Abstract
In this study, we aimed to investigate the influence of soil preservation approaches, especially cryopreservation and high temperature-drying on the sequencing quality of its microbial community and the background microbial diversity information of fairy ring soil from Leucocalocybe mongolica. Through DNA metabarcoding surveys based on 16S rDNA and ITS (Internal Transcribed Spacer) barcodes, we observed that the bacterial abundance was notably changed when the soil samples were exposed in room temperature for 4 h, whereas the fungal composition was not significantly changed. Moreover, the soil samples preserved their major microbial structures even after high temperature-drying for 12 h, whereas their microbial diversity was influenced. Overall, a total of 9283 and 1871 OTUs (Operational Taxonomic Units) were obtained from soil bacteria and fungi, respectively, and we observed that Chthoniobacteraceae and Tricholomataceae were the dominant bacterial and fungal families in the fairy ring soil, respectively. Our study reveals the impact of soil processing methods on the microbial community compositions contributes to the understanding of fairy ring ecology.
Supplemental data for this article is available online at https://doi.org/10.1080/13102818.2021.1996272 .
Introduction
Soil biodiversity is mostly attributed to its rich contents of microorganisms, but currently most of these microbial taxa remain undescribed, which provides the importance for cultivation and edaphology studies [Citation1]. Sequencing technology is considered as the most effective approach on studying soil microbial diversity, but the sequencing results were differential and usually influenced by different soil processing methods [Citation2]. Moreover, the preservation process of soil samples has been reported to affect microbial DNA extraction and thus, directly determining the sequencing quality [Citation3–7]. The internal microbial diversity in soil, as a living biotic environment, after sampling can gradually or drastically change from its original habitat over time. In this case, cryopreservation is one of the common methods applied for soil processing, whereas the impacts of different storage temperature and duration on soil microbial sequencing results were controversial, in which most of the studies supported that only a little interference could be introduced [Citation4, Citation5, Citation8–11], while few others highlighted the existed interferences on soil microbial sequencing [Citation6, Citation12]. The divergence in these studies is possibly due to the differences in their research background or the targeted soil types, therefore, all of them possess significance as references on the basis of specific research objectives.
Since the soil storage temperature can influence the quality of high-throughput sequencing [Citation6, Citation12], there is possibility that cryopreservation for the first few hours after sampling might also have such impact, though extremely low temperature and fast preservation are commonly the best ways to ensure the authenticity of soil samples. Similar sequencing results were observed previously when comparing the soil samples preserved in liquid nitrogen (< −80 °C) and in a freezer (−20 °C) [Citation11]. However, no study has directly assessed the influence of immediate cryopreservation, which is usually needed for sampling at remote locations. On the other hand, efficient and radical method, with time-saving and high safety properties, in preserving soil samples other than ethanol processing [Citation3, Citation13], air-drying [Citation8] and freeze-drying [Citation7] is scarce. Drying at high temperature is a promising approach for long-term preservation, which is widespread applied in the biodiversity surveys for microbial DNA of the preserved specimen [Citation14].
The fairy ring is a unique mycological growth pattern usually shown in grasslands and formed by certain fungi living in the soil of special grassland habitat, which has the ecological values in promoting the yield of crops [Citation15]. Leucocalocybe mongolica, as one of the representative fairy ring fungi with a unique taxonomic status (belongs to a monotypic genus), is an incomplete domestication of a rare wild mushroom, which is edible, possesses medicinal values and only lives in fairy soil [Citation16]. Therefore, it is of a great significance to investigate fairy ring soil with L. mongolica and its microbial compositions, through which we could advance the studies of both fairy rings and L. mongolica domestication.
As soil serves as an unstable environment [Citation1, Citation2], large-scale and multi-point sampling from different geographical environments is crucial for studying the ecological diversity of fairy rings in the soil. Therefore, soil preservation methods, such as high temperature-drying approach, are critical to be evaluated for their influences on detecting the main microbial diversity structures of the soil samples. In addition, the microbial diversity of fairy ring soils could provide important reference values for the subsequent large-scale investigation, due to the limited numbers of studies and the inconsistent sequencing results [Citation17, Citation18].
