Abstract
Psidium guajava is a new crop cultivated in Greece, with a potential pharmaceutical and ornamental uses. An efficient and reliable protocol for in vitro clonal propagation of P. guajava from seeds was used. Five different media were examined. Shoot proliferation succeeded on a new basal medium (BB) supplemented with a modified vitamin solution (vit BB), without plant growth regulators. In addition, shoots on media supplemented with BB vitamin solution performed better than in Murashige and Scoog (MS) and Woody Plant (WP) media. When BB medium was used, the greatest shoot number resulted in 4 mg L−1 6-benzyladenine (BA) with a score of 2.8. Regenerated single shoots were rooted in the BB medium, supplemented with 0.1 ΝΑΑ (mg L−1) and producing 2.2 roots of 4.6 cm, along with 3.1 cm of adventitious shoots. Acclimatization was accomplished in three phases, using peat-moss, vermiculite and perlite (1:1:1), with a total survival of 83.3%. To examine the genetic diversity of the plant material, Inter Simple Sequence Repeat (ISSR) and Start Codon Targeted (SCoT) molecular markers were used. The results indicated a low diversity, along with high percentage of polymorphism for both ISSR and SCoT analyzes.
Introduction
Guava (Psidium guajava) is a perennial species originating in tropical America. Due to the plant’s high productivity and adaptability, guava quickly spread to most of the tropics and subtropics, mainly in America, Asia and Australia [Citation1–3] and recently in the European Mediterranean region. In Greece, it is reported as a crop in the South and East Aegean Islands and Crete, a cool subtropical era. In Greece, there have been reported no serious problems with pests and diseases, although the root-knot nematode Meloidogyne enterolobii isolated from guava groves in Egypt [Citation4] is a well-known pathogen of guava, causing important losses in this crop [Citation5] and it is regulated as a quarantine nematode in the Mediterranean region (EPPO).
Guava is a very valuable fruit, and its nutritional benefits place it among the superfruits. It is a natural source of iron, calcium, phosphorus, vitamins A and B and pectin [Citation6, Citation7]. It also contains high-grade antioxidants such as lycopene, carotenoids, tannins, phenols, triterpenes, flavonoids, essential oils, saponins, lectins and polyphenols [Citation6, Citation8]. Apart from its nutritional value, guava has many medicinal properties. In folk medicine fruits, leaves and bark are used for a variety of illnesses [Citation9]. As reviewed by Jamieson et al. [Citation6], many countries in Latin America, the Caribbean, India, Africa and Indonesia have used guava-derived products to treat various communicable and non-communicable ailments, such as gastrointestinal disease, hepatic damage, bacterial and fungal infection, fever, rheumatism, respiratory illness, cough, diabetes, pain, wounds, mouth ulcers, uterine bleeding, blennorrhagia, menstrual disorders, amenorrhea, and as an emmenagogue [Citation9–11]. These therapeutic effects are thought to be due to the antioxidant, anti-inflammatory, antimicrobial, immunomodulatory, ntihyperglycemic, and antihyperlipidemic effects of numerous phytochemicals present in P. guajava [Citation11–13]. With vitamin C content 4 times that of oranges, many studies report the anticancer potential of P. guajava tested in breast, prostate, blood, colorectum, gynecological, lung, oral, sarcoma, liver, neural, kidney, skin and stomach [Citation6, Citation14]. Having white-colored, large, faintly fragrant flowers and aromatic, evergreen leaves, guava is also used as an ornamental plant [Citation15]. Guava can grow well in a wide range of climatic and edaphic conditions, from sea level to 2,100 m of altitude. However, optimum growth conditions include temperatures between 20 and 30 °C, annual rainfall from 1,000 to 2,000 mm well distributed throughout a year, soils with good drainage and 5 to 7 pH level [Citation2, Citation3, Citation16–18].
Similar to other species [Citation19], there are various challenges in the nurseries that produce seedlings for rootstocks that will be used for grafting of well-known varieties. Conventional propagative methods of guava are limited because, like other woody species, there is a long juvenile growth period and self-incompatibility [Citation20]. In addition, seed multiplication will result in genetically heterogeneous individuals that can grow in the same orchard or in separate ones [Citation21]. Therefore, seed propagation is not recommended in commercial orchards to increase productivity [Citation22]. Vegetative propagation of guava is one of the most important practices that influence the vigor, yield and quality of the fruits. Asexually, guava can be successfully propagated by stooling, inarching, layering, cutting, grafting and budding [Citation23]. According to recent research, the inarching significantly influenced the survival percent and growth parameters of guava among layering and stooling [Citation24].
There are several advantages using tissue cultured rootstocks, instead of conventional tools for guava propagation. Rapid clonal propagation in controlled conditions, efficient propagation with less initial plant material, better growth and development of healthy plants [Citation25, Citation26]. Also, clonal propagation reduces plant-to-plant variation and ensures uniform population of unique clones [Citation1, Citation27]. Although there are several problems associated with in vitro culture of guava like browning of culture medium due to phenolic compounds and microbial contamination, the future of guava industry lies in the development of an efficient protocol that produces healthy plant material through micropropagation [Citation3].
