaD+) have also been carried out. Experimental trials were conducted for evaluation of its hemolytic, gelatinase and DNase activities. Results clearly demonstrated the lack of any kind of hemolytic, gelatinase and DNase activity in the developed recombinant LAB strain, thus proving its safety for the human consumption (Unpublished data). P. acidilactici BD16 (alaD+) has greatly enhanced the key wine attributes like aroma, flavour and fragrance, mouthfeel and overall acceptability. GC-MS-based wine metabolomics has facilitated the detection of a large number of flavour enhancers and therapeutic ingredients in the secondary fermented wines.
Wine metabolomics is a rapidly emerging field that allows the holistic identification of valuable compounds which are responsible for enhancing key wine characteristics and their therapeutic potential [Citation73]. Wine metabolomics involves the integration and application of various omics techniques for the characterisation and identification of the desired wine metabolites. The most commonly used instrumental techniques for metabolomic analysis include LC-MS, ESI-MS, GC-MS, NMR, etc. [Citation74]. Nowadays, GC-MS has been extensively used for metabolomics studies which allows sensitive and precise detection of biomolecules based on their interaction with the mobile phase (carrier gas) and the stationary phase [Citation1]. GC-MS-based wine metabolomics offers a great advantage over other chromatographic techniques due to its high resolving power, high accuracy and sensitivity, high reproducibility and easy separation of small volatile as well as semi-volatile compounds [Citation75,Citation76].
Cui et al. developed a LC-MS based method for the identification of organic acids and polyphenols in the red wine after decantation. About 20 organic acids and phenols were identified and the effect of other factors such as temperature, time, and light intensity on the decantation was also investigated [Citation77]. Schmutzer and coworkers also developed a simplified GC-MS protocol for the determination of important wine phenolics and flavonoids. Compounds like tyrosol, hydroxyl-anisic acid, hydroxyl-tyrosol, the ethyl ester of gallic acid and gallic acid were detected in the wines [Citation78]. Similarly, Angioni and others developed a GC-MS-based technique for the qualitative and quantitative determination of volatile compounds present in wines. A total of 24 compounds imparting aroma and flavour were identified using GC-MS which could be beneficial for enhancing the key quality and organoleptic attributes of various wines [Citation79]. Further, in 2015, the Silva research group has developed an ESI-MS based simple and efficient method for quick estimation of organic acids such as citric acid, malic acid, tartaric acid, quinic acid and galacturonic acid which contribute immensely in improving the organoleptic properties of wines [Citation80].
A highly sensitive and selective method for the quantification of wine polyphenols was developed by Lambert et al. [Citation81]. Using the UHPLC-QqQ-MS technique, authors were able to quantify up to 152 phenolic compounds in just 30 min [Citation81]. Recently, the approaches like an untargeted headspace solid-phase microextraction coupled with gas chromatography time-of-flight mass spectrometry and an untargeted reverse-phase ultra-high-performance liquid chromatography mass spectrometry, were used to study the volatile and non-volatile metabolite profiles of Pinot Noir wine. Results suggest that the non-volatile metabolites contribute to a greater extent in improving the quality and sensory attributes of wines in comparison to the volatile ones [Citation82]. However, most of the previous methods aimed at the selective identification and quantification of the wine phenolics and organic acids, thus ignoring many important classes that could also have significant contributions in improving organoleptic and therapeutic characteristics of the fermented beverages.
