Abstract
Potato virus Y (PVY) is one of the most dangerous pathogens that cause serious damage to crop production. Fighting plant viral infections is crucial to limit these damages and save millions of dollars for farmers. Gene suppression of selected virus genes is one of the modern, innovative and effective approaches to block viral replication and infection. The virus, in turn, has developed its own strategy to avoid this gene suppression through mutations in the attacked genes of the virus genome. For this reason, blocking several important viral genes simultaneously led to better results in blocking viral replication. In this study, we achieved an epigenetic control of viral infection of PVY N/NTN of potato plants cultivar Agria by applying a combination of ds/siRNAs specific for two different conservative regions from the PVY genome, CI-6K2-NIa and HC-Pro. The potato plants that received the ds/siRNAs remained virus-free, suggesting that the CI-6K2-NIa and HC-Pro regions could serve as effective targets for control of PVY.
Introduction
The genus Potyvirus is the largest in family Potyviridae and includes more than 230 virus species in 12 genera, accounting for about 30% of all known plant viruses (ICTV, Citation2021). The type representative of the genus, Potato virus Y (PVY), is of a great economic importance for Bulgaria and it is the subject of this study. The symptoms caused by the virus vary widely and depend on the strain, the variety of the host plant, the climatic conditions and the geographical region. The most common symptoms of disease that it causes on potatoes are: mosaic and wrinkling of the leaves, weak to strong mottling of the leaves, often leading to deformation and wrinkling of the petura, chlorosis and necrosis, vein necrosis, necrotic spots and circles on tubers, drying and falling of the eaves. In tobacco, tomato, and pepper, leaf spotting, mosaic, wrinkling and vein necrosis, as well as leaf drop, are observed (Van der Vlugt et al. Citation1993; Glais et al. Citation1996; Petrov, Citation2012a).
The development of effective techniques to control diseases caused by Potyviruses is essential to reduce production losses in agriculture. Induced post-transcriptional RNA gene silencing (PTGS) is one of the defense mechanisms of plants against pathogens (Vance and Vaucheret, Citation2001; Martinez et al. Citation2002; Jorgensen Citation2003). By creating inducer molecules (specific small interfering RNAs (siRNAs) against conserved genetic regions of Potyviruses), we can induce PTGS, leading to inhibition of viral replication and induction of the natural plant resistance to the virus. In this approach, the plant genome is not changed, and the natural unique genetic inheritage can be preserved (Martinez et al. Citation2002; Petrov, Citation2012a, Petrov et al. Citation2015, Petrov et al. Citation2018a, Petrov et al. Citation2019).
The aim of the study was to elaborate effective techniques for controlling Potyvirus diseases based on RNA interference and induction of host plant defense mechanisms against Potyviruses. Unlike previous studies we targeted two genetic regions simultaneously. The collected data will contribute to reducing production losses and increasing productivity in agriculture.
Materials and methods
Virus
We used virus strain PVYN/NTN from the collection of the Institute of Soil Science, Agrotechnologies and Plant Protection “N. Pushkarov” (ISSAPP), Agricultural Academy.
Plants
The plant material for this experiment was Solanum tuberosum cv. Agria grown at ISSAPP. Twenty-one plant pots were used in this study. The plants were grown in phytotronic rooms at a temperature range of 21-25 °C and a photoperiod of 8:16 (light to dark) to avoid overgrowth of the landmass.