Accordingly, we aimed to discuss the following three issues in this study: (i) whether the sequencing results could be affected by immediate in situ soil cryopreservation after sampling, (ii) whether the microbial structure in the soil sample could be preserved after high temperature-drying and (iii) to obtain the microbial diversity information and major species distribution patterns of bacteria and fungi in fairy ring soil of L. mongolica. We used the same fairy ring soil sample and divided it by three groups, with six biological replicates for each group. The sub-samples were processed with cryopreservation in situ, with initial 4 h exposure at room temperature, and with further 12 h of drying at high temperature (). 16S rDNA and ITS (Internal Transcribed Spacer) barcodes for bacteria and fungi analyses and DNA metabarcoding surveys for all the soil samples were further performed. Finally, we discussed the three issues mentioned above based on the analyses of microbial community compositions and diversity.
Table 1. General metabarcoding sequencing results of the soil samples.
Materials and methods
Materials
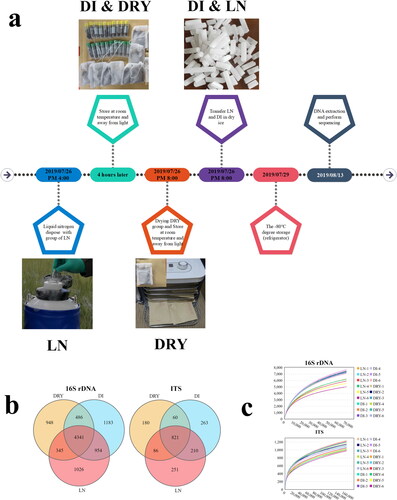
The soil samples were collected from a L. mongolica fairy ring, located in a prairie in Baorixile Town, HulunBuir City, Inner Mongolia autonomous region, China (49°29’N, 119°59’E, and elevation of 691 m). The pH of sampled soil was 6.4. The sampling area was divided into 3 parts, as Dark zone (Blue flags), Dead zone (Yellow flags) and Outer zone (Red flags), then 8 groups of totally 24 spots were evenly selected for sampling (). For each sampling spot, the soil was collected from the top-layer in a depth of 0–10 cm and a diameter of 5 cm using soil sample collector (). Then, the collected soil sample was screened by using a sieve with a 0.3 mm filter (). The soil samples were respectively packed with sterile cryo-storage tubes or paper bags for storage in cold or room temperature conditions, respectively (). The samples were further divided into 3 groups, designated as DRY (DRY-1 to DRY-6), DI (DI-1 to DI-6) and LN (LN-1 to LN-6), each with 6 biological replicates. Samples in the DRY group were kept at room temperature for 4 h after sampling, followed by flash drying for 12 h at 60 °C and long-term storage at room temperature in dry and dark conditions. Samples in the DI group were kept at room temperature for 4 h after sampling, transferred for cryopreservation in dry ice and preserved in cryogenic refrigerator (−80 °C) for long-term storage. Samples in the LN group were immediately transferred into liquid nitrogen for 4 h, then transferred for cryopreservation in dry ice and finally preserved in cryogenic refrigerator (−80 °C) for long-term storage (). The timeline of sample processing is shown in .
Figure 1. Differences in preservation methods and sequencing results of soil samples across the three groups. (a) The timeline of sample processing. (b) The Venn diagram of OTUs from metabarcoding sequencing, in which 16S rDNA represents bacteria and ITS represents fungi. (c) The rare faction curves of the 18 samples from 16S rDNA and ITS metabarcoding, in which the x-axis represents sequence volume and the y-axis represents OTU numbers.
DNA extraction, PCR amplification and sequencing
Microbial DNA was extracted using the HiPure Soil DNA Kits (Magen, Guangzhou, China) according to the manufacturer’s protocols. The 16S rDNA V3-V4 region of the ribosomal RNA gene was amplified by PCR using primers 341F: CCTACGGGNGGCWGCAG; 806R: GGACTACHVGGGTATCTAAT for bacteria [Citation19] and ITS3_KYO2: GATGAAGAACGYAGYRAA; ITS4: TCCTCCGCTTATTGATATGC for fungi [Citation20]. PCR was run under the following conditions: 94 °C for 2 min, 30 cycles of 98 °C for 10 s, 62 °C for 30 s and 68 °C for 30 s, and a final extension at 68 °C for 5 min. The PCR reactions were performed in triplicate, in a 50 μL mixture containing 5 μL of 10× KOD Buffer, 5 μL of 2 mM dNTPs, 3 μL of 25 mM MgSO4, 1.5 μL of each primer (10 μM), 1 μL of KOD Polymerase and 100 ng of template DNA. The amplicons were extracted from 2% agarose gels, purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions and quantified using ABI StepOnePlus Real Time PCR System (Life Technologies, Foster City, CA, USA). The purified amplicons were pooled in equimolar and paired-end sequenced (PE250) on an Illumina platform (Novaseq 6000 sequencing) according to the standard protocols.