Organogenesis is a known model of regeneration using in vitro explants. Since the regeneration of tissues from in vivo sources faces various obstacles, tall explant seedlings are a convenient solution, as they produce less contamination and less phenolic compounds [Citation25, Citation26, Citation28]. Clonal propagation of Guava from seedlings and Grafted Plants was attempted by Loh and Rao [Citation29] and Papadatou et al. [Citation30], where cultures were established from shoot tips excised from seedlings. Mohamed-Yasseen et al. [Citation31]reported a propagation culture system from seedling, where rooted plantlets were acclimatized into a greenhouse. Regeneration of adventitious shoots from hypocotyl explants of seedlings and their acclimatization have been reported in 2002 by Sign et al. According to their results, the rate of shoot organogenesis was low, but still the protocol was suggested as useful for transformation studies or for propagating elite genotypes
Most of the guava clonal propagation studies have used Murashige and Scoog (MS) or Woody Plant (WP) culture medium [Citation32, Citation33]. However, a new promising medium (BB basal medium, BBb) and a modified vitamin solution (vit BB) was reported by Tsoktouridis et al. [Citation34], giving promising results in Thymus sibthorpii. Apart from the explant type, the success of tissue culture is based on plant growth regulators included in the culture medium and their concentrations. Cytokinin levels and especially 6-benzyladenine (BA) have been shown to play a crucial role on the propagation of guava and other subtropical species [Citation20, Citation29–32].
With little data available regarding the micropropagation of Psidium guajava, the aim of this study was to develop a powerful micropropagation protocol for ex situ conservation using for the first time a promising culture medium This is an initial step toward the effort of P. guajava commercialization in pomology and alimentary industries as a new crop with valuable pharmaceutical, culinary and ornamental properties from the field to the market [Citation6, Citation13, Citation15, Citation35].
Materials and methods
Plant material
The plant material was maintained at the local living plant collection of the Laboratory of Subtropical Plants and Tissue Culture in Chania, Institute of Olive Tree, Subtropical Crops and Viticulture, Hellenic Agricultural Organization DIMITRA. The mother plants of P. guajava of the collection were maintained in the field and received annually fertilization (7-14-21 + 2Fe) in November and water-soluble fertilizer when necessary. The plants produced fruits, mature in November.
In vitro establishment and culture conditions
The procedure was performed by analogy to Tsoktouridis et al. [Citation34]. Seeds taken from a single fruit of P. guajava mother plant were initially surface disinfected using 70% (v/v) ethanol (Disolol 97%; Chem-Lab, Zedelgem, Belgium) for 3 to 4 min, followed by 15% (v/v) sodium hypochlorite solution (NaOCl; Chem-Lab), supplemented with 160 µ L−1 of Tween® 20 per 100 mL solution, shaken on a rotary shaker overnight and rinsed three times with sterile distilled water.
All seeds were transferred in 150 × 200 mm test tubes containing 10 mL of MS [Citation36] medium supplemented with 25 g L−1 sucrose (Duchefa, Haarlem, The Netherlands) and solidified with 6 g L−1 Agar (Fluka, BioChemika). The medium was free of hormones. The pH was adjusted to 5.8 using 1 mol L−1 HCl or 1 mol L−1 KOH (Chem-Lab) prior to gelling agent addition and autoclaving at 121 °C for 20 min. Cultures were maintained at 25 ± 1 °C and a 16-h photoperiod under cool white fluorescent light (40 μmol m−2 s−1 photosynthetic photon flux) (TL-D 58 W/54-765, Philips, Carquefou, France).
Shoot proliferation experiments
Shoot explants from the germinated seeds were transferred into Magenta® B-cap-sealed, 62.4 mm × 95.8 mm, 200 mL glass (baby food jars) (Sigma-Aldrich®), containing 30 mL of the same medium. Explants that proliferated clusters of shoots were dissected into single shoots and the procedure was repeated every 4 weeks, until adequate plant material was produced for further experimentation.
For the first experiment, the following culture media were used; MS basal medium [Citation36], WP basal medium [Citation37] and a new medium (BB basal medium, BBb) [Citation34, ) alone, or with a modified vitamin solution (vit BB), referred as MS + vit BB, MS full, WP + vit BB, WP full, BB full (BBb + vit BB).
Table 1. Components of BB full [Citation34] culture medium for in vitro experiments (BB basal medium and BB vitamins solution).
The first experiment was based on the selection of MS, WP and BB media combined with BB vitamins instead of their vitamin’s solution (MS + vit BB, MS full, WP + vit BB, WP full, BB full), free of hormones, supplemented with 25 g L−1 sucrose and solidified with 6 g L−1 Agar, pH adjusted, and autoclaved as before. Mainly shoot tips (meristem tips) and in some cases shoot internodes (both with at least two leaves) were used as explants. The experiment was based on a completely randomized design and consisted of five treatments with four replications per treatment averaged over four explants (4 baby food jars x 4 explants per jar). The explants were transferred to fresh culture medium after four weeks. The results were evaluated 8 weeks after the beginning of the experiment and measurements concerned the number of shoots and roots, shoot and root length per explant and treatment.
In the following experiments (2, 3 and 4), 2- to 3-week-old in vitro-formed proliferated shoot tips were used as initial explants. In the second experiment, explants were transferred into the baby food jars, each containing 35 mL of BB culture medium supplemented with BA. The experiments for shoot proliferation were based on a completely randomized design and consisted of five treatments (0, 1, 2, 3 or 4 mg L−1 BA) with four replications per treatment, averaged over four explants (4 baby food jars x 4 explants per jar).
