Abstract
Potato virus Y (PVY) is the reason for significant losses in the potato crop yearly. The recent technologies in molecular biology have given insights on possibilities for control of viral infections at epigenetic level. In the present study, we obtained a DNA template complementary to a key sequence at the NIb genetic region of PVY. Potato leaves were inoculated with dsRNAs and siRNAs complementary to NIb 48 h before inoculation with PVY strain N/NTN. The treated plants did not develop any signs of disease. Double antibody sandwich еnzyme-linked immunosorbent assay (DAS-ELISA) of the plants treated with Nib-dsRNAs showed optical density values commensurate to the cut-off value. The Nib-siRNAs obtained in this study were twice as effective, with DAS-ELISA values which were below the cut-off and similar to the values of the healthy plants. Inducing posttranscriptional gene silencing in crops presents a promising utility for control of virus diseases and for prevention of crop losses, being non-inheritable, non-toxic and adaptable to a specific crop.
Introduction
Potato (Solanum tuberosum L.) is one of the leading crops in the worldwide production. In the past two decades, the overall global yield has increased slowly but constantly from 321.80 million (M) tonnes in 2000 to 359.07 M tonnes in 2020. 2005 was a key year, when the world potato sector underwent significant changes. It was the first time when the potato production of the developing countries exceeded the production in the developed world. Since then, China has become the leading potato producer, followed by India (The Food and Agriculture Organisation, FAO [Citation1,Citation2]). Another noticeable change was marked by the year 2000. In the period 1990–2000, the production areas constantly grew in size from 17.66 to 19.775 M ha, while the actual potato production varied with ups and downs in the range from 148.14 to 164.60 M t. After the peak in the size of the world harvested areas in 2000, their enlargement was ceased, and a new trend in the global potato production was settled—reducing the production plots and raising the potato yield quantity per plot [Citation3]. This new trend, however, presents new challenges to farmers, as the sustainable growth also demands environmentally friendly fertilizers and above all, environmentally friendly products for plant protection.
Potyviruses are the reason for significant losses in different Solanaceae crops, potatoes included. The most widely spread and among the most devastating ones is Potato virus Y (PVY) [Citation4–6]. Damage to potatoes caused by PVY includes but is not limited to mosaic, chlorosis, leaf wrinkling and deformation, necrosis and necrotic spots or rings on all plant parts (leaves, stem, tubers, etc.), defoliation and drying [Citation7–9]. Necrotic lesions of potatoes are most often caused by the PVYNTN [Citation9–11] and PVYN/NTN groups of strains, the latter being particularly devastating [Citation12,Citation13].
Control of virus diseases is difficult and indirect, mainly focused on control of the vectors and production of virus-free seeds [Citation14]. Yet, the scientific community continue their attempts to find or elaborate means for direct control of the virus. The recent technologies in molecular biology have given insights on some possibilities for control of viral infections at epigenetic level [Citation15–17]. In this study, we aimed to control plant infection with PVY in potatoes by inducing posttranscriptional gene silencing (PTGS) as a result of targeting the NIb viral gene region.
Materials and methods
Material
The virus strain was PVYN/NTN from the virus collection of the Institute of Soil Science, Agrotechnologies and Plant Protection “N. Pushkarov” (ISSAPP). The plant material for this experiment was grown at ISSAPP. Twenty-one plant pots with potatoes cv. Djeli were used in this study. The plants were grown in phytotronic rooms at a temperature range 21–25 °C and a photoperiod of 8:16 (light to dark) to avoid overgrowth of the landmass.