Production of dsRNAs and siRNAs in vitro
RNeasy Plant Mini Kit (Qiagen Inc.) was used for isolation of total RNA. DNA templates were obtained by polymearase chain reaction (PCR) using Phusion High-Fidelity DNA polymerase. The primer pairs were specific for the genetic regions coding 1) the nuclear inclusion protein a (NIa) and 2) the HC-Pro of PVY (). The first primer pair was: o6266c F 5′- CTC CTG TGC TGG TAT GTC CT −3′ and S5585m R 3′- GGA TCT CAA GTT GAA GGG GAC −5′ (Lorenzen, Citation2006), and the second: HC-Pro dsRNA F 5′- TAG GAT TCT GTC GAA TGC CGA CAA TTT T −3′ and HC-Pro dsRNA R 3′- TGC AGA CCA ACT CTA TAA TGT TT −5′ (Petrov et al. Citation2015), flanked by T7 promoter sequences in the 5′ end and Phi6 qRdRP promoter sequences in the 3′ end. The PCR program was performed in a thermocycler Auto-Q Server (LKB, UK) under the following conditions: preliminary denaturation at 95 °C for 5 min; 35 cycles of amplification at 95 °C for 10 s, 58 °C for 10 s and 72 °C for 20 s; and final elongation at 72 °C for 10 min. Each DNA template was in vitro transcribed by T7 viral RNA polymerase to ssRNA and subsequently replicated to dsRNA by phague Phi6 qRdRP through Replicator RNAi Kit (Thermo Fisher Inc.). The resulting dsRNAs were visualised by standart 2% agarose gel-electrophoresis at 90 V, for 40 min and stained with ethidium bromide, under ultraviolet light. GeneRuler 100 bp Plus DNA Ladder (Thermo Scientific Inc.) was used. The produced dsRNAs were cleaved to small nucleotide fragments (siRNAs) by the enzyme PowerCut Dicer (a recombinant endoribonuclease from Giardia intestinalis) (Petrov, Citation2012a; Citation2012b).
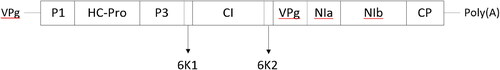
Figure 1. The organisation of PVY viral genome. The boxes represent the genetic regions of PVY encoding the virus proteins: P1, protein 1; HC-Pro, helper component proteinase; P3, the third protein; CI, protein of cylindrical inclusion; 6K1 and 6K2, 6 K proteins; VPg, viral genome-linked protein; NIa, nuclear inclusion protein a; NIb, nuclear inclusion protein b (RNA-dependent-RNA polymerase); CP, coat protein (based on the findings of Riechmann et al. Citation1992; Revers et al. Citation1999).
Inoculation of potato plants
Potato plants were inoculated according to Nooordam (Citation1973) in the shade. Leaves were besprinkled with distilled water and then, dusted with carborundum with 400-600 mesh size particles. The homogenate for inoculation was prepared from 1 g of virus-infected plant material homogenised in 1 mL of 0.1 mol/L cooled to 4 °C potassium sodium phosphate (PSP) buffer pH 8.0, containing 0.2% Na2SO3 and 0.2% ascorbic acid. The plants were processed by gentle rubbing of the leaves with this homogenate and incubation for 3-5 min before washing out with water.
A similar mechanical inoculation technique was applied for the treatments with dsRNAs and siRNAs 48 h before inoculation with the virus. Approximately 10-15 mL of RNAs solution per plant were used depending on the size of the plant and the number of leaves. Positive controls were infected-only plants. Negative controls were non-infected and water-treated-only plants. Experiments were performed in triplicate. Seven days after inoculation with the virus, the plants were inspected for symptoms of infection and serological analysis was carried out to determine the presence of PVY viral infection.
Serological diagnostic test
DAS-ELISA (Double Antibody Sandwich Еnzyme Linked Immunosorbent Assay) using the commercial kit of LOEWE (Biochemica GmbH, Germany) and the method of (Clark & Adams (Citation1977) was carried out seven days after virus inoculation of the potato plants. The plant leaf samples were ground in diluted extraction buffer (1:10, v/v) containing 1% polyvinyl pyrrolidone. The ELISA plates were loaded with polyclonal antiserum (immunoglobulin G, IgG) for PVY in a series of dilutions in 0.05 mol/L carbonate buffer and incubated at 37 °C for 4 h. The unbound components were triple washed out with PBS-T buffer for 5 min. Then the samples were loaded and the plates were once again incubated at 4 °C for 16 h. After triple washing, the alkaline-phosphatase conjugate for PVY was added and the plates were incubated for another 4 h at 37 °C. The colourimetric substrate was p-nitrophenyl phosphate (Sigma) in diethanolamine buffer (pH 9.8) (1 mg/mL). The colour reaction was held in the light at room temperature and stopped with 3 N NaOH. The adsorption was measured at 405 nm in a multifunctional detector DTX 880 (Beckman Coulter, USA). The DAS-ELISA assay was carried out for four leaf samples from each pot. The threshold value (cut-off) was set at three times the value of the negative healthy control.
Data analysis
Data are presented as mean values of the measurements per pot and the standard deviation (±SD) was calculated.