In the third experiment, explants were transferred into the baby food jars, each containing 35 mL of BB culture medium supplemented with 0.1 mg L−1 NAA and 0, 0.5, 1, 2 or 4 mg L−1 BA. The experiment for shoot proliferation was based on a completely randomized design and consisted of five treatments (0, 0.5, 1, 2 or 4 mg L−1 BA) with four replications per treatment, averaged over four explants (4 baby food jars x 4 explants per jar). The number of shoots and roots, shoot and root length per explant and treatment were measured 8 weeks after the beginning of the experiment.
Root proliferation experiment
For the fourth experiment, explants were transferred into the baby food jars, each containing 35 mL of BB culture medium supplemented with 0.5 mg L−1 NAA and 0, 0.1, 0.5, 1.0, 1.5 mg L−1 IAA or IBA. The experiments for root proliferation were based on a completely randomized design and consisted of nine treatments with four replications per treatment, averaged over four explants (4 baby food jars x 4 explants per jar).
Acclimatization and plant establishment ex vitro
In the fifth experiment, the in vitro-rooted plants were transferred to baby food jars containing hormone-free BB medium, solidified with 3 g L−1 Agar. The cultures were maintained at 25 ± 1 °C and 16-h photoperiod under cool white fluorescent light (40 μmol m−2 s−1) for 2 weeks before transfer for further acclimatization in greenhouse conditions.
The in vitro-rooted acclimatized micro-plants in vessels were carefully rinsed with tap water to remove the adhering medium and then they were transferred into 30 cm x 50 cm plastic seedling trays (Tray 50 × 30 cm 60 Hole Insert, Alegre growshops, Athens, Greece) filled with soil substrate mixture consisting of peat moss (Klasmann, TS2), vermiculite and perlite (1:1:1 v/v). The seedling trays were then placed in a nylon tunnel with adjustable relative humidity (RH), from 75 to 82% RH in the first week and 55 to 62% RH during the second week. After 2 weeks, the trays of P. guajava plants were transferred to a greenhouse bench (25 ± 4 °C temperature, 50 ± 5% RH) for two more weeks, where they were irrigated by sprinkling. The plants were transplanted into 1-L pots containing soil substrate consisting of peat moss, vermiculite and soil (1:1:1 v/v) and moved to the nursery outdoors, where the acclimatization of the plants was completed. The number and percentage (%) of successfully acclimatized plants were recorded 2 months later.
Genetic characterization using ISSR and SCoT molecular markers
Nine guava genotypes were examined, obtained from the in vitro germinated seeds. DNA extraction and isolation was performed from young guava leaves, using a NucleoSpin® Plant II Kit (Macherey-Nagel) according to the manufacturer’s instructions. The obtained DNA was quantified by a standard spectrophotometric measurement at 260 nm in an Eppendorf BioPhotometer; and its integrity was assayed by gel electrophoresis in a 1% agarose gel. Samples were then diluted to 20 ng μL−1 work concentrations.
Inter simple sequence repeat (ISSR) procedure
The ISSR assay was performed as previously described [Citation38], with some modifications. Briefly, six oligonucleotide primers complementary to simple sequence repeats (UBC807, UBC810, UBC811, UBC835, UBC840, UBC891) were used for polymerase chain reaction (PCR) amplification conducted in 25 μL reaction volume containing the following reagents: 0.5 μL of dNTPs (10 mmol L−1), 2.5 μL of 10xbuffer, 1 μL of Primer (10 mmol L−1), 0.5 μL of template DNA, 0.2 μL of Taq polymerase (5 U/μL) and 20.3 μL of sterile dd H2O. Binary data points denote the presence/absence of each distinguishable band across all samples for the same primer in both replicate sets of amplifications. PCR amplifications were performed in an Eppendorf Mastercycler EP Thermal Cycler Range as follows: Initial DNA denaturation was performed at 95 ◦C for 5′, followed by 35 cycles at: 95 ◦C of 30′’ for DNA denaturation, at 48 – 56 °C (all 52 ◦C except UBC811 at 48 ◦C) for 90′’ for the annealing of the primers and at 72 ◦C for 90 s for the extension of the chain. When the 35 cycles were completed, the temperature was maintained at 72 ◦C for 5′. Amplification products were separated by electrophoresis in a 1.5% agarose gel and stained with Midori green direct by NIPPON Genetics. A 1-kb DNA ladder (New England Biolabs) was used as a size marker. Gel images were documented in a FastGene FAS-DIGI PRO (NIPPON Genetics).
Start codon targeted (SCoT) procedure
According to SCoT procedure, five oligonucleotide primers complementary to codon targeted polymorphisms (SCoT1, SCoT13, SCoT33, SCoT34, SCoT61) were used for PCR amplification conducted in 25 μL reaction volume containing the following reagents: 0.5 μL of dNTPs (10 mmol L−1), 2.5 μL of 10xbuffer, 1 μL of Primer (10 mmol L−1), 1 μL of template DNA, 0.2 μL of Taq polymerase (5 U μL−1) and 19.3 μL of sterile dd H2O. Binary data points denote the presence/absence of each distinguishable band across all samples for the same primer in both replicate sets of amplifications. PCR amplifications were performed in an Eppendorf Mastercycler EP Thermal Cycler Range as follows: Initial DNA denaturation was performed at 95 ◦C for 5′, followed by 35 cycles: at 95 ◦C of 30′’ for DNA denaturation, at 50 ◦C for 90′’ for the annealing of the primers and at 72 ◦C for 90′’ for the extension of the chain. When the 35 cycles were completed, the temperature was maintained at 72 ◦C for 5 min. Amplification products were separated by electrophoresis in 1.5% agarose gel and stained with Midori green direct by NIPPON Genetics. A 1-kb DNA ladder (New England Biolabs) was used as a size marker. Gel images were documented in a FastGene FAS-DIGI PRO (NIPPON Genetics).