Production of dsRNAs and siRNAs in vitro
Virus RNA was extracted via RNEasy Plant Mini Kit (QIAGEN GmbH, Germany) for isolation of total RNA from a sample. PCR using Phusion High-Fidelity DNA polymerase was used to obtain a DNA template. Primers used were specific for the genetic region coding the nuclear inclusion protein b (NIb) of PVY For 5′-TAA TAC GAC TCA CTA TAG GGC TCA TCA TCA GAA GCA CAT ACA-3′ and Rev 3′-GGA AAA AAA TAC CAA CAG CGA ACG ATA CG-5′ [Citation18], flanked by T7 promoter sequences in the 5′ end and Phi6 qRdRP promoter sequences in the 3′ end. The PCR program was performed in an Auto-Q Server (LKB, UK) thermocycler under the following conditions: preliminary denaturation at 95 °C for 5 min; 35 cycles of amplification at 95 °C for 10 s, 58 °C for 10 s and 72 °C for 20 s; and final elongation at 72 °C for 10 min. Replicator RNAi Kit (Thermo Fisher Scientific Inc., USA) was used for in vitro transcription and replication of the DNA template for production of dsRNAs. The steps included transcription of DNA by T7 viral RNA polymerase to ssRNA and subsequent replication of ssRNA to dsRNA by phague Phi6 qRdRP. The resulting dsRNAs were visualized by standard agarose gel-electrophoresis containing 2% agarose gel at 90 V, for 40 min, stained with ethidium bromide, under ultraviolet light. GeneRuler 100 bp Plus DNA Ladder (Thermo Fisher Scientific Inc., USA) was used. The enzyme PowerCut Dicer (a recombinant endoribonuclease from Giardia intestinalis) was used to cleave dsRNAs to small nucleotide fragments which are the siRNAs [Citation13,Citation19].
Inoculation of potato plants
Inoculation of plants was performed according to Noordam [Citation20]. Plants were processed in the shade. Leaves were besprinkled with distilled water and then, dusted with carborundum with size of the particles 400–600 meshes. Virus-containing homogenate for inoculation was prepared from 1 g of infected plant material homogenized in 1 mL of 0.1 mol/L cooled to 4 °C potassium sodium phosphate buffer pH 8.0, containing 0.2% Na2SO3 and 0.2% ascorbic acid. Inoculations were attained by gentle rubbing of the leaves with this homogenate and incubation for 3–5 min before washing out with water. Treatments with dsRNAs and siRNAs were achieved by a similar mechanical inoculation technique 48 h before inoculation with the virus. Positive controls were infected-only plants. Negative controls were non-infected and water-treated-only plants. Treatments with the referent compounds dsRNAs and siRNAs specific for the S-segment of Phi6 served as additional negative controls regarding the specificity of the produced RNAs. Experiments were performed in triplicate. Symptoms of infection were observed seven days after inoculation with the virus and serological analysis was carried out to determine the presence of PVY viral infection.
Double antibody sandwich еnzyme linked immunosorbent assay (DAS-ELISA)
The method of Clark and Adams [Citation21] and the commercial kit of LOEWE (Biochemica GmbH, Germany) were used in the diagnostic test which was carried out 14 days after the treatment of the potato plants. Analysis was performed according to the instructions of the manufacturer. The plant samples were grounded in diluted extraction buffer (1:10, v/v) containing 1% polyvinyl pyrrolidone. The ELISA plates were loaded with polyclonal antiserum (immunoglobulin G, IgG) for PVY in a series of dilutions of 0.05 mol/L carbonate buffer and incubated at 37 °C for 4 h. The unbound components were triple washed out with PBS-T buffer for 5 min. The samples were loaded and incubated at 4 °C for 16 h. After triple washing, the alkaline-phosphatase conjugate for PVY was added and the plates were once again incubated at 37 °C for 4 h. The colorimetric substrate was p-nitrophenyl phosphate (Merck KGaA, Germany) in diethanolamine buffer (pH 9.8) (1 mg/mL). The colour reaction was held in the light at room temperature and stopped with 3 N NaOH. The adsorption was measured at 405 nm in a multifunctional detector DTX 880 (Beckman Coulter, USA). The threshold value (cut-off) was set at three times the value of the negative control.
Results
The nuclear inclusion protein b (NIb) is a multifunctional protein of the Potyviruses. Besides being the key enzyme for the viral replication, it also interferes with the early defense reactions of the plant host [Citation18,Citation22], as discussed in the next section, which makes it a good choice as a target for PTGS.