The present study highlights the importance of P. acidilactici BD16 (alaD+) in enhancing key wine attributes like aroma, flavour and fragrance, mouthfeel and overall acceptability. GC-MS-based metabolomics has facilitated the detection of a large number of aroma and flavour enhancers such as 3-butynol, 2-hepatnol, acetaldehyde, alaninol, aminopentols, benzene methanol, octanoic acid and ribitol etc. in the fermented beverages. Therapeutic ingredients such as L-alanine, L-2,4-diaminobutyric acid, N-methylallylamine, 2-butaneamine, 2-propaneamine, 4-amino-1-pentanol, 8-azanonane, acetanilide, actinobolin, adamantanemethylamine, aminononadecane, amphetamine, azastreptonigrin, benzeneethanamine, buthetamate citrate, dextroamphetamine, guanosine, heptadecanenitrile, propanediamine, isopropyluriedoacetate, meglumine, methylaminolauric acid, nortriptyline, octanoic acid, rimantadine and xanthatin were also detected in the secondary fermented wines. The presence of anti-depressants, modulators of the CNS and anti-cancer, anti-microbial and anti-viral agents, has convincingly highlighted the significance of the study.
Conclusions
To conclude, none of the previous reports has emphasised the nature of microbial biotransformations taking place during secondary fermentation of wines and hence never correlated the metabolic activities with wine characteristics. However, in the present study, P. acidilactici BD16 (alaD+) induced metabolic biotransformations have been addressed by adopting a GC-MS-based metabolite procedure and by manual integration approach. But, there are some limitations of the study as well. For example, despite the GRAS status of the P. acidilactici BD16 (alaD+), we cannot recommend it for final inclusion in an industrial procedure such as secondary wine fermentation until its complete genetic characterisation to rule out the presence of any kind of virulence character. Therefore, additional experiments at the molecular level need to be carried out to decipher its genotypic and phenotypic characteristics. Further, preclinical investigation in the healthy volunteers is also proposed to strengthen its safety for various food-based applications. In the future, authors will try to overcome these limitations so that substitution of the developed strain in the industrial bioprocess can be facilitated. However, the present study has provided sufficient experimental shreds of evidence to strengthen the role of P. acidilactici BD16 (alaD+) in secondary wine fermentation and has opened up new avenues for its industrial utilisation.
Consent to participate
All the 10 healthy volunteers who participated in the sensory evaluation of wines samples have given their consent to participate.
Acknowledgements
Authors acknowledge Indian Council of Medical Research (ICMR), New Delhi, India for providing Senior Research Fellowship (SRF) to Ms. Anshula Sharma, vide no. 2017-361-6/CMB/BMS.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Disclosure statement
All the authors declare no conflict of interest.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Alanon ME, Pérez-Coello MS, Marina ML. Wine science in the metabolomics era. TRAC-Trend Anal Chem. 2015;74:1–20.
- Kaur B, Kumar B, Kaur G, et al. Application of recombinant Pediococcus acidilactici BD16 (fcs+/ech+) in malolactic fermentation. Appl Microbiol Biotechnol. 2015;99(7):3015–3028.
- Pavlidou E, Mantzorou M, Fasoulas A, et al. Wine: an aspiring agent in promoting longevity and preventing chronic diseases. Diseases. 2018;6(3):73.
- Davis CR, Wibowo D, Eschenbruch R, et al. Practical implications of malolactic fermentation: a review. Am J Enol Viticult. 1985;36(4):290–301.
- Du Toit M, Engelbrecht L, Lerm E, et al. Lactobacillus: the next generation of malolactic fermentation starter cultures—an overview. Food Bioprocess Technol. 2011;4(6):876–906.
- Liu CL, Wang JM, Chu CY, et al. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem Toxicol. 2002;40(5):635–641.
- Volschenk H, Van Vuuren HJJ, Viljoen-Bloom M. Malic acid in wine: origin, function and metabolism during vinification. S Afr J Enol Vitic. 2006;27:123–136.
- Hatti-Kaul R, Chen L, Dishisha T, et al. Lactic acid bacteria: from starter cultures to producers of chemicals. FEMS Microbiol Lett. 2018;365(20):fny213.
- Rodríguez RLG, Mohamed F, Bleckwedel J, et al. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in Northern Argentina. Front Microbiol. 2019;10(1091):1091.
- Sharma A, Gupta G, Ahmad T, et al. Tailoring cellular metabolism in lactic acid bacteria through metabolic engineering. J Microbiol Methods. 2020;170:105862.