Statistical analysis
Data resulting from the two experiments of shoot proliferation and rooting of P. guajava were analyzed according to the Completely Randomized Design (CRD). The experiments were performed once. Separation of means was done with the Duncan’s multiple comparisons procedure at significance levelα = 0.05 (p ≤ 0.05). All statistical analyses were performed with the SPSS® v.20.0 statistical software (IBM, Armonk, NY).
The presence of alleles was scored as ‘1’ and the absence as ‘0’′. Only clear, reproducible bands were scored, manually. The fragments ranged in size from 500 to 3100 bases for ISSRs and 650–4000 for SCoTs. All analyses were performed using GenAlex 6.5 [Citation39] and Microsoft® Excel 2010/XLSTAT©-Pro software (Version 2013.4.07, Addinsoft, Inc., Brooklyn, NY, USA). Nei’s genetic distance [Citation40] was determined among the genotypes and was used for grouping of the genotypes by UNJ (Un-weighted Neighbor Joining) cluster method [Citation41]. The Nei’s coefficient [Citation42] was calculated to evaluate the genetic variation within the population based on the performed method. The data were processed to generate the observed number of alleles (na), effective number of alleles (ne), Shanon’s information index (I), diversity (h), unbiased diversity (uh), and the percentage of polymorphic loci (%P). Principal Coordinate Analysis (PCoA) analysis was also performed, using GenAlex software.
Results
Disinfection and establishment of in vitro cultures of P. guajava
The most appropriate concentration of NaOCl for P. guajava seeds was 15% (v/v), which led to a 97.3% of disinfection (107 out of 110 seeds). The disinfected seeds from a single fruit, were placed in the basal medium MS without any growth regulators to ensure sterility, until germination. After 3 months, 96% of the seeds germinated (). Shoot explants from the germinated seeds were recultured until the production of adequate plant material for further experimentation and initiation of the next experiments on shoot proliferation.
Shoot proliferation of P. guajava
To study the most appropriate culture medium for guava development, five different media were prepared. After 8 weeks, the results showed that the shoot number was significantly higher in BB full medium by 1.2 shoots (). The shoot numbers in the other treatments were not statistically significant compared to BBb + vit BB. The shoot length in MS + vit BB was statistically higher in comparison to MS full and WP full media. There were non-significant differences between MS + vit BB, WP + vit BB and BB full in terms of shoot length. However, the shoots on media supplemented with BB vitamins solution performed slightly better than in MS full and WP full media. No hormones were added to the media. As roots derived from the shoot explants in all treatments during the experiment, root induction was recorded as well. WP + vit BB media produced a significantly higher root number (1.7), compared to WP full, MS full and BB full media. The root length reached 3.9 cm in WP + vit BB, which was significantly higher from the other treatments. Based on the shoot number on BB full medium and the performance of the explants on BB vitamins solution, we recommend BB full medium for shoot proliferation of P. guajava, although the differences were not statistically significant compared to all other media ().
Table 2. 1st experiment: Effect of different growth regulator-free basal media on Psidium guajava explants development.
In vitro-maintained shoots of P. guajava were used for a two-stages organogenesis experiment, with the initial experiment using the BB full medium supplemented with BA at 0, 1, 2, 3 or 4 mg L−1. The results showed shoot induction under the effect of all treatments when BA was added (). The greatest shoot number resulted in 4 mg L−1 BA with a score of 2.8 (). Although the treatments with 1, 2 or 3 mg L−1 of BA had non-significant differences among them, the shoot number was statistically lower than that in the treatment with 4 mg L−1 BA. On the contrary, the shoot length showed the opposite trend to that of shoot number: the greatest shoot length was observed in the control treatment, followed by 1, 2 and 3 mg L−1 of BA. The poorest results were scored at 4 mg L−1 BA. Root induction was only observed at the control treatment. BA addition in the medium resulted in yellowish callus formation on the base of the explants ().
Table 3. 2nd experiment: Effect of different BA concentrations on the organogenesis of Psidium guajava explants, using BB full medium.
Another experiment was set up to study the proliferation of Psidium guajava. The selection of treatments was based on the previous BA concentrations in combination with 0.1 mg L−1 NAA. Evaluations of five treatments () showed that the best combination for shoot proliferation of P. guajava was 2.0 BA + 0.1 NAA mg L−1, but still, the longest shoots and roots were induced in the control treatment. Callus appeared on the explants when BA was added in the media. The evaluation resulted in non-significant differences between the treatments of 0.5, 1.0, 2.0 and 4.0, with a score between 1 and 1.3.
Table 4. 3rd experiment: Effect of different BA concentration combined with NAA on the organogenesis of Psidium guajava explants, using BB full medium.