Results
The generated dsRNAs complementary to the HC-Pro gene were approximately 1412 bp in length as described before (Petrov et al. Citation2018a). The resulting dsRNAs complementary to the CI-6K2-NIa genetic region were approximately 689 bp in length without the added promoters. The dsRNA fragments and the resulting pool of CI-6K2-NIa-specific siRNAs were mixed with the resulting dsRNAs and the pool of HC-Pro-specific siRNAs, respectively. We treated viable potato plants with the resulting solutions.
The observation for viral disease symptoms and the diagnostic tests for the presence or absence of PVY infection were performed seven days after inoculation with the PVYN/NTN strain. The positive infected controls showed clear symptoms of viral disease - mosaic of leaves, peripheral necrosis of leaves and necrotic spots of the stems, and necrosis of tubers. Plants treated with combinations of siRNAs and dsRNAs remained visually healthy. No negative effects were observed.
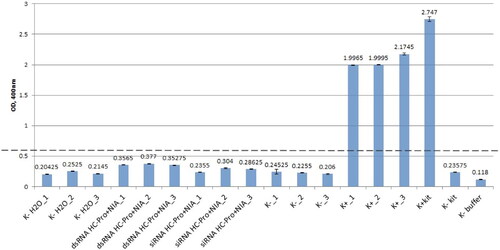
DAS-ELISA of the plants treated with the combination of siRNAs specific for NIa and HC-Pro showed optical density (OD) values in the range of 0.235-0.304, which were comparable to the OD values of the samples from the healthy (not treated and not inoculated) plants − 0.206-0.245, and the values of the water-only-treated plants (not inoculated with virus) − 0.204-0.252 (). The other approach, which used the combination of dsRNAs specific for NIa and HC-Pro resulted in OD values in the range of 0.356-0.377, which were only a little higher and also significantly below the cut-off (0.618) (). The OD values of the samples from the virus-inoculated and not treated plants were approximately 2.0 and above, which is more than five times higher than the highest registered OD value of a sample from a dsRNA treated plant (). These results indicated that a combination of dsRNAs/siRNAs specific for two different conserved genetic regions of PVY successfully conferred immunity to the virus in plants.
Figure 2. DAS-ELISA of potato plants after treatment with combinations of dsRNAs and siRNAs, specific for HC-Pro and Nia of PVYN/NTN and after infection. K- H2O, healthy control plants treated with pure water; dsRNAs HC-Pro-NIA, potato plants treated with a mixture of dsRNAs specific for the HC-Pro-region and dsRNAs specific for the Nia-region of PVY, and infected; siRNAs HC-Pro-NIA, potato plants treated with a mixture of siRNAs specific for the HC-Pro-region and siRNAs specific for the Nia-region of PVY, and infected; K- healthy potato plants, not treated and not infected; K + infected with PVY potato plants, not treated; K + kit, positive control of the ELISA kit; K- kit, negative control of the ELISA kit; K- buffer, negative buffer control of the ELISA. 1, 2 and 3 refer to the number of the pot. Note: Values are means from four replicates of each pot; error bars show standard deviation (±SD).
We suggest that applying a combination of dsRNAs specific for two different conserved genetic regions of PVY can have an additive effect.
The OD values in the DAS-ELISA of the plants were significantly below the cut-off value. With single application of HC-Pro-specific dsRNAs in our previous study, the OD values of the new leaves of the plants treated with HC-Pro-specific dsRNAs were just below the cut-off and 1.3-2 times higher than the OD values of the treated with HC-Pro-specific siRNAs (Petrov et al. Citation2015). Here, the effect was more pronounced: the combination of HC-Pro-specific dsRNAs with other target-specific dsRNAs lowered the extinction values to only 1.1-1.6 times higher than the OD values of the plants treated with HC-Pro-specific siRNAs, making them comparable to the values of the healthy plants and the plants treated with water.This result is extremely important, because it expands the possibilities for the production and application of RNA fragments for RNA interference. To the best of our knowledge, investigating the combined effect of ds/siRNAs specific to two different conserved genetic regions of PVY to control viral infection is a pilot approach.