Quality control, reads assembly and operational taxonomic units (OTUs) analysis
(a) Read filtering: Raw data containing adapters or low quality reads were further filtered according to the following rules using FASTP [Citation21]: (1) removing reads containing more than 10% of unknown nucleotides (N) and (2) removing reads containing less than 50% of bases with low quality (Q-value > 20). (b) Read assembly: The cleaned paired-end reads were merged as raw tags using FLSAH (version 1.2.11) [Citation22] with minimum overlap of 10 bp and mismatch error rate of 2%. (c) Raw tag filtering: Noisy sequences of raw tags were filtered by QIIME (version 1.9.1) pipeline [Citation23] under specific filtering conditions [Citation24] to obtain the high quality clean tags. (d) OTUs analysis: The effective tags were clustered into of OTU ≥ 97% similarity using UPARSE (version 9.2.64) pipeline [Citation25]. The tag sequence with the highest abundance was selected as the representative sequence within each cluster. Between groups-Venn analysis was performed in R project VennDiagram package (version 1.6.16) [Citation26].
Community compositional analysis
The representative sequences were classified into organisms by a naive Bayesian model using RDP classifier (version 2.2) [Citation27] based on SILVA database (version 132) [Citation28] for bacterial taxonomy and UNITE database (version 8.0) [Citation29] for fungal taxonomy with confidence threshold values ranged from 0.8 to 1. The abundance statistics of each taxonomy were visualized using Krona (version 2.6) [Citation30]. The stacked bar plot of the community composition was visualised in R project ggplot2 package (version 2.2.1) [Citation31]. The heat map of species abundance was plotted using pheatmap package (version 1.0.12, https://cran.r-project.org/web/packages/pheatmap/) in R project (version 2.5.3). The comparison of species among groups was computed by Kruskal-Wallis H test and Welch’s t test in R project Vegan package (version 2.5.3) [Citation32].
Alpha diversity analysis
Chao1, Simpson, and all other alpha diversity indices were calculated in QIIME (version 1.9.1) [Citation23]. OTU rarefaction curves were plotted in R project ggplot2 package (version 2.2.1) [Citation31]. The comparisons of alpha indices among groups were performed by Kruskal-Wallis H test in R project Vegan package (version 2.5.3) [Citation32].
Beta diversity analysis
Sequence alignment was performed using Muscle (version 3.8.31) [Citation33], and phylogenetic tree was constructed using FastTree (version 2.1) [Citation34]. Then weighted unifrac distance matrix was generated by GuniFrac package (version 1.0) in R project [Citation35]. The NMDS of weighted unifrac distances was generated in R project Vegan package (version 2.5.3) [Citation32] and plotted in R project ggplot2 package (version 2.2.1) [Citation31]. The statistical analyses of Kruskal-Wallis H test and Anosim test were calculated in R project Vegan package (version 2.5.3) [Citation32].
Results
Sequencing analysis
A total of 5,350,348 metabarcoding tags were obtained from sequencing data. The clustering analyses of the soil samples showed that there were 7171 and 1161 OUTs in average for bacteria (based on 16S rDNA) and fungi (based on ITS), respectively (). The Venn diagram exhibited that 46.76% (4341/9283) bacterial and 43.88% (821/1871) fungal OTUs were shared among the three groups of soil samples (). The leveled-off rarefaction curves of all the soil samples for both bacteria and fungi indicated the near saturation of the metabarcoding sequencing (). Generally, we found that the OTUs number of bacteria in the fairy ring soil was significantly higher than that of fungi.