Effect of hormones on the rooting of P. guajava micro-shoots
The eight different concentrations (four of IAA and four of IBA) used for rooting were not statistically significant compared to the control as refers to root number, but significant differences were detected in root length between the last treatment (1.5 IBA + 0.5 NAA mg L−1) and the following three; 1.0 IAA, 1.5 IAA, 0.5 IBA (+0.5 NAA mg L−1) (, ). However, the explants continued to grow during this stage and therefore the shoot length per explant was calculated. Shoot length in the last treatment (1.5 IBA + 0.5 NAA mg L-1) was statistical greater than in 1.0 IAA and 0.5 IBA (+0.5 NAA mg L−1) (). The rooted micro-plants had excellent general appearance and were ready for ex vitro acclimatization under greenhouse conditions.
Table 5. 4th experiment: Effect of different IAA or IBA concentrations in combination with 0.5 mg L−1 NAA. on rooting of Psidium guajava micro-plants, established in BB full culture medium. The number and length of roots was counted for each treatment. In addition, shoot proliferation was also observed during rooting, therefore the number and height of the proliferated shoots was noted as well.
Acclimatization of P. guajava micro-plants
The 144 micro-plants obtained from the baby food jars and transplanted in the soil substrate presented 89.5% success after the first two weeks in the nylon tunnel and 93% on a greenhouse bench (). In the third phase for hardening the plants outdoors in the main nursery, all plants that were transplanted to 1 L pots had 100% survival, declining to a total survival of 83.3% of the initial plant material, after acclimatization completed.
Table 6. 5th experiment: Percent acclimatization success of Psidium guajava micro-plants derived from in vitro acclimatization in Magenta vessels and transferred to greenhouse.
Genetic diversity
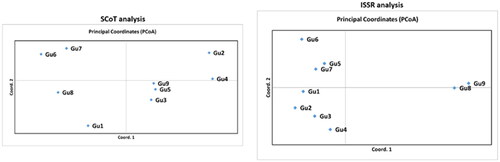
The next step in our experiments was GenAlEx analysis for the allelic patterns of the examined population. The results for ISSR showed that the mean number of different alleles (Na) was 1.889, the number of effective alleles (Ne) was 1.587, Shanon’s information Index (I) was 0.498 and the unbiased expected heterozygosity (uHe) was 0.379. In the SCoT analysis, the mean number of different alleles (Na) was 1.961, the number of effective alleles (Ne) was 1.651, Shanon’s information Index (I) was 0.547 and the unbiased expected heterozygosity (uHe) was 0.418. Moreover, the percentage of polymorphic loci for ISSR was 88.89%, while for SCoT was 96.10%. According to PCoA analysis for ISSR, the samples were classified in two groups. Most of the samples were concentrated on the left side of the plot, while samples Gu8 and Gu9 formed another group in the right part of the plot. According to PCoA analysis for SCoT, the samples were scattered around the plot, forming 1 group at each quadrant. The first group comprised of the samples Gu6 and Gu7 in the upper left part of the plot, samples Gu1 and Gu8 in the bottom left part, Gu2 and Gu4 in the upper right part and Gu3, Gu5, Gu9 in the bottom right part ( and ).
Discussion
In vitro culture of guava
Several subtropical species are now available for cultivation in new areas, such as Southern Greece, due to climate changes. Both the increased interest in consumption of fruit with medicinal value and the need to produce plant material rapidly for commercial purposes have resulted in the development of vegetative propagation methods, such as plant tissue culture techniques for the production chain [Citation43].
It is still very difficult to establish guava plants in vitro for asexual propagation because of deficient sterilization techniques and subsequent contamination of explant tissues. Therefore, in the present study, we selected in vitro seed germination and juvenile plantlets as initial material for guava cultivars, in accordance with Chandra and Mishra [Citation25], Usman et al. [Citation26] and Papadatou et al. [Citation30]. Using various amounts of antioxidants, such as PVP, ascorbic acid and citric acid [Citation1], plant tissues can be successfully disinfected and established in vitro. In the present study, we treated seeds of P. guajava with ethanol and soaked them in a 15% (v/v) NaOCl solution overnight. This protocol should be followed as a standard procedure for seed material, to allow in vitro establishment with low risk of contamination.
Generally, MS basal medium in full or half strength is used for guava micropropagation [Citation1, Citation26, Citation32, Citation44]. Hence Papadatou et al. [Citation30] used OM to proliferate guava seedlings, with positive results. The present study evaluated five different medium formulations for P. guajava. This study is the only report of that extent to demonstrate that a new basal medium is suitable for the in vitro regeneration of guava plants.
The five basal media in this study were designed based on combinations between standard MS or WP basal medium and the new medium (BB) used by Tsoktouridis et al. [Citation34] which had been routinely used for the micropropagation of subtropical species. Compared to MS, the BB medium has lower nitrogen and boron content, six more vitamins and much higher concentrations of phosphorus, calcium, magnesium, copper, manganese, molybdenum and zinc (). By combining the BB vitamin solution with these two basal media, we obtained new media with intermediate concentrations of compounds between the BB and standard MS or WP media (). Compared to standard MS medium used in micropropagation of guava, BB medium more appropriate for the micropropagation of P. guajava, as regards shoot number. In addition, this new MS + vit BB medium led to longer new derived shoots, showing that a lower concentration of nitrogen, higher concentrations of phosphorus, calcium, magnesium, copper, manganese, molybdenum, zinc, and 6 additional vitamins had a positive effect on guava propagation. Although there were non-significant differences between MS + vit BB, WP + vit BB and BB full in terms of shoot length, the explants showed that the addition of BB vitamins solution acted positively.