In the present study we obtained the DNA template complementary to a key sequence at the NIb genetic region of PVY, purified it and used it for production of gene-specific dsRNAs and siRNAs as described in the ‘Material and methods’ section. The obtained dsRNAs had a length of approximately 220 bp (). The dsRNA fragments and the resulting pool of siRNAs were used for treatment tests on viable potato plants. dsRNAs and siRNAs produced by the same protocol but specific for the S-segment of bacteriophage Phi6 served as referent compounds for the specificity of the RNAs.
Figure 1. dsRNAs, specific for the NIb genetic region of PVY visualized by gel electrophoresis. L, Ladder (GeneRuler 100 bp Plus DNA Ladder (Thermo Scientific Inc.); S, Sample.

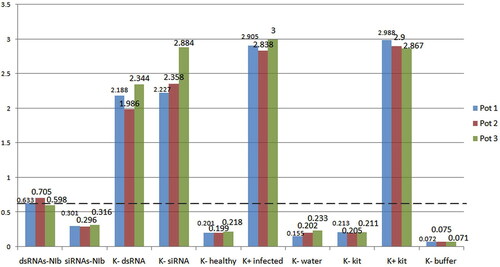
Potato leaves were inoculated with dsRNAs and siRNAs, complementary to the NIb gene region of PVY, before inoculation with PVYN/NTN. Observation for symptoms of viral disease and diagnostic tests for presence or absence of PVY infection were performed seven days after the inoculation with the virus. The positive infected controls exhibited clear symptoms of viral disease–mosaic and peripheral necrosis of leaves, necrotic spots of the stems, as well as necrosis of tubers which spreads in depth. The same symptoms were observed on the plants treated with dsRNAs and siRNAs which were not specific for PVY genetic regions. The plants treated with dsRNAs and siRNAs specific for Nib of PVY did not develop any signs of disease. DAS-ELISA of the plants treated with Nib-dsRNAs showed optical density (OD) values commensurate to the cut-off value (0.629). The Nib-siRNAs obtained in this study were twice as effective with DAS-ELISA values which were below the cut-off and similar to the values of the healthy plants and water-treated plants. The OD values of the plants treated with dsRNAs and siRNAs not specific for PVY were high above the cut-off and three times and more the values of the plants treated with NIb-specific dsRNAs. These values indicated a successful viral infection and lack of protective effect of the dsRNAs and siRNAs which were not specific for PVY ().
Figure 2. DAS-ELISA of potato plants after treatment with PVYN/NTN-NIb-specific dsRNAs and siRNAs after infection.
Note: dsRNAs-NIb, Potato plants treated with dsRNAs specific for the HIb-region of PVY, and infected; siRNAs-NIb, Potato plants treated with siRNAs specific for the HIb-region of PVY, and infected; K − dsRNA, Potato plants treated with dsRNA specific for the S-segment of Phi6, and infected; K − siRNA, Potato plants treated with siRNA specific for the S-segment of Phi6, and infected; K − healthy, non-treated healthy potato plants; K + infected, potato plants inoculated with virus only; K − water, non-treated healthy potato plants inoculated with water only; K − kit, negative control of the ELISA kit; K + kit, positive control of the ELISA kit; K − buffer, negative buffer control of the ELISA kit.
Discussion
The nuclear inclusion protein b (NIb) represents the RNA-dependent RNA polymerase (RdRp) of the Potyviruses, which is essential for formation of the viral replication complex [Citation23–27]. It is partially localized in the endoplasmic reticulum membranes and can interact with other proteins of the host cell, thus promoting the viral replication cycle [Citation25,Citation26,Citation28]. NIb has a nuclear translocation activity and accumulates in the nucleus, forming together with NIa amorphous or crystalline nuclear inclusions in the host cells [Citation23,Citation29–31]. Mutations in NIb that disrupt this nuclear translocation activity prevent viral genome replication [Citation23]. NIb interacts with other host proteins, such as the heat shock proteins (HSPs) that are involved in promoting the virus infection cycle [Citation18]. Moreover, it is also supposed that NIb possesses other functions, like participating in the early plant defense reactions by interacting with proteins from the small ubiquitin-related modifier (SUMO) pathway to suppress NPR1-mediated immunity response [Citation18,Citation22]. All these activities of HIb make it a good target for PTGS.