Microbial community composition
Based on SILVA [Citation28] and UNITE [Citation29] databases, we analyzed the differences in the community compositions of soil bacteria and fungi among LN, DI and DRY groups. shows the microbial taxonomic structures for soil samples at the levels of phylum and family. For bacteria in LN, DI and DRY groups (, ), the top 10 most abundant phyla were Actinobacteria (22.3, 24.72 and 24.25%), Verrucomicrobia (22.91, 16.69 and 19.22%), Proteobacteria (15.93, 18.64 and 19.64%), Planctomycetes (16.2, 14.59 and 13.75%), Acidobacteria (10.25, 11.79 and 11.17%), Chloroflexi (3.74, 4.07 and 3.51%), Gemmatimonadetes (3.27, 3.63 and 3.73%), Bacteroidetes (1.43, 1.71 and 1.6%), Rokubacteria (0.65, 0.74 and 0.8%) and Patescibacteria (0.91, 0.86 and 0.4%); while the top 10 most abundant families included Chthoniobacteraceae (20.68, 15.02, and 17.78%), Gemmataceae (6.54, 5.55 and 6.52%), Pyrinomonadaceae (3.5, 4.09 and 3.52%), Tepidisphaerales sp.(3.91, 3.66 and 3.0%), Gemmatimonadaceae (2.95, 3.29 and 3.49%), Xanthobacteraceae (2.66, 2.92 and 3.82%), Sphingomonadaceae (2.34, 2.98 and 3.57%), Solirubrobacteraceae (2.32, 2.45 and 2.48%), Solirubrobacterales sp. (2.22, 2.45 and 2.54%), and Pirellulaceae (2.92, 2.35 and 1.89%). For fungal compositions in LN, DI and DRY groups (, Supplemental Tables S4 and S5), Basidiomycota (81, 82.1 and 68.49%), Ascomycota (17.04, 16.3 and 29.04%), and Anthophyta (1.2, 1.04 and 0.97%) were the dominant phyla; while Tricholomataceae (76.99, 78.41 and 65.05%), Geoglossaceae (1.11, 1 and 1.42%), Aspergillaceae (0.92, 0.79 and 1.45%), Clavariaceae (1.1, 1.02 and 0.76%) and Helotiales sp. (0.57, 0.56 and 1.02%) were the dominant families. Overall, we detected similar microbial compositional distribution patterns among the 3 groups of fairy ring soil.
Figure 2. OTUs taxonomic analysis of the soil samples across the three groups. (a–d) Taxonomy of bacteria (a,c) and fungi (b,d) at phylum (a,b) and family (c,d) levels, in which the x-axis represents the sample identification number (ID) and the y-axis represents the relative abundance. (e,f) Heatmap of the top 10 families of bacteria (e) and fungi (f), in which the x-axis represents the sample ID and the y-axis represents the family. (g,h) The Kruskal-Wallis test of the top 5 families of bacteria (g) and fungi (h), in which the x-axis represents the relative abundance and the y-axis represents the family. Tricholomataceae was excluded from the results of fungi.
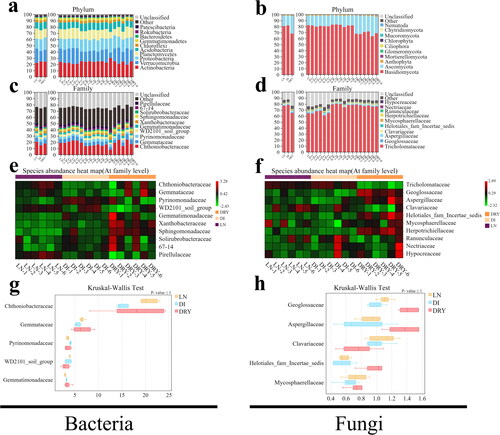
Table 2. Alpha diversity indices for the soil samples.
However, based on the heat maps (), when species were compared separately across all soil samples, we observed differences in the microbial abundance among the individual samples as well as across the groups. The relative abundances of the top 10 families of both bacteria and fungi in the DRY group exhibited various distribution patterns, which indicates that high temperature-drying process might affect the constancy of soil microbial sequencing. On the other hand, the relative abundances in 3 of the top 5 families, i.e. Chthoniobacteraceae, Gemmataceae and Pirellulaceae, in the LN group were higher than those in the DI group, whereas a higher abundance of Pyrinomonadaceae was found in the DI group (), indicating that the difference in 4 h initial preservation of soil samples could influence their microbial sequencing consistency. Moreover, the relative abundance of Tricholomataceae was lower in the DRY group when compared to those in LN and DI groups, while 4 of the top 5 families, i.e. Geoglossaceae, Aspergillaceae and Helotiales sp., were relatively higher in the DRY group but Clavariaceae showed the opposite result.