Discussion
Viruses of the family Potyviridae are spread by insects in a non-persistent way that suck the sap of plants infected with viruses. Тransmission of the virus takes place in seconds even with the test stings of the insects when choosing food. Human activity can also contribute to the spread of the infection through the planting material when it is not grown according to phytosanitary rules. There are well-established rules and practices for protecting plants from viruses, which are subject to supplementation given the progress in agro-technologies and knowledge in the field of plant virology. Plant protection practices can be aimed at killing the pathogen, its vector or host. This is done through the use of physical impact, for example, thermotherapy of planting material, the use of chemical substances (insecticides) against virus vectors (infectious vectors) or wild plant species - virus vectors (Petrov, Citation2012a).
Another way to reduce damage from viral diseases is to develop cultivars that are resistant or tolerant to viral pathogens. In practice, such varieties react less to pathogens, in some cases they remain hidden carriers of the infection, but in general they give a profitable yield from the point of view of quantity and quality. Agrotechnical practices, such as the destruction of weeds and self-sowing, the rotation of agricultural species in the cultivated area, the observance of sowing dates and spatial isolation, are important for reducing the risk of the emergence and spread of viral diseases (Petrov, Citation2012a).
The need to implement comprehensive pathogen control through strategies based on advanced methods of plant health in varietal maintenance and the provision of virus-free planting material is important for practice. Scientific studies on PTGS of PVY genes are scarce, and the combined application of siRNAs against different targets in the virus genome is almost absent in the scientific literature. Although the mechanisms of PTGS have been studied since the 1990s, the knowledge has been used predominantly for construction of transgenic plants (Lin et al. Citation2007; Eamens et al. Citation2008; Prins et al. Citation2008; Ibrahim and Aragão, Citation2015; Lindbo and Dougherty, Citation2005; Jiang et al. Citation2022). The induction of resistance to PVY is mainly based on the expression of sense or antisense sequences complementary to a given fragment of the viral genome. The multifunctional Potyvirus protein HC-Pro, which also has protease activity, is involved in virus transmission by aphids, thereby significantly increasing pathogenicity and suppressing the natural process of PTGS in the host plant (Kasschau and Carrington, Citation1998; Quenouille et al. Citation2013). Systemic spread and replication of PVY were effectively blocked in newly grown leaves by dsRNAs complementary to a capsid protein fragment in transgenic potato cv. Spunta. The obtained results prove the presence of siRNAs and strong resistance to PVYN, PVYO and PVYNTN virus strains (Missiou et al. Citation2004). An inverted hairpin RNA (ihRNA)-generating construct was introduced into transgenic potatoes cv. Atlantic and cv. Ranger Russet, which generated high levels of expression of siRNAs against the PVY capsid protein (McCue et al. Citation2012).
As a much better alternative to transgenic plants, which pose many risks to the ecosystem and human health, our research is based only on the regulation of gene expression of important viral genes, without introducing foreign genetic information into the plants themselves; a process known as epigenetic regulation of gene expression by PTGS. In the literature, experimental studies on RNAi for virus control in plants are scarce. Resistant tobacco plants were obtained after application of dsRNAs targeting the replicase gene (IR 54) of Pepper mild mottle virus (PMMoV) and the HC gene of Plum pox virus (PPV) (Tenllado et al., Citation2003). Much later, hairpin RNA (hpRNAs) constructs targeting different hot-spot sequences of Tobacco mosaic virus (TMV) and PVY genomes were assessed by Sun et al. (Citation2010). In further studies, Petrov (Citation2012b) achieved 82% reduction of disease development caused by PVYO in tobacco by inoculation of constructed dsRNAs complementary to the HC-Pro gene of the virus. PTGS as achieved successfully in potatoes by inoculation of dsRNAs or siRNAs targeting the same gene against PVY strains N, NTN and N/NTN (Petrov et al. (Citation2015 and (Petrov et al., Citation2018b)). Virus-free potato plants were also obtained after treatment with dsRNAs and siRNAs complementary to the NIb region of PVY (Petrov et al. Citation2022).