- Ayivi RD, Gyawali R, Krastanov A, et al. Lactic acid bacteria: Food safety and human health applications. Dairy. 2020;1(3):202–232.
- Raj T, Chandrasekhar K, Kumar AN, et al. Recent biotechnological trends in lactic acid bacterial fermentation for food processing industries. SMAB. 2021;1–27.
- Sharma A, Singh RS, Gupta G, et al. Metabolic engineering of enzyme-regulated bioprocesses. In: Advances in enzyme technology. Amsterdam, The Netherlands: Elsevier Publications; 2019a;293–323.
- Sharma A, Gupta G, Ahmad T, et al. Enzyme engineering: current trends and future perspectives. Food Rev Int. 2019b;37(2):121–154.
- Börner RA, Kandasamy V, Axelsen AM, et al. Genome editing of lactic acid bacteria: opportunities for food, feed, pharma and biotech. FEMS Microbiol Lett. 2019;366(1):fny291.
- Liu J, Chan SHJ, Chen J, et al. Systems biology–a guide for understanding and developing improved strains of lactic acid bacteria. Front Microbiol. 2019;10(876):876.
- Sharma A, Noda M, Sugiyama M, et al. Metabolic engineering of Pediococcus acidilactici BD16 for heterologous expression of synthetic alaD gene cassette and L-alanine production in the recombinant strain using fed-batch fermentation. Foods. 2021a;10(8):1964. 1.
- Sharma A, Noda M, Sugiyama M, et al. Production of functional buttermilk and soymilk using Pediococcus acidilactici BD16 (alaD+). Molecules. 2021b;26(15):4671.
- Braverman ER, Pfeiffer CE, Blum K, et al. Alanine: the hypoglycemia helper. In: The healing nutrients within: Facts, findings and new research on amino acids. 3rd ed. CA, United States of America: Accessible Publishing Systems Pty Ltd; 2009;461.
- Brennan L, Shine A, Hewage C, et al. A nuclear magnetic resonance-based demonstration of substantial oxidative l-alanine metabolism and l-alanine-enhanced glucose metabolism in a clonal pancreatic beta-cell line: metabolism of L-alanine is important to the regulation of insulin secretion . Diabetes. 2002;51(6):1714–1721.
- Tessem MB, Swanson MG, Keshari KR, et al. Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1H HR-MAS spectroscopy of biopsy tissues. Magn Reson Med. 2008;60(3):510–516.
- Dave UC, Kadeppagari RK. Alanine dehydrogenase and its applications - A review. Crit Rev Biotechnol. 2019;39(5):648–664.
- Leuchtenberger W, Huthmacher K, Drauz K. Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol. 2005;69(1):1–8.
- Oprea E, Ruta LL, Farcasanu IC. Pharmacological aspects and health impact of sports and energy drinks. In: Sports and energy drinks. Duxford, UK: Woodhead Publishing; 2019. p. 65–129.
- Noda M, Miyauchi R, Danshiitsoodol N, et al. Expression of genes involved in bacteriocin production and self-resistance in Lactobacillus brevis 174A is mediated by two regulatory proteins. Appl Environ Microbiol. 2018;84(7):e02707–17.
- Wada T, Noda M, Kashiwabara F, et al. Characterization of four plasmids harboured in a Lactobacillus brevis strain encoding a novel bacteriocin, brevicin 925A, and construction of a shuttle vector for lactic acid bacteria and Escherichia coli. Microbiology (Reading). 2009;155(Pt 5):1726–1737.
- Kaur B, Chakraborty D, Kumar B. Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl Microbiol Biotechnol. 2014a;98(20):8539–8551.
- Son HS, Hong YS, Park WM, et al. A novel approach for estimating sugar and alcohol concentrations in wines using refractometer and hydrometer. J Food Sci. 2009;74(2):C106–C111.