The strategy implemented in the present study was based on the scientific data on the PVY genome, the viral replication and the molecular mechanisms of plant defense responses to an infection. PTGS mechanisms have been studied for more than 25 years [Citation17,Citation32–34]. However, many of the investigations concern the invention of transgenic plants [Citation16,Citation32,Citation34–36]. A small part of the research focuses on the development of techniques with potential for direct plant disease control, but only a limited number of studies experimentally applied RNAi for virus control in the plants [Citation15,Citation19,Citation37–41]. Sun et al. [Citation38] used hairpin RNA (hpRNAs) constructs to target different hot-spot sequences of Tobacco mosaic virus and PVY genomes. The hpRNAs targeting NIb in their experiment gave the best results in acquiring resistance to PVY in tobacco. Tenllado et al. [Citation42] managed to acquire resistant tobacco plants by applying dsRNAs targeting the replicase gene (IR 54) of Pepper mild mottle virus (PMMoV) and the HC gene of Plum pox virus (PPV) through crude preparations of a mutated bacterial carrier. Petrov [Citation19] applied dsRNAs targeting the HC-Pro of PVYO and achieved blocking of the disease in 82% of the tested tobacco plants. Further studies [Citation15,Citation40,Citation41] obtained virus-free potato plants by inoculation of constructed dsRNAs and/or siRNAs specific for the HC-Pro region of PVYN, PVYNTN and PVYN/NTN, respectively. The authors observed that the old leaves remained infected but later defoliated, while the newly formed ones remained virus-free, suggesting the blocking of the systemic spread of the virus in the plants [Citation15,Citation40]. In the present research we obtained virus-free potato plants after treatment with dsRNAs and siRNAs complementary to the NIb region of PVY. NIb, like HC-Pro, proved to be a tempting target for inducing PTGS in plants as a means for plant disease protection.
Inducing PTGS in crops presents a promising utility for control of virus diseases and for prevention of crop losses [Citation41,Citation43]. Unlike transgenic plants, this technique presents a considerably shorter time to implement on-site, as no propagation of plants is involved. As a consequence, it is much more cost-effective [Citation37,Citation38,Citation42] and has the advantage to be adapted towards a specific pathogen regardless of the specific crop or variety [Citation43]. In recent years, concerns have been raised for the use of transgenic plants due to their possible negative ecological impact, such as heterologous encapsidation, complementation, synergy and genetic flow between organisms [Citation37,Citation42]. The ds/siRNAs introduced into the plant cells do not embed in the plant genome so they are not inherited, thus making it possible to retain sensitive crop varieties with valuable properties[Citation40,Citation43]. Moreover, ds/siRNAs do not pose treat for human health like chemical compounds, pesticides, allergens, etc. [Citation43] and do not accumulate in the environment [Citation38].
Conclusions
Application of dsRNAs and siRNAs targeting the NIb gene region of PVY successfully induced PTGS in potato plants cv. Djeli. Both molecular products were effective in interfering with viral systemic spread in the plants. The obtained results are promising for further research.
Funding
This study was supported by the National Science Fund of Bulgaria under contract DНТС India 01/1.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- The Food and Agriculture Organization (FAO). International year of the potato. Potato world [Online]. 2008. [cited 07 Feb 2022]. Available from: https://www.fao.org/potato-2008/en/world/.
- The Food and Agriculture Organization (FAO). FAOSTAT. Data. Crops and livestock products [Online]. 2021. [cited 07 Feb 2022]. Available from: https://www.fao.org/.