Adventitious shoot induction of explants is the usual mode of regeneration in guava. According to the literature, addition of the cytokinins BA, 2,4-D or kinetin, in combination with the auxins NAA, IBA, indole-3-acetic acid (IAA), or gibberellic acid (GA3) in the basal medium is the common procedure for in vitro shoot proliferation of guava [Citation32, Citation33, Citation45, Citation46]. Different responses of in vitro regeneration using several combinations of the above plant growth regulators mainly depend on the type of explant, medium composition and type of plant growth regulators. BA and BAP were more effective than kinetin in inducing shoot proliferation in guava [Citation47]. Amin and Jaiswal [Citation48] reported plantlet formation from mature tissue of guava, using only BA. Our results supported this, as BB full medium supplemented only with 1, 2, 3 or 4 mg L−1 BA showed excellent results for shoot proliferation. However, absence of BA with 0 or 0.1 mg L−1 NAA resulted in shoot elongation ( and , Control). Therefore, BB full medium supplemented with 4 mg L−1 BA, was the optimum basal medium, producing an average of 2.8 shoots per explant of P. guajava ().
In vitro rooting of guava is not a simple procedure, especially when in vitro seedlings are used as initial plant material. The standard auxins IBA, NAA or IAA are widely used and induce roots for many different guava cultivars, mainly from mature tree explants [Citation1, Citation3, Citation49], although some authors have reported rooting in the absence of plant growth regulators during guava micropropagation [Citation26, Citation49]. Combinations of the above auxins were used in the present investigation for P. guajava, producing a mean number of 4.7 roots induced from the bases of micro-shoots at the concentration of 0.5 mg L−1 NAA (). Noticeably, the shoots continued to proliferate during the rooting phase of P. guajava, yielding a mean number of 1.2 micro-shoots for the treatment of 0.5 IAA + 0.5 NAA (mg L−1) ().
Acclimatization is the most important stage in plant tissue culture and is completed after in vitro regeneration and the plants’ transfer under natural environmental conditions. The effect of environmental factors on acclimatization and growth of rooted plantlets is very important, affecting processes, such as evaporation and respiration [Citation3]. By analogy to the previous report in T. sibthorpii [Citation34], the three phases of acclimatization used in the present study for the micro-plants of P. guajava focused on the gradual reduction of relative humidity. The procedure led to an optimistic survival rate of 83.3%, using peat-moss:vermiculite:perlite (1:1:1) as potting mixture. According to the literature, the survival rate of guava explants is based on root formation and potting mixture. The response rates of shoot tip explants were low in the study by Amin and Jaiswal [Citation48], due to low root formation. Sand, soil and FYM mixture seems adequate for high percentage of guava plantlets survival [Citation32, Citation46, Citation50]. Rai et al. [Citation47] reported that almost 90% of guava plants acclimatized in sand and compost potting mixture.
Genetic structure of guava assessed by molecular markers
The long juvenile phase and the high heterozygous nature of guava plants are the causes that traditional building is not used for the varietal development [Citation51–53] minimizing yield. Thus, genomics could apply new methods and techniques to find answers to existing breeding barriers in woody perennial fruit crops like guava. Molecular markers are a powerful genomic tool for genetic diversity studied between crop species that could be introduced at each stage of varietal improvement programs. Among molecular markers, SSRs tend to be used more frequently in plant genomic studies, towing to their high reproducibility, multi-allelic nature, co-dominant inheritance, high abundance and extensive genome coverage [Citation54, Citation55].
A few studies have been carried out to comprehend the genetic variability of guava germplasm collections. In 2012, a study assessed the potential of SSR and ISSR markers to assay the genetic stability of in vitro regenerated plants. The results suggested that due to high reproducibility and reliability, SSR and ISSR markers could successfully be used for the determination of the genetic homogeneity among tissue culture raised plants [Citation56]. In 2014, researchers tried to analyze both quantitative and qualitative traits in 132 elite Pakistani guava accessions, in order to assess possibilities for improving guava fruit yield and quality. They found that these cultivars were genetically diverse and contributed significantly to variation of the morphological traits studied, suggesting also that reliable markers should be selected to analyze further the genetic composition of this germplasm [Citation57]. Moreover, six ISSR primers could distinguish 16 closely related varieties, which have a good reference value [Citation58], whereas screening with nine ISSR primers to examine the genetic diversity of 36 guava germplasm resources indicated that the genetic basis of the collected germplasm resources was narrow [Citation59]. SSR markers can specifically differentiate seedling strains with an unclear parental origin and on target identify cultivars that could provide reference value and scientific basis for seed cultivation [Citation60]. Analysis of the genetic diversity in guava plants using SSR markers revealed that high genetic diversity existed among genotypes including wild species, while these markers could be used in genomic-assisted guava breeding [Citation52]. Apart from ISSR, RAPD and SSR markers, a recent study was the first to employ genome-wide single nucleotide polymorphisms (SNPs) to explore the genetic diversity of 48 guava accessions collected from Mexico and other continents [Citation61]. A parallel aim of the present study was to evaluate the genetic diversity of the produced seedling progenies of guava plants obtained from one fruit, using ISSR and SCoT molecular markers. The results indicated a conformity between the analyzes from GenAlEx for ISSRs and SCoTs. According to the PCoA plot for ISSRs, most samples were clustered in the left part, forming 2 groups, except for samples Gu8 and Gu9, which were grouped separately in the right part of the plot. As for SCoTs, the samples were dispersed around the 4 quadrants, forming 4 groups. According to GenAlEx analysis, the results showed low diversity in our population, since the unbiased expected heterozygosity (uHe) was 0.379 for ISSR, and 0.418 for SCoT. Moreover, Shanon’s information Index (I) was also very low, with a value of 0.498 for ISSR and 0.547 for SCoT. Additionally, the number of different alleles (Na) was 1.889 for ISSR and 1.961 for SCoT, while the number of effective alleles (Ne) was 1.587 for ISSR and 1.651 for SCoT, also indicating low diversity. Finally, the percentage of polymorphism for ISSR and SCoT analyzes was high for both, with a value of 88.89% and 96.10% respectively.