- Park WM, Park HG, Rhee SJ, et al. Properties of wine from domestic grape, vitis labrusca cultivar. Campbell’s early, fermented by carbonic maceration vinification process. Korean J Food Sci Technol. 2004;36(5):773–778.
- Shah SA, Rathod IS, Kanakia D. Colorimetry method for estimation of glycine, alanine and isoleucine. Indian J Pharm Sci. 2007;69(3):462–464.
- Shibatani T, Kakimoto T, Chibata I. Stimulation of L-asparate beta-decarboxylase formation by L-glutamate in Pseudomonas dacunhae and improved production of L-alanine. Appl Environ Microbiol. 1979;38(3):359–364.
- Kaur B, Kumar B, Kaur N, et al. Role of Lactobacillus fermentum as a starter culture for malolactic fermentation to improve quality of white wines. World J Pharm Pharm Sci. 2014b;3(3):1687–1712.
- Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 2009;34(9):739–751.
- Halket JM, Waterman D, Przyborowska AM, et al. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J Exp Bot. 2005;56(410):219–243.
- Iqbal Z, Mehmood HK, Hussain M, et al. Antioxidant activity of essential oil from the leaves and stems of murraya koenigii. WJPR. 2017;6(7):267–273.
- Mariani TJ, Budhraja V, Mecham BH, et al. A variable fold change threshold determines significance for expression microarrays. Faseb J. 2003;17(2):321–323.
- Kovač A, Majce V, Lenaršič R, et al. Diazenedicarboxamides as inhibitors of D-alanine-D-alanine ligase (DDL). Bioorg Med Chem Lett. 2007;17(7):2047–2054.
- Uma M, Jothinayaki S, Kumaravel S, et al. Determination of bioactive components of plectranthus amboinicus lour by GC-MS analysis. N Y Sci J. 2011;4(8):66–69.
- El-Emary TI. Synthesis and biological activity of some new pyrazolo [3, 4-b] pyrazines. J Chin Chem Soc. 2006;53(2):391–401.
- Rassem H, Nour AH, Yunus RM. GC-MS analysis of bioactive constituents of hibiscus flower. Aust J Basic Appl Sci. 2017;11:91–97.
- Ara I, Shinwari MMA, Rashed SA, et al. Evaluation of antimicrobial properties of two different extracts of Juglans regia tree bark and search for their compounds using gas chromatography-mass spectrum. Int J Biol. 2013;5(2):92.
- Stanek J, Frei J, Mett H, et al. 2-Substituted 3-(aminooxy)propanamines as inhibitors of ornithine decarboxylase: synthesis and biological activity. J Med Chem. 1992;35(8):1339–1344.
- Dias HR, Batdorf KH, Fianchini M, et al. Antimicrobial properties of highly fluorinated silver(I) tris(pyrazolyl)borates. J Inorg Biochem. 2006;100(1):158–160.
- Wijekoon MJO, Bhat R, Karim AA, et al. Chemical composition and antimicrobial activity of essential oil and solvent extracts of torch ginger inflorescence (Etlingera elatior jack.). Int J Food Prop. 2013;16(6):1200–1210.
- Obeidat M, Abu-Romman S, Odat N, et al. Antimicrobial and insecticidal activities of n-butanol extracts from some streptomyces isolates. Research J of Microbiology. 2017;12(4):218–228. doi:10.3923/jm.2017.218.228.
- Roy CL, Naresh S, Sunil KS, et al. GCMS and FTIR analysis on the methanolic extract of red Vitis vinifera peel. World J Pharm Sci. 2018;7(8):1110–1123.
- Seebacher W, Wolkinger V, Faist J, et al. Synthesis of 3-azabicyclo[3.2.2]nonanes and their antiprotozoal activities. Bioorg Med Chem Lett. 2015;25(7):1390–1393.
- Lamba A. Antimicrobial activities of aldehydes and ketones produced during rapid volatilization of biogenic oils [thesis]. USA: University of Missouri-Rolla; 2007.