Additionally, in order to further clarify the differences we observed by visualisation in the heat map, the Kruskal-Wallis and Welch’s t-tests of bacteria () and fungi () were performed. The Kruskal-Wallis test result revealed that most of the top abundant families were insignificantly different and two of the dominant families of bacteria and fungi, i.e. Chthoniobacteraceae and Tricholomataceae, showed the biggest difference, in which Chthoniobacteraceae (P-value = 0.038) exhibited 5.66% differences in DI (LN:DI) and 2.9% differences in DRY (LN:DRY), while Tricholomataceae (P-value = 0.002) exhibited −1.43% differences in DI (LN:DI) and 11.94% in DRY (LN:DRY). The Welch’s t-test revealed that, when comparing the difference between LN and DI groups, 3 of the top 5 bacterial families exhibited significant difference, including Gemmataceae (0.99% difference, P-value = 0.008), Pyrinomonadaceae (−0.59% difference, P-value = 0.001) and Gemmatimonadaceae (−0.54% difference, P-value = 0.004) (). When comparing the difference between LN and DRY groups, 4 of the top 5 fungal families exhibited significant difference, including Geoglossaceae (−0.31% difference, P-value = 0.001), Aspergillaceae (−0.53% difference, P-value = 0.02), Clavariaceae (0.34% difference, P-value = 0.02) and Helotiales sp. (−0.45% difference, P-value = 0.003) (). To be noted, the fungal family Tricholomataceae was excluded from the Kruskal-Wallis test due to its exorbitant abundance. Besides, soil samples in the DRY group were associated with less repeatability in bacterial abundances (such DRY-1,2 vs DRY-3,5; ), and less differences in fungal abundances were observed between LN and DI groups (). Therefore, the Welch’s t-tests for bacterial abundance of LN vs DRY and for fungal abundance of LN vs DI were not performed. Overall, the abundance pattern of fungal community was moderately affected by different soil preservation methods, but the pattern of soil bacterial community was significantly influenced by different preservation methods.
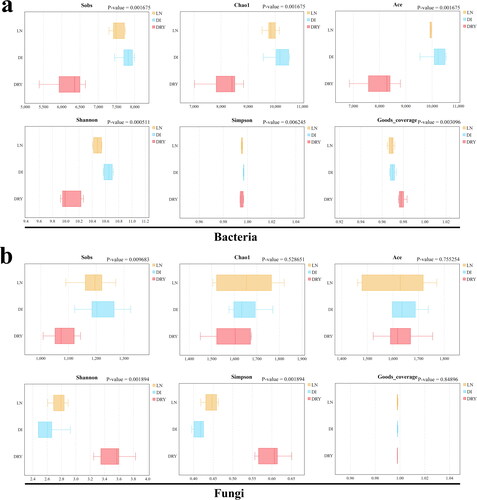
Alpha diversity analysis
We further sought to better understand the changes of both bacterial and fungal diversity in the soil samples introduced by in situ cryopreservation. As shown in and , a total of 6 alpha diversity indices were estimated, including Sobs (observed species, equal to the number of OTUs), Chao1, Ace, Shannon, Simpson and Goods_coverage. For bacteria, among all 18 soil samples, we detected Sobs values at a range of 5386 (DRY-6) to 7992 (DI-1), Chao1 from 7204 (DRY-6) to 10530 (DI-1), Ace from 6857 (DRY-6) to 10537 (DI-3), Shannon from 9.92 (DRY-5) to 10.72 (DI-1) and Simpson and Goods_coverage indices all exceeded 0.95 (). Among the 6 indices, Sobs (P-value = 0.002), Chao1 (P-value = 0.002) and Ace (P-value = 0.002) were significantly different across soil groups, in which the DRY group exhibited the lowest values ( and ), suggesting that drying soil at high temperature could reduce both the richness and diversity of bacterial species. For fungal alpha diversity, we observed Sobs ranging from 1009 (DRY-6) to 1325 (DI-3), Chao1 from 1450 (DRY-6) to 1822 (DI-1), Ace from 1463 (LN-6) to 1771 (LN-1), Shannon from 2.48 (DI-2,DI-3) to 3.83 (DRY-5), Simpson from 0.4 (DI-2,DI-3) to 0.65 (DRY-5) and Goods_coverage values all exceeded 0.99 (). The soil samples in the DRY group also had the lowest Sobs value (P-value = 0.01) but the highest Shannon value (P-value = 0.002), indicating that the high temperature-drying process could reduce the richness of fungal species but increase their diversity ( and ).