Conclusions
This is the first time Psidium guajava is studied in Greece, as a promising crop acclimatized in subtropical South regions of the country. A new culture medium for micropropagation of guava seedlings is demonstrated herein, which could be further exploited in propagation and breeding programs, as well as finding applications in applied biotechnology of this species. ISSR and SCoT markers have the potential to depict accurately the genetic distances of the produced germplasm and therefore could be applied effectively in future breeding studies as well.
Supplemental Material
Download PDF (112.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available in ‘figshare’ [https://figshare.com/s/3b500ba06775dfae80d8], reference number 1.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Liu X, Yang G. Clonal propagation of guava (Psidium guajava L) on nodal explants of mature elite cultivar. IJPB. 2011;2(1):e2.
- Paull RE, Bittenbender HC. 2006. Psidium guajava guava. In: Janick L, Paull RE, editors. The encyclopedia of fruit and nuts. Cambridge: Cambridge University Press; p. 541–549.
- Pereira FMP, Usman M, Mayer NA, et al. Advances in guava propagation. Rev Bras Frutic. 2016:39.
- Ibrahim D, Zawam H, El-Deriny M, et al. First report of Meloidogyne enterolobii Yang & Eisenback, 1983 (guava root-knot2 nematode) infecting guava (Psidium guajava) in Egypt. Plant Disease. 2022;106(0000):1–7.
- Castagnone-Sereno P, Castillo P. 2020. Meloidogyne enterolobii (Pacara earpod65 tree root-knot nematode); [accessed Sep 2022]. Available from: https://www.cabi.org/isc/datasheet/3323866.
- Jamieson S, Wallac CE, Das N, et al. Guava (Psidium guajava L.): a glorious plant with cancer preventive and therapeutic potential. Crit Rev Food Sci Nutr. 2021;21:1–32.
- Singh G. Strategies for improved production in guava. Vol. 26. Proceeding of 1st International Guava Symposium; 2005. Lucknow, India: CISH; p. 39.
- Jimenez-Escrig A, Rincon M, Pulido R, et al. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J Agric Food Chem. 2001;49(11):5489–5493.
- Gutiérrez RM, Mitchell S, Solis RV. Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2008;117(1):1–27.
- Daswani PG, Gholkar MS, Birdi TJ. Psidium guajava: a single plant for multiple health problems of rural Indian population. Pharmacogn Rev. 2017;11(22):167–174.
- Díaz-de-Cerio E, Verardo V, Gómez-Caravaca AM, et al. Health effects of Psidium guajava L. leaves: an overview of the last decade. IJMS. 2017;18(4):897.
- Deguchi Y, Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond). 2010;7(1):9.9.
- Khare CP. 2007. Indian medicinal plants. Berlin (Heidelberg): Springer-Verlag; p. 522–523.
- Kumar A, Paudel S, Pandey A, et al. 2022a. Guava leaf essential oil as a potent antioxidant and anticancer agent: validated through experimental and computational study. Antioxidants https://www.researchgate.net/publication/364657605_Guava_Leaf_Essential_Oil_as_a_Potent_Antioxidant_and_Anticancer_Agent_Validated_through_Experimental_and_Computational_Study.
- Medina NNR, Herrero JV-I. Guava (Psidium guajava L.) cultivars: an important source of nutrients for human health. In: Nutritional composition of fruit cultivars. Academic Press; 2016; vol. 13: p. 287–315.
- Menzel CM. Guava: an exotic fruit with potential in Queensland. Queensland Agricult J. 1985;111(2):93–98.
- Pontikis CA. 1996. Psidium guajava L.(guava). In: Trees IV. Berlin, Heidelberg: Springer; p. 308–320.
- Yadava UL. Guava (Psidium guajava L.): an exotic tree fruit with potential in the southeastern United States. HortSci. 1996;31(5):789–794.
- Tzatzani TT, Basdeki E, Ladikou EV, et al. Seed germination traits of loquat (Eriobotrya japonica Lindl.) as affected by various Pre-Sowing treatments (cutting of cotyledons, removal of perisperm, moist chilling and/or exogenous application of gibberellin). Phyton. 2020;89(3):645–656.
- Ali N, Mulwa RMS, Norton MA, et al. Micropropagation of guava (Psidium guajava L). J Hortic Sci Biotechnol. 2003;78(5):739–741.
- Martinez-De Lara J, Barrientos-Lara MC, Reyes-De Anda AC, et al. Diversidad fenotipica y genetica en huertas de guayabo de calvillo. RevFitotecMex. 2022;27(3):243–249.