HC-Pro is a multifunctional protein of the Potyviruses with key functions for the spread of the virus. HC-Pro is involved in the transportation of virions at long distances through the aphid vectors and within the plant. At the same time the protein is the first reported Potyvirus suppressor of plant gene silencing (Kasschau and Carrington, Citation1998; Quenouille et al. Citation2013). Another promising target for PTGS is the CI-6K2-NIa genetic region. CI-6K2-NIa encompasses the sequences encoding three proteins: the cylinder inclusion (CI) protein, which is a helicase with seven conserved motifs (Kadaré and Haenni, Citation1997), the 6 K protein, which is required for genome replication (Schaad, Citation1997), and the Nuclear inclusion protein a (NIa). CI and NIa are major components of the membrane-bound CI/VPg/NIa/NIb replication complex (Shukla, Citation1998). These features define the CI-6K2-NIa and HC-Pro genetic regions of the virus as suitable for inducing gene silencing and to complement the results for evaluating the effectiveness of different molecules for inducing resistance. Thus, the combined effect of specific dsRNAs and siRNAs targeting two genetic regions of PVY to control virus disease by gene silencing was assessed in potato cv. Agria. Gene silencing against PVYN/NTN was achieved by applying combinations of dsRNAs and siRNAs specific for the HC-Pro region of PVY and specific for the CI-6K2-NIa region of the virus. It has been found that a possible insufficient efficiency of dsRNAs specific for a given genetic region can be overcome by the combined administration of dsRNAs specific for two different genetic regions. The presented results are first of their kind regarding the application of a combination of siRNAs against different genetic regions of PVY.
Conclusions
By applying a combination of ds/siRNAs specific for two different conservative regions from the PVY genome, CI-6K2-NIa and HC-Pro, an epigenetic control of viral infection of PVY N/NTN of potato plants cultivar Agria was achieved. This strategy ensures the cultivation of virus-free plants by effective blocking of the systemic spread of the virus and inhibiting viral replication in the host. The use of a combination should be adequate in cases when the application of dsRNAs specific for a single region is not sufficient for full protection of the plants against viral infection.
Data availability statement
The data supporting the findings in this study are available from the corresponding author upon reasonable request.
Disclosure statement
The authors report no conflict of interest.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Clark MF, Adams AN. Chracterisation of the microplate method of enzyme linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977;34(3):475–483.
- Eamens A, Wang M-B, Smith NA, et al. RNA silencing in plants: yesterday, today, and tomorrow. Plant Physiol. 2008;147(2):456–468.
- Glais L, Kerlan C, Tribodet M, et al. Molecular characterization of potato virus Y N isolates by PCR-RFLP. Eur J Plant Pathol. 1996;102(7):655–662.
- Ibrahim AB, Aragão FJL. 2015. Chapter 5. RNAi-Mediated resistance to viruses in genetically engineered plants. In K.S. Mysore and M. Senthil-Kumar (eds.), Plant gene silencing: methods and protocols, methods in molecular biology., vol. 1287, Humana Press, New York. DOI
- ICTV (International Committee on Taxonomy of Viruses). 2021. Virus Taxonomy: 2021 Release. Available from https://ictv.global/taxonomy. Accessed 05.10.2022.
- Jiang L, Mu R, Wang Z, et al. Silencing P25, HC-Pro and Brp1 of potato virus (viroid) using artificial microRNA confers resistance to PVX, PVY and PSTVd in transgenic potato. Potato Res. 2022. https://doi.org/10.1007/s11540-022-09580-x.
- Jorgensen RA. 2003. Sense co-suppression in plants: past, present and future. In G.J. Hannon (ed.), RNAi: a guide to gene silencing. Cold Spring Harbor Laboratory Press, New York, pp. 15–22. ISBN 978-087969641-2
- Kadaré G,Haenni AL. Virus-encoded RNA helicases. J Virol. 1997;71(4):2583–2590.
- Kasschau KD, Carrington JC. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell. 1998;95(4):461–470.
- Lin S-S, Henriques R, Wu H-W, et al. Strategies and mechanisms of plant virus resistance. Plant Biotechnol Rep. 2007;1(3):125–134.
- Lindbo JA, Dougherty WG. Plant pathology and RNAi: a brief history. Annu Rev Phytopathol. 2005;43:191–204.
- Lorenzen JH, Piche LM, Gudmestad NC, et al. A multiplex PCR assay to characterize potato virus Y isolates and identify strain mixtures. Plant Dis. 2006;90(7):935–940.
- Martinez J, Patkaniowska A, Urlaub H, et al. Single-stranded antisense siRNAs guide target RNA cleavagein RNAi. Cell. 2002;110(5):563–574.
