Abstract
The biological variation of cardiac troponins and the existence of a circadian/diurnal rhythm have been poorly evaluated as only the recently developed high-sensitivity assays (hs-cTn) allow the detection of circulating concentrations of both cardiac troponin I (cTnI) and T (cTnT) in reference subjects and under the cut-off values. Available data seem to demonstrate a circadian rhythm for cTnT but not for cTnI but these data need to be confirmed and in particular it should be better clarified if possible variation may affect currently recommended rapid diagnostic algorithms for safe rule-in and rule-out strategies. Here we report the results obtained using a hs-cTnI assay in 35 subjects who underwent serial samples collection over time.
Introduction
Cardiac troponin I (cTnI) and T (cTnT) are components of the contractile apparatus of myocardial cells and are expressed almost exclusively in the heart. cTnI and cTnT are the preferred biomarkers for the evaluation of myocardial injury, and high-sensitivity (hs)-cTn assays are recommended for the routine clinical use [Citation1], while other biomarkers, e.g. creatine kinase MB isoform (CK-MB), are less sensitive and less specific [Citation2]. Myocardial injury is defined as being present when blood levels of cTns are increased above the 99th percentile upper reference limit (URL). Various causes have been suggested for the release of the structural proteins from the myocardium, including normal turn-over of myocardial cells, apoptosis, cellular release of cTns degradation products, increased cellular wall permeability, formation and release of membranous blebs, and myocyte necrosis [Citation3,Citation4].
According to the fourth definition of myocardial infarction (MI), detection of an elevated cTns value above the 99th percentile upper reference limit (URL) is defined as myocardial injury [Citation1]. The injury is considered acute if there is a rise and/or fall of cTn values, thus highlighting the need for serial sampling. Since the development and the introduction of high-sensitive cTn assays (hs-cTns), reliable and accurate measurement of cTns concentrations around the 99th percentile, and even more interesting, within the reference interval, became possible, thus increasing the diagnostic efficiency for MI presentation. In particular, hs-cTns improved the reliability of safe rule-in and rule-out strategies which, however, are still based on the dynamic changes in serial sampling that should discriminate acute myocardial ischemia from persistent cTns elevations [Citation5].
It should be clearly underlined, therefore, that intra- and inter-individual variability in cTn concentrations should be carefully taken into account for a correct interpretation of the difference between two serial samples [Citation6]. However, an accurate evaluation of the biological variation of cTns, has recently become possible thanks to the development of hs-cTn assays characterized by significantly improved quality specifications, in particular the analytical sensitivity and precision at low levels of concentrations. Furthermore, hs-cTn assays allows to achieve a fundamental goal that is the detection of the cTns lowest concentration with a total coefficient of variation (CV) of 20% or less (LOQ). This analytical performance allows the reliable measurement of cTn values below the cut-off and in ‘reference’ subjects.
Circadian and diurnal rhythm of cardiac troponins
There are few literature data on the biological variation of cardiac troponins and, in particular, on the existence of a circadian/diurnal rhythm. In 2017, Fournier et al. demonstrated a circadian rhythm for hs-cTnT in young males and females suggesting caution in interpreting results from serial samples if collected at different hours, and emphasizing differences between samples collected in the early morning and afternoon [Citation7]. Klinkenberg and colleagues evaluated the diurnal rhythm of cardiac troponins, both cTnT and cTnI and possible consequences for the diagnosis of acute myocardial infarction [Citation8]. In the cited study, hs-cTnT, but not hs-cTnI, exhibited a diurnal rhythm, characterized by gradually decreasing concentrations throughout daytime, rising concentrations during nighttime, to peak concentrations in the morning. The diagnostic accuracy [area under the receiver-operation characteristics curve (AUC)] of hs-cTnT at presentation, after 1 h, and for the combination of absolute changes with concentration at admission, were very high and comparable among patients presenting early morning and at evening (all AUC 0.93). Hs-cTnI exhibited no diurnal rhythm with no differences in AUC among early morning and evening presenters. The authors’ conclusions were: ‘rhythmic diurnal variation of hs-cTnT is a general phenomenon that is not seen with hs-cTnI. While the diurnal hs-cTnT rhythm does not seem to affect the diagnostic accuracy of hs-cTnT for AMI, it should be considered when using hs-cTnT for screening purposes’.
The aim of our work was to evaluate the cTnI variability measured with a high sensitivity assay focusing on the short-term variation which is of interest for adopting rapid diagnostic algorithms (0/1 h, 0/2 h and 0/3 h), as recommended by the European Society of Cardiology (ESC) in clinical practice guidelines [Citation9].