- Hunt DE, Narkates AJ. Effect of actinobolin on nucleic acid and protein synthesis in Streptococcus faecalis. J Dent Res. 1971;50(6):1610–1615.
- McGahen JW, Neumayer EM, Grunert RR, et al. Influenza infections of mice: ii. curative activity of α‐methyl‐1‐adamantanemethylamine hcl (rimantadine hcl. Ann NY Acad Sci. 1970;173(1 Second Confer):557–581.
- Chen D, Wang Z, Zhang Y, et al. An amine: hydroxyacetone aminotransferase from Moraxella lacunata WZ34 for alaninol synthesis. Bioprocess Biosyst Eng. 2008;31(4):283–289.
- Runti C, De MN, Fabrissin S. Antiviral chemotherapeutic agents. XVIII. Adamantane derivatives of amphetamine. Their potential interest as autonomic and antiparkinson agents. Farmaco Ed Sc. 1975;30(4):260–275.
- Altaee N, Kadhim MJ, Hameed IH. Characterization of metabolites produced by E. coli AND analysis of its chemical compounds using GC-ms. Int J Curr Pharm Rev Res. 2017;7(6):13–19.
- Api AM, Belsito D, Bhatia S, et al. RIFM fragrance ingredient safety assessment, benzyl alcohol, CAS registry number 100-51-6. Food Chem Toxicol. 2015;84:S1–S14.
- Zan R, Hubbezoğlu I, Özdemir AK, et al. Antibacterial effect of different concentration of boric acid against Enterococcus faecalis biofilms in root canal. J Marmara Univ Dent Fac. 2013;1:76–80.
- Lu J, Chen ZW. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from drosophila virilis. Peptides. 2010;31(1):44–50.
- Ray AS, Yang Z, Chu CK, et al. Novel use of a guanosine prodrug approach to convert 2’,3’-didehydro-2’,3’-dideoxyguanosine into a viable antiviral agent. Antimicrob Agents Chemother. 2002;46(3):887–891.
- Lanznaster D, Dal-Cim T, Piermartiri TC, et al. Guanosine: a neuromodulator with therapeutic potential in brain disorders. Aging Dis. 2016;7(5):657–679.
- Kavitha R, Uduman MAM. Identification of bioactive components and its biological activities of abelmoschas moschatus flower Extrtact-A Gc-Ms study. IOSR J Appl Chem. 2017;10:19–22.
- Shalini K, Sharma PK, Kumar N. Imidazole and its biological activities: a review. Der Chemica Sinica. 2010;1(3):36–47.
- Bastin J, Boominathan M. Comparative analysis of the crude extract and active fraction of allium sativum against Streptococcus pnemoniae isolate from chronic illness patients. IJIPLS. 2011;1(2):28–35.
- Hussain AZ, Kumaresan S. GC-MS analysis and antibacterial evaluation of acalypha indica. Asian J Plant Sci Res. 2013;3(6):46–49.
- Denke MA, Grundy SM. Comparison of effects of lauric acid and palmitic acid on plasma lipids and lipoproteins. Am J Clin Nutr. 1992;56(5):895–898.
- Akpuaka A, Ekwenchi MM, Dashak DA, et al. Biological activities of characterized isolates of n-hexane extract of azadirachta indica A. Juss (neem) leaves. Nat Sci. 2013;11(5):141–147.
- Li H, Sumarah MW, Topp E. Persistence of the tricyclic antidepressant drugs amitriptyline and nortriptyline in agriculture soils. Environ Toxicol Chem. 2013;32(3):509–516.
- Salim EI, Wanibuchi H, Morimura K, et al. Inhibitory effects of 1,3-diaminopropane, an ornithine decarboxylase inhibitor, on rat two-stage urinary bladder carcinogenesis initiated by N-butyl-N-(4-hydroxybutyl)nitrosamine. Carcinogenesis. 2000;21(2):195–203.