- The Food and Agriculture Organization (FAO). FAOSTAT. Compare data. Crops and livestock products [Online]. 2021b. [cited 07 Feb 2022]. Available from: https://www.fao.org/.
- Kovachevski I. Potato virus in tobacco (in Bulgarian). Notes Biol Inst. 1951;1:123–142.
- Hull R. Matthew’s plant virology, 4th ed. London: Academic Press, A Harcourt Science and Technology Company; 2001. p. 33.
- Rybicki EP. A top ten list for economically important plant viruses. Arch Virol. 2015;160(1):17–20.
- Buchen-Osmond C. Potato Virus Y in Plant Viruses Online: Descriptions and Lists from the VIDE. 1987. Available from: http://pvo.bio-mirror.cn/descr652.htm.
- Van der Vlugt RA, Leunissen J, Goldbach R. Taxonomic relationships between distinct potato virus Y isolates based on detailed comparisons of the viral coat proteins and 3’ non-translated regions. Arch Virol. 1993;131(3–4):361–375.
- Petrov N, Stoyanova M, Gaur RK. Chapter 3. Characterization and control of potato virus Y in the crops. In: Gaur RK, Khurana SMP, Dorokhov Y, editors. Plant viruses: diversity, interaction and management. Boca Raton, FL, USA: Taylor & Francis Group, LLC, CRC Press, 2018. р. 41–64.
- Chrzanowska M. New isolates of the necrotic strain of potato virus Y (PVYN) found recently in Poland. Potato Res. 1991;34(2):179–182.
- Shukla DD, Ward CW, Brunt AA. The potyviridae. Wallingford: CAB International; 1994. p. 516.
- Petrov N, Hristova D, Heinze C, et al. Identification of the virus, causing necrotic ring spots on potato tubers in Bulgaria. Plant Sci. 2008;45:407–411.
- Petrov N. Potato virus Y (PVY) of solanaceae crops [in Bulgarian] [PhD thesis]. Sofia, Bulgaria: Institute of Soil Science, Agrotechnologies and Plant Protection “N. Pushkarov”, Agricultural Academy; 2012.
- Radcliffe E, Ragsdale D. Aphid-transmitted potato viruses: the importance of understanding vector biology. Am J Pot Res. 2002;79(5):353–386.
- Petrov N, Teneva A, Stoyanova M, et al. Blocking the systemic spread of potato virus Y in the tissues of potatoes by posttranscriptional gene silencing. Bul J Agric Sci. 2015;21(2):288–294.
- Ibrahim AB, Aragão FJL. Chapter 5. RNAi-mediated resistance to viruses in genetically engineered plants. In: Mysore KS and Senthil-Kumar M, editors. Plant gene silencing: methods and protocols, methods in molecular biology. Vol. 1287, New York, NY: Humana Press; 2015.
- Moyo L, Ramesh SV, Kappagantu M, et al. The effects of potato virus Y-derived virus small interfering RNAs of three biologically distinct strains on potato (Solanum tuberosum) transcriptome. Virol J. 2017;14(1):129.
- Kozieł E, Surowiecki P, Przewodowska A, et al. Modulation of expression of PVYNTN RNA-dependent RNA polymerase (NIb) and heat shock cognate host protein HSC70 in susceptible and hypersensitive potato cultivars. Vaccines. 2021;9(11):1254.
- Petrov N. Inhibition of the virus replication of PVY by siRNAs. Proceedings of the Third Congress of Virology (Days of Virology in Bulgaria) with Int. Part., Sofia; 2012b. p. 101–105.
- Noordam D. Identification of plant viruses. Methods and experiments. Wageningen: Centre for Agricultural Publishing and Documentation (Pudoc); 1973.
- Clark MF, Adams AN. Chracterisation of the microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. Gen Virol. 1977;34(3):475–483.
- Shen W, Shi Y, Dai Z, et al. The RNA-Dependent RNA polymerase NIb of potyviruses plays multifunctional, contrasting roles during viral infection. Viruses. 2020;12(1):77.