Subjects and methods
Ethics statement
Written informed consent was obtained from all subjects.
Subjects
Thirty-five subjects (20 females and 15 males) recruited from the hospital personnel were included in the study. The inclusion criteria comprise the age (between 18 and 80 years) and the absence of hypertension, drug treatment cardiomyopathy, renal failure, obesity (BMI > 40 kg/m2), insulin therapy. Participants were asked to refrain from exhaustive physical activities and exercise training 2 days before the test period. After an overnight fast, from 8.30 to 9.00 am the participants were restricted to the laboratory environment; their blood pressure and heart rate were measured, and blood was collected (time 0) by venipuncture in two PSTII tubes (lithium heparin with gel separator, Becton Dickinson) and one K2EDTA tube (Becton Dickinson) for BNP measurement (Quidel BNP, UniCel DxI 800, Beckman Coulter Inc. Brea, CA,USA). The plasma lithium heparin blood drawing was obtained after 1, 2, 3 and 7 h in respect to time 0. All tubes were centrifuged within 5 min from collection (3500 g for 10 min), aliquoted and stored at −80 °C before analyses. From each subject, all aliquots for hs-cTnI biological variability study were thawed the same day and measured in the same analytical run) in duplicate using a commercially available high-sensitivity troponin I method (Access hs-TnI) automatized on UniCel DxI 800 instrument (Beckman Coulter Inc. Brea, CA, USA). In order to reduce the preanalytical variability, the pretreatment of aliquots samples has been standardized before analysis, thawing the aliquots at room temperature (RT), mixing accurately by inversion and centrifuging at 12000 g for 5 min to remove fibrin formations. Other data and the statistical analyses performed are reported in the original published paper [Citation10].
Results
Preliminary studies to provide evidence of the accuracy of the assay, and in particular, the imprecision, demonstrate a coefficient of variation (CV) ranging from 15.5% (n = 20; hs-cTnI = 2.2 ng/L) to 7.7% (n = 20; hs-cTnI = 4.79 ng/L), confirming the LOB and LOD declared by the manufacturer, 1.7 ng/L and 2.3 ng/L, respectively.
The baseline levels at time 0 (between 8 and 9 am) were significantly higher than those measured at different times of the day in the overall population, as well as in the subjects subdivided by gender. The graphical representation of the circadian biological rhythmicity () demonstrates a comparable pattern regardless of the gender. The variations in hs-cTnI concentrations measured at a different time of day were found to be statistically significant with respect to time 0, for male at 2 h (p < 0.001), 3 h (p < 0.001) and 7 h (p < 0.001) (rank-transformed analysis of variance (ANOVA), Bonferroni’s adjusted pairwise comparisons), as shown in . Further, similar hs-cTnI variations were found in individuals with age < 50 years, whilst a less pronounced variability was observed for subjects with more than 50 years of age (). The predicted amplitude of the variations in cTnI concentrations observed during the time of evaluation is around 0.3–0.4 ng/L, and therefore, does not affect the clinical interpretation of the results. The results of the biological variability in the overall population as well as according to gender () demonstrate that the intra-individual variation (expressed as CVI) is about 4.1%, whereas the inter-individual variation (CVG) is about 17.28%, the RCV 22.77% and the Individuality Index (ratio of the within-subject biological variation to the between-subject variation) 0.54.
Figure 1. Circadian biological variation rhythmicity in females (left panel) and males (right panel).
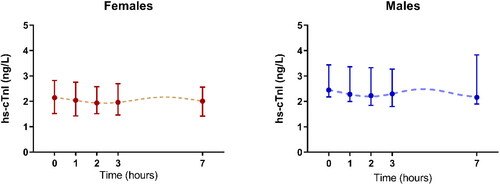
Table 1. Hs-cTnI levels, measured at different time points.
Table 2. Analytical (CVA), intra- and inter-individual (CVI and CVG) biological variabilities and reference change value (RCV), obtained by applying the Nested ANOVA analyses to data either in original scale or by applying a log transformation.
Discussion
The results of our study [Citation10], aimed to evaluate not only the biological variability but also the circadian rhythm of troponin I measured with a high-sensitivity assay in a population of healthy subjects, demonstrate that troponin I exhibits a diurnal rhythm, characterized by gradually decreasing values throughout daytime, with the peak concentrations (2.35 ng/L in overall population) being observed in the morning [Citation10]. The circadian variability in males and females is over a 5.4 h interval, suggesting that the troponin I concentrations peak about 3 times during the day (24 h), even if the interval of time monitored in our study (7 h) does not allow to obtain complete information about the nocturnal rhythmicity [Citation11].