Beta diversity analysis
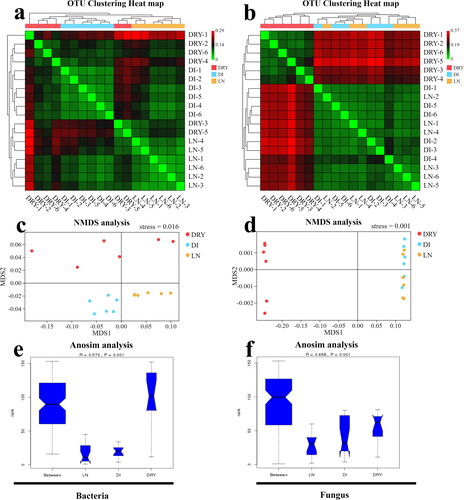
Finally, beta diversity analysis was performed in order to understand the variation of soil microbes within an individual group and across different groups. The beta diversity based on weighted unifrac distance index from OTUs is shown in . For bacteria, as shown in the heat map (), the soil samples in LN and DI groups clustered in their own branches, while the samples in the DRY group failed to cluster, and these findings were further confirmed by the non-metric multi-dimensional scaling (NMDS) analysis shown in . The analysis of similarities (Anosim), shown in , also revealed that, compared to the DRY group, LN and DI soil groups had lower intra-group differences (R-value = 0.573, P-value < 0.001), inferring the moderate inter- and intra-group differences across the three groups. For fungi, as shows, we observed that the soil samples clustered into two branches, in which the LN group clustered with the DI group, while the DRY group clustered alone (). Similar results were found in the NMDS analysis (). The P-value of Anosim was less than 0.001 (), which indicates that the diversity differences in soil fungi among groups were greater than those in soil bacteria. Overall, soil bacterial diversity in LN and DI groups formed two complete clusters, and compared to the DRY group, they contained less intra-group difference; for fungi, soil bacterial diversity in LN and DI groups could not be significantly distinguished by OTU genetic distance, but they were clustered separately from the DRY group.
Figure 4. Beta diversity analysis of the soil samples across the three groups. (a,b) Heat map of weighted unifrac distance index based on UPGMA clustering from bacteria (a) and fungi (b), in which the bottom x-axis and right y-axis represent the sample ID, and the upper x-axis and left y-axis represent UPGMA clustering results. (c,d) The NMDS plot of bacteria (c) and fungi (d), in which MDS1 represents abundance distance matrix and MDS2 represents evolutionary distance matrix. (e,f) The map of Anosim analysis, in which the x-axis represents the group ID, the y-axis represents the relative difference grade, the R-value represents the difference level, and the P-value represents the significance of the difference.
Discussion
In this study, we performed community compositional analysis as well as alpha and beta diversity analyses to assess the influences of three different preservation processes of soil samples on their microbial DNA metabarcoding sequencing results. We found that the initial 4 h of soil processing were important for the quality of microbial sequencing, whereas high temperature-drying could not seriously influence the sequencing results. Meanwhile, we have also obtained abundant background information on soil microbial composition and diversity in the habitat environment of the fairy ring of L. mongolica.
Four-hour soil processing at room temperature could influence the microbial sequencing. First, we observed that the soil microbial community of the DI group was different compared with the LN group, in terms of the abundance of major distributed families, especially Chthoniobacteraceae, Gemmataceae, Solirubrobacterales sp. and Pirellulaceae, and differences were also found within the biological replicates (). Secondly, we detected that the soil bacteria in the DI group diverged significantly from those in the LN group through UPGMA clustering analysis (), with all biological replicates from both groups clustered into their own branches. Finally, the analyses of NMDS () and Anosim () validated the differences between LN and DI groups. Previously, Schnecker found that when soil samples were stored at 4 °C, the biomarker bacterial populations changed [Citation12], and similarly we also revealed that if the soil samples were not immediately in situ cryo-preserved, the sequencing results of soil bacterial diversity could change, by as short as 4 h initial storage at room temperature. However, for fungi we did not find any substantial influence of initial room temperature-preservation on the quality of DNA metabarcoding sequencing, indicated by the similar community fungal compositions and diversity in LN and DI groups (), which is consistent with the studies by Cui et al. [Citation5]. In addition, the Ace index in bacteria showed that the LN group contained less intra-group difference within the biological replications than that of the DI group (). Since the Ace index could cover the microbial species with low abundance (OTUs number below 10 but more than 1) [Citation23], this result indicates that the low abundance-species in the soil samples were well preserved by in situ cryopreservation. Furthermore, our study is related to a recent study by Delavaux [Citation11], which also supported that low temperature-preservation of soil samples is important for the accuracy of microbial metabarcoding sequencing. However, compared to the study by Delavaux, our investigation was focussed more on the influence of the temperature during initial soil sample processing instead on the difference introduced by low temperature-storage (cooler or liquid nitrogen). In general, the community composition in the soil samples was influenced by the initial preservation method, but at a low degree (), possibly due to the shared soil source in LN and DI groups. At the same time, given that the initial duration of sample processing in the DI group was very short, the microbial differences between LN and DI groups also revealed the accuracy of our sequencing results.