- Asif HM, Sultana S, Akhtar N. A panoramic view on phytochemical, nutritional, ethanobotanical uses and pharmacological values of trachyspermum ammi linn. Asian Pac J Trop Biomed. 2014;4:S545–S553.
- Tao L, Sheng X, Zhang L, et al. Xanthatin anti-tumor cytotoxicity is mediated via glycogen synthase kinase-3β and β-catenin. Biochem Pharmacol. 2016;115:18–27.
- Amal KH, Sabeeh H, Ahmed MA. In vitro activity of alkaloids extracted from chlorophyta and cyanophyta against the hydatid disease compared with albendazole. Thi-Qar Med J. 2011;5(3):56–70.
- Singh S, More PK, Mohan SM. Curry leaves (murraya koenigii linn. Sprengal)-a mircale plant. Ind J Sci Res. 2014;4(1):46–52.
- Karlsten R, Hartvig P. High frequency of cough after intravenous bolus injection of Ketogan (ketobemidone + N,N-dimethyl-3,3-diphenyl-1-methylallylamine chloride) for postoperative pain relief. Acta Anaesthesiol Scand. 1992;36(2):193–194.
- Nibret E, Youns M, Krauth‐Siegel RL, et al. Biological activities of xanthatin from xanthium strumarium leaves. Phytother Res. 2011;25(12):1883–1890.
- Laranjo M, Potes ME, Elias M. Role of starter cultures on the safety of fermented meat products. Front Microbiol. 2019;10(853):853–811.
- Papagianni M, Anastasiadou S. Pediocins: the bacteriocins of pediococci. Sources, production, properties and applications. Microb Cell Fact. 2009;8(3):3–16.
- Diamantidou D, Zotou A, Theodoridis G. Wine and grape marc spirits metabolomics. Metabolomics. 2018;14(12):159.
- Dambergs R, Gishen M, Cozzolino D. A review of the state of the art, limitations, and perspectives of infrared spectroscopy for the analysis of wine grapes, must, and grapevine tissue. Appl Spectrosc Rev. 2015;50(3):261–278.
- Gromski PS, Muhamadali H, Ellis DI, et al. A tutorial review: metabolomics and partial least squares-discriminant analysis-a marriage of convenience or a shotgun wedding. Anal Chim Acta. 2015;879:10–23.
- Khakimov B, Gürdeniz G, Engelsen SB. Trends in the application of chemometrics to foodomics studies. Acta Aliment. 2015;44(1):4–31.
- Cui Y, Li Q, Liu Z, et al. Simultaneous determination of 20 components in red wine by LC-MS: application to variations of red wine components in decanting. J Sep Sci. 2012;35(21):2884–2891.
- Schmutzer G, Avram V, Coman V, et al. Determination of phenolic compounds from wine samples by GC/MS system. J Rev Chim. 2012;9:855–858.
- Angioni A, Pintore GA, Caboni P. Determination of wine aroma compounds by dehydration followed by GC/MS. J AOAC Int. 2012;95(3):813–819.
- do Nascimento Silva FL, Schmidt EM, Messias CL, et al. Quantitation of organic acids in wine and grapes by direct infusion electrospray ionization mass spectrometry. Anal Methods. 2015;7(1):53–62.
- Lambert M, Meudec E, Verbaere A, et al. A high-throughput UHPLC-QqQ-MS method for polyphenol profiling in rosé wines. Molecules. 2015;20(5):7890–7914.
- Sherman E, Coe M, Grose C, et al. Metabolomics approach to assess the relative contributions of the volatile and non-volatile composition to expert quality ratings of pinot noir wine quality. J Agric Food Chem. 2020;68(47):13380–13396.
- Altaee N, Kadhim MJ, Hameed IH. Characterization of metabolites produced by E. coli and analysis of its chemical compounds using GC-MS. Int J Curr Pharm Rev Res. 2017;7(6):13–19.
Web resources
- database: https://go.drugbank.com/.
- database: https://pubchem.ncbi.nlm.nih.gov/.