Further studies are therefore needed to confirm the obtained data in serial sampling measurements over a 24 h period, particularly in the late evening and in different days to confirm the evidence of a circadian rhythm for cTnI in times of the day different from those already studied [Citation6–8].
As highlighted in the previous paper [Citation10], the most important result is that the amplitude of the variations in cTnI concentrations observed during the time evaluated is statistically significant, but it is not relevant from the clinical point of view. Of particular interest seems to be the cTnI concentrations observed in the population studied that are close to or lower than LOD declared by the manufacturer (2.3 ng/L). Nevertheless, as demonstrated by the analytical performance observed during the study, the method adopted shows a highly satisfactory precision (CVA about 8%) also in serial samples collected at short intervals (1–2 h). These results, in turn, explain the obtained CVI (4.1%) that is lower than that calculated in other studies [Citation12,Citation13) for the same method, and stress the relevance of delta change (expressed as reference change value—RCV) in patients with borderline values of cTnI at baseline (zero time). In addition, a relevant finding is related to the index of individuality (II = 0.54) which, being lower than 0.6, highlights the scarce value of the reference interval when evaluating cTnI concentrations in general populations.
Conclusions
The results obtained in our study confirm the presence of a diurnal rhythm for cTnI with higher values in the early morning and a statistically significant decrease in the late morning and afternoon. However, the observed differences do not affect the clinical interpretation of serial sampling when adopting the recommended rapid diagnostic algorithms (0/1 h, 0/2 h and 0/3 h). Further studies are required to investigate the biological variation of cTnI with serial sampling measurements over the 24 h, particularly in the late evening and in different days, to confirm the evidence of a circadian rhythm for cTnI. In addition, the same investigation should be performed using high sensitivity assays for cTnT measurement providing data about the possible clinical significance of the already described circadian rhythm for hs-cTnT in young males and females.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [MP], upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Executive group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) task force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231–2264.
- Goodman SG, Steg PG, Eagle KA, et al. GRACE investigators. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: lessons from the global registry of acute coronary events (GRACE). Am Heart J. 2006;151(3):654–660.
- Mair J, Lindahl B, Hammarsten O, et al. European society of cardiology (ESC) study group on biomarkers in cardiology of the acute cardiovascular care association (ACCA). How is cardiac troponin released from injured myocardium? Eur Heart J Acute Cardiovasc Care. 2018;7(6):553–560.
- Thygesen K, Alpert JS, Jaffe AS, et al. The universal definition of myocardial infarction. Chapter 37. In: Tubaro M, Vranckx P, Price S, Bonnefoy E, Vrints C, editors. The ESC textbook of intensive and acute cardiovascular care. Oxford (UK): Oxford University Press; 2021. p. 463–478.
- Giannitsis E, Katus HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol. 2013;10(11):623–634.
- Wu AH, Lu QA, Todd J, et al. Short- and long-term biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009;55(1):52–58.
- Fournier S, Iten L, Marques-Vidal P, et al. Circadian rhythm of blood cardiac troponin T concentration. Clin Res Cardiol. 2017;106(12):1026–1032.
- Klinkenberg LJ, Wildi K, van der Linden N, et al. Diurnal rhythm of cardiac troponin: consequences for the diagnosis of acute myocardial infarction. Clin Chem. 2016;62(12):1602–1611.
- Roffi M, Patrono C, Collet JP, ESC Scientific Document Group, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-Segment elevation of the European society of cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
- Zaninotto M, Padoan A, Mion MM, Marinova M, et al. Short-term biological variation and diurnal rhythm of cardiac troponin I (access hs-TnI) in healthy subjects. Clin Chim Acta. 2020;504:163–167.
- Clerico A, Padoan A, Zaninotto M, et al. Clinical relevance of biological variations of cardiac troponins. Clin Chem Lab Med. 2021;59(4):641–652.
- Clerico A, Ripoli A, Masotti S, et al. Evaluation of 99th percentile and reference change values of a high-sensitivity cTnI method: a multicenter study . Clin Chim Acta. 2019; 493:156–161.
- Di Pietro M, Dipalo M, Rocchi MBL, et al. Assessment of access hsTnI 99th percentiles upper reference limits following IFCC recommendations. Clin Chim Acta. 2019; 492:26–28.