- Li XH, Valdez P, Olvera RE, et al. Functions of the tobacco etch virus RNA polymerase (NIb): subcellular transport and protein-protein interaction with VPg/proteinase (NIa). J Virol. 1997;71(2):1598–1607.
- Adams MJ, Antoniw JF, Fauquet CM. Molecular criteria for genus and species discrimination within the family potyviridae. Arch Virol. 2005;150(3):459–479.
- Wei T, Huang TS, McNeil J, et al. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J Virol. 2010;84(2):799–809.
- Mine A, Okuno T. Composition of plant virus RNA replicase complexes. Curr Opin Virol. 2012;2(6):669–675.
- Revers F, García JA. Molecular biology of potyviruses. Adv Virus Res. 2015;92:101–199.
- Wang X, Ullah Z, Grumet R. Interaction between zucchini yellow mosaic potyvirus RNA-dependent RNA polymerase and host poly-(A) binding protein. Virology. 2000;275(2):433–443.
- Restrepo MA, Freed DD, Carrington JC. Nuclear transport of plant potyviral proteins. Plant Cell. 1990;2(10):987–998.
- Martin MT, Gélie B. Non-structural plum pox potyvirus proteins detected by immunogold labelling. Eur. J. Plant Pathol. 1997;103(5):427–431.
- Ivanov KI, Eskelin K, Lohmus A, et al. Molecular and cellular mechanisms underlying potyvirus infection. J Gen Virol. 2014;95(Pt 7):1415–1429.
- Lin S-S, Henriques R, Wu H-W, et al. Strategies and mechanisms of plant virus resistance. Plant Biotechnol Rep. 2007;1(3):125–134.
- Wadsworth S, Dunoyer P. Chapter 1. Plant RNA-silencing immunity and viral counter-defence strategies. In Bouarab K, Brisson N, Daayf F, editors. Molecular Plant-Microbe Interactions; 2009. p. 1–35. UK: CAB Int. ISBN 9781845935740.
- Lindbo JA, Dougherty WG. Plant pathology and RNAi: a brief history. Annu Rev Phytopathol. 2005;43(1):191–204.
- Eamens A, Wang M-B, Smith NA, et al. RNA silencing in plants: yesterday, today, and tomorrow. Plant Physiol. 2008;147(2):456–468.
- Prins M, Laimer M, Noris E, et al. Strategies for antiviral resistance in transgenic plants. Mol Plant Pathol. 2008;9(1):73–83.
- Sun Z-N, Song Y-Z, Yin G-H, et al. HpRNAs derived from different regions of the NIb gene have different abilities to protect tobacco from infection with potato virus Y. J Phytopathol. 2010;158:566–568.
- Sun Z-N, Yin G, Song Y-Z, et al. Bacterially expressed double-stranded RNAs against hot-spot sequences of tobacco mosaic virus or potato virus Y genome have different ability to protect tobacco from viral infection. Appl Biochem Biotechnol. 2010b;162(7):1901–1914.
- Iqbal MS, Muhammad HN, Wattoo JI, et al. Prediction of host-derived miRNAs with the potential to target PVY in potato plants. Front Genet. 2016;7:159.
- Petrov N, Stoyanova M, Andonova R, et al. Induction of resistance to potato virus Y strain NTN in potato plants through RNAi. Biotech Biotechnol Equip. 2015b;29(1):21–26.
- Petrov N, Stoyanova M, Stoev A, et al. Induction of ecological resistance to potato virus Y in potato varieties through silencing of main viral genes. Acta Microbiol Bulg. 2021;37(2):95–97.
- Tenllado F, Martínez-García B, Vargas M, et al. Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol. 2003;3(1):3.
- Petrov N, Stoyanova M, Gaur RK. Chapter 23. Post-transcriptional gene silencing as a tool for controlling viruses in plants. In Khurana SMP, Gaur RK, editors. Plant biotechnology: progress in genomic era. 1st ed. Singapore: Springer Verlag; 2019. p. 527–542.