- McCue KF, Ponciano G, Rockhold DR, et al. Generation of PVY coat protein siRNAs in transgenic potatoes resistant to PVY. Am J Pot Res. 2012;89(5):374–383.
- Missiou A, Kalantidis K, Boutla A, et al. Generation of transgenic potato plants highly resistant to potato virus Y (PVY) through RNA silencing. Mol. Breeding. 2004;14(2):185–197.
- Nooordam D. Identification of plant viruses. Methods and experiments. Centre for Agricultural Publishing and Documentation (Pudoc), Wageningen, 1973. ISBN 90 220 0464 3.
- Petrov N,Stoyanova M,Stoev A, et al. Resistance to potato virus Y in potatoes induced at epigenetic level. Acta Microbiol Bulg. 2018b;34(3):174–179.
- Petrov N. 2012a. Potato virus Y (PVY) of solanaceae crops. [in bulgarian]. PhD thesis, institute of soil science, agrotechnologies and plant protection “N. Pushkarov., Agricultural Academy, Sofia, Bulgaria.
- Petrov N. 2012b. Inhibition of the virus replication of PVY by siRNAs. Proc. of the Third Congress of Virology (Days of Virology in Bulgaria) with Int. Part., Sofia, 101–105.
- Petrov N, Stoyanova M, Andonova R, et al. Induction of resistance to potato virus Y strain NTN in potato plants through RNAi. Biotech. Biotechnol. Equip. 2015;29(1):21–26.
- Petrov N, Stoyanova M, Gaur RK. 2018a. Chapter 3. Characterization and control of potato virus Y in the crops. In R.K. Gaur, S.M.P. Khurana, Y. Dorokhov (Еds.) Plant viruses: diversity, interaction and management. CRC Press, Taylor&Francis Group, Boca Raton, FL. рp. 41–64. ISBN 9781138061514.
- Petrov N, Stoyanova M, Gaur RK. 2019. Chapter 23. Post-transcriptional gene silencing as a tool for controlling viruses in plants. In Khurana S.M.P., R.K. Gaur (eds.), Plant biotechnology: progress in genomic era. 1st ed., Springer Verlag, Singapore. ISBN 978-981-13-8498-1, p.527–542.
- Petrov NM, Stoyanova MI, Stoev AV, et al. Induction of gene silencing of NIb gene region of potato virus Y by dsRNAs and siRNAs and reduction of infection in potato plants cultivar djeli. Biotechnol. Biotechnol. Equip. 2022;36(1):159–164.
- Prins M, Laimer M, Noris E, et al. Strategies for antiviral resistance in transgenic plants. Mol Plant Pathol. 2008;9(1):73–83.
- Quenouille J, Vassilakos N, Moury B. Potato virus Y: a major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Mol Plant Pathol. 2013;14(5):439–452.
- Revers F, Le Gall O, Candresse T, et al. New advances in understanding the molecular biology of plant/potyvirus interactions. MPMI. 1999;12(5):367–376.
- Riechmann J, Lain S, Garcia JA. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 1992;73(1):1–16.
- Schaad M, Jensen PE, Carrington JC. Formation of plant RNA virus replication complex on membranes: role of an endoplasmic reticulum-targeted viral protein. Embo J. 1997;16(13):4049–4059.
- Shukla DD, Ward CW, Brunt AA, et al. 1998. Potyviridae family. In: description of plant Viruses, No. 366. Association of Applied Biologists (AAB).
- Sun Z-N, Yin G, Song Y-Z, et al. Bacterially expressed double-stranded RNAs against hot-spot sequences of tobacco mosaic virus or potato virus Y genome have different ability to protect tobacco from viral infection. Appl Biochem Biotechnol. 2010;162(7):1901–1914.
- Tenllado F,Martínez-García B,Vargas M, et al. Crude extracts of bacterially expressed dsrna can be used to protect plants against virus infections. BMC Biotechnol. 2003;3:3. PMC: 12659646
- Van der Vlugt RA, Leunissen J, Goldbach R. Taxonomic relationships between distinct potato virus Y isolates based on detailed comparisons of the viral coat proteins and 3’non-translated regions. Arch. Virol. 1993;131(3-4):361–375.
- Vance V, Vaucheret H. RNA silencing in plants—defense and counterdefense. Science. 2001;292(5525):2277–2280.