High temperature-drying could not influence the major microbial community. We have observed that the major microbial community in the soil samples was not significantly changed after 12 h drying at 60 °C, reflected by the shared similar relative abundances of the top 5 bacterial and fungal families between the DRY soil group and LN/DI soil group (). While previous studies have investigated the influences of long time storage, normal drying and freeze-drying on soil microbial community [Citation3, Citation5, Citation7–10], our finding demonstrates that high temperature-drying can serve as an efficient approach for soil sample processing, although the drying process reduced microbial alpha diversity () and leads to less repeatability in the analyses of soil bacteria and fungi ( and ). Overall, the approach of high temperature-drying is only limited in preserving the major microbial community composition of the soil samples, whereas the method could not ensure high-precision of the sequencing results. In addition, this approach is also not suitable for ensuring high repeatability within biological replicates and preserving microbial species with low abundances. Therefore, in situ cryopreservation is a better processing method for high-throughput sequencing study of soil microbial ecosystem.
Through DNA metabarcoding sequencing analysis with the barcodes of 16S rDNA and ITS in this study, we obtained a basic background information of the soil microorganisms in fairy ring of L. mongolica and identified the major distribution patterns of both bacteria and fungi in the soil. First, we observed that the total number of OTUs obtained from the soil samples was close to the maximum number of Chao1 and Ace indices for both bacteria and fungi, indicating that a nearly complete DNA barcoding background information of soil microbes was obtained through 18 repeated sequencing ( and ). In relation to relevant studies form the fairy ring soil of Floccularia luteovirens and Agaricus gennadii, we were able to obtain higher numbers of bacterial OTUs (ranging from 5386 to 7992; ) in fairy ring of L. mongolica compared with 348 to 569 OTUs sequenced from the fairy ring soil of Floccularia luteovirens [Citation18], and we showed a similar fungal OTUs in fairy ring of L. mongolica (ranging from 1009 to 1325; ) with the OTUs in the fairy ring soil of Agaricus gennadii (ranging from 1242 to 1398) [Citation17]. These suggest that the OTU numbers of soil bacteria were more unstable than those of soil fungi. Second, we found that the bacteria and fungi in the fairy ring soil were dominated by the family of Chthoniobacteraceae and Tricholomataceae, respectively, whose abundances were much higher than the following families (Gemmataceae and Geoglossaceae, respectively) in all the soil samples. In fact, Chthoniobacteraceae in the phylum of Verrucomicrobia was previously reported to be one of the dominant soil bacterial taxa based on 16S rRNA studies and its relative abundance in average was between 0% and 20% in various soil environments [Citation36, Citation37]. Moreover, Chthoniobacteraceae is also one of the major bacterial families in several other environments, such as mine area and water [Citation38, Citation39], and it contributed in nitrogen metabolism of certain ecosystems [Citation40], potentially indicating its important functions in fairy ring ecology. On the other hand, Tricholomataceae exhibited extremely high abundance in all the soil samples in this study (), from which we speculated that the dominance was attributed to the mycelium of L. mongolica during its fruiting body formation.
Conclusion
In this study, we assessed the importance of in situ cryopreservation and high temperature-drying methods for fairy ring soil sample processing. Then we analyzed the microbial structure for both the distribution pattern and the relative abundance of the soil samples from L. mongolica fairy ring, which could provide references for further ecological studies on fairy ring ecosystem and ex situ conservation of L. mongolica.
Authors’ contributions
B.T. conceived the study. M.D. performed research and wrote the manuscript.
Supplemental Material
Download Zip (76.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The raw amplicon sequencing dataset is available in the NCBI Sequence Read Archive under BioProject ID: PRJNA635768.
Additional information
Funding
References
- Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol. 2017;15(10):579–590.
- Nesme J, Achouak W, Agathos SN, et al. Back to the future of soil metagenomics. Front Microbiol. 2016;7:73.
- Harry M, Gambier B, Garnier-Sillam E. Soil conservation for DNA preservation for bacterial molecular studies. Eur J Soil Biol. 2000;36(1):51–55.
- Brandt FB, Breidenbach B, Brenzinger K, et al. Impact of short-term storage temperature on determination of microbial community composition and abundance in aerated forest soil and anoxic pond sediment samples. Syst Appl Microbiol. 2014;37(8):570–577.
- Cui H, Wang C, Gu Z, et al. Evaluation of soil storage methods for soil microbial community using genetic and metabolic fingerprintings. Eur J Soil Biol. 2014;63:55–63.
- Tatangelo V, Franzetti A, Gandolfi I, et al. Effect of preservation method on the assessment of bacterial community structure in soil and water samples. FEMS Microbiol Lett. 2014;356(1):32–38.