- Pereira F. Factors affecting guava production and quality with special reference to são paulo, Brazil. In: International Symposium on the Culture of Subtropical and Tropical Fruits and Crops (vol. 275, pp. 103–110).
- Amin MN, Jaiswal VS. Micropropagation as an aid to rapid cloning of a guava cultivar. Sci Hortic. 1988;36(1–2):89–95.
- Kumar A, Kumar A, Tripathi SK, et al. Impact of different vegetative propagation techniques in guava (Psidium guajava L.) cv. Dhawal under Western U.P. conditions. Biol Forum – An Int J. 2022b;14(3):814–817.
- Chandra R, Mishra M. Biotechnological interventions for improvement of guava (Psidium guajava L). Acta Horticulturae, The Hague. 2005;735(1):117–125.
- Usman M, Butt M, Fatima B. Enhanced in vitro multiple shoot induction in elite Pakistani guava cultivars for efficient clonal plant multiplication. Afr J Biotechnol. 2012;11(44):10182–19187.
- Rai MK, Akhtar N, Jaiswal VS. Somatic embryogenesis and plant regeneration in Psidium guajava L. cv. Banarasi local. Sci Hortic. 2007;113(2):129–133.
- Ali N, Mulwa RMS, Norton MA, et al. Radical disinfestation protocol eliminates in vitro contamination in guava (psidium guajava L). Plant Cell Tiss Organ Cult. 2007;91(3):295–298.
- Loh CS, Rao AN. Clonal propagation of guava (Psidium guajava L.) from seedlings and grafted plants and adventitious shoot formation in vitro. Sci Hortic. 1989;39(1):31–39.
- Papadatou P, Pontikis CA, Ephtimiadou E, et al. Rapid multiplication of guava seedlings by in vitro shoot tip culture. Sci Hortic. 1990;45(1-2):99–103.
- Mohamed-Yasseen Y, Barringer SA, Schnell RJ, et al. In vitro shoot proliferation and propagation of guava (Psidium guajava L.) from germinated seedlings. Plant Cell Rep. 1995;14(8):525–528.
- Kala S, Sharma S, Kajla S, et al. In vitro multiplication of guava rootstocks: Psidium guajava cv. Lucknow-49 and Psidium friedrich sthalianum (Chinese guava). Indian J Ecol. 2017;44(Special Issue 5):488–493.
- Singh KK, Singh SP. A review: micropropagation of guava (Psidium spp.). HIJ. 2018;2(6):462–467.
- Tsoktouridis G, Krigas N, Sarropoulou V, et al. Micropropagation and molecular characterization of Thymus sibthorpii benth.(lamiaceae), an aromatic-medicinal thyme with ornamental value and conservation concern. In Vitro Cell Dev Biol-Plant. 2019;55(6):647–658.
- Patel P, Ellis K, Sunkara R, et al. Development of a functional food product using guavas. FNS. 2016;07(10):927–937.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497.
- McCown BH, Lloyd G. Woody plant medium (WPM)—A mineral nutrient formulation for microculture of woody plant species. HortScience. 1981;16:453–453.
- Boutsika A, Sarrou E, Cook CM, et al. Evaluation of parsley (Petroselinum crispum) germplasm diversity from the Greek gene bank using morphological, molecular and metabolic markers. Ind Crops Prod. 2021;170:113767.
- Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in excel. Population genetic software for teaching and research – an update. Bioinformatics. 2012;28(19):2537–2539.
- Nei M, Chakraborty R. Genetic distance and electrophoretic identity of proteins between taxa. J Mol Evol. 1973;2(4):323–328.
- Perrier X, Flori A, Bonnot F. 2003. Methods of data analysis. In: Hamon P, Seguin M, Perrier X, Glaszmann JC, editors. Genetic diversity of cultivated tropical plants. Montpellier, France: Cirad; p. 31–63.
- Lynch M, Milligan BG. Analysis of population genetic structure with RAPD markers. Mol Ecol. 1994;3(2):91–99.
- Singh G, Sahare H, Maninderdeep M. Recent trends in guava propagation-a review. Biosci, Biotech Res Asia. 2019;16(1):143–154.
- Mishra M, Jalil SU, Sharma N, et al. An agrobacterium mediated transformation system of guava (Psidium guajava L.) with endochitinase gene. Crop Breed Appl Biotechnol. 2014;14(4):232–237.
- Jaiswal VS, Amin MN. 1986. In vitro shoot proliferation and plant formation from somatic tissues of guava. VI Intl. Congr. Plant Tissue Culture, University of Minnesota, Minneapolis. Minneapolis: University of Minnesota; p. 279.
- Kumar R, Tiwari JP, Mishra KK. In vitro cloning of chinese guava (psidium friedrich sthalianum) (berg.) nierdz. Progressive Horticulture. 2006;38(1):15–21.
- Rai MK, Jaiswal VS, Jaiswal U. Shoot multiplication and plant regeneration of guava (Psidium guajava L.) from nodal explants of in vitro raised plantlets. J Fruit Ornam Plant Res. 2009;17(1):29–38.
- Amin MN, Jaiswal VS. Rapid clonal propagation of guava through in vitro shoot proliferation on nodal explants of mature trees. Plant Cell Tiss Organ Cult. 1987;9(3):235–243.
- Rai MK, Asthana P, Jaiswal VS, et al. Biotechnological advances in guava (Psidium guajava L.): recent developments and prospects for further research. Trees. 2010;24(1):1–12.
- Kumari A