- Weißbecker C, Buscot F, Wubet T. Preservation of nucleic acids by freeze-drying for next generation sequencing analyses of soil microbial communities. J Plant Ecol. 2017;10(1):81–90.
- Klammer S, Mondini C, Insam H. Microbial community fingerprints of composts stored under different conditions. Ann Microbiol. 2005;55(4):299–305.
- Lauber CL, Zhou N, Gordon JI, et al. Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol Lett. 2010;307(1):80–86.
- Rubin BE, Gibbons SM, Kennedy S, et al. Investigating the impact of storage conditions on microbial community composition in soil samples. PLoS One. 2013;8(7):e70460.
- Delavaux CS, Bever JD, Karppinen EM, et al. Keeping it cool: soil sample cold pack storage and DNA shipment up to 1 month does not impact metabarcoding results. Ecol Evol. 2020;10(11):4652–4664.
- Schnecker J, Wild B, Fuchslueger L, et al. A field method to store samples from temperate mountain grassland soils for analysis of phospholipid fatty acids. Soil Biol Biochem. 2012;51(2):81–83.
- Rissanen AJ, Kurhela E, Aho T, et al. Storage of environmental samples for guaranteeing nucleic acid yields for molecular microbiological studies. Appl Microbiol Biotechnol. 2010;88(4):977–984.
- Na Q, Bau T. New species of Mycena (Mycenaceae, Agaricales) with colored lamellae and three new species records from China. Phytotaxa. 2018;361(3):266–278.
- Shantz HL, Piemeisel R. Fungus fairy rings in Eastern Colorado and their effect on vegetation. J Agric Res. 1917;11(5):191–246.
- Xiao-Dan Y. Leucocalocybe, a new genus for Tricholoma mongolicum (Agaricales, Basidiomycota). Afr J Microbiol Res. 2011;5(31): 5750-5756.
- Yang C, Zhang F, Liu N, et al. Changes in soil bacterial communities in response to the fairy ring fungus Agaricus gennadii in the temperate steppes of China. Pedobiologia. 2018;69:34–40.
- Xing R, Yan H, Gao Q, et al. Microbial communities inhabiting the fairy ring of Floccularia luteovirens and isolation of potential mycorrhiza helper bacteria. J Basic Microbiol. 2018;58(6):554–563.
- Guo M, Wu F, Hao G, et al. Bacillus subtilis improves immunity and disease resistance in rabbits. Front Immunol. 2017;8:354.
- Toju H, Tanabe AS, Yamamoto S, et al. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS One. 2012;7(7):e40863.
- Chen S, Zhou Y, Chen Y, et al. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i90.
- Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963.
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336.
- Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10(1):57–59.
- Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998.
- Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 2011;12(1):35.
- Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267.
- Pruesse E, Quast C, Knittel K, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35(21):7188–7196.
- Nilsson RH, Larsson K-H, Taylor AFS, et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019;47(D1):D259–D64.
- Ondov BD, Bergman NH, Phillippy AM. Interactive metagenomic visualization in a web browser. BMC Bioinformatics. 2011;12(1):385.
- Wickham H, Chang W. Ggplot2: an implementation of the grammar of graphics. R package version 07; 2008. Available from: http://CRAN.R-project.org/package=ggplot2 13.10.2019
- Oksanen J, Blanchet FG, Kindt R, et al. Vegan: community ecology package. R package version 1.17-4; 2010. Available from: http://CRAN.R-project.org/package=vegan 13.10.2019
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797.
- Price MN, Dehal PS, Arkin AP . FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490.
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235.
- Janssen PH. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol. 2006;72(3):1719–1728.
- Sangwan P, Chen X, Hugenholtz P, et al. Chthoniobacter flavus gen. nov., sp. nov., the first pure-culture representative of subdivision two, Spartobacteria classis nov., of the phylum Verrucomicrobia. Appl Environ Microbiol. 2004;70(10):5875–5881.
- Fernandes CC, Kishi LT, Lopes EM, et al. Bacterial communities in mining soils and surrounding areas under regeneration process in a former ore mine. Braz J Microbiol. 2018;49(3):489–502.
- Hou D, Huang Z, Zeng S, et al. Environmental factors shape water microbial community structure and function in shrimp cultural enclosure ecosystems. Front Microbiol. 2017;8:2359.
- Cernava T, Erlacher A, Aschenbrenner IA, et al. Deciphering functional diversification within the lichen microbiota by meta-omics. Microbiome. 2017;5(1):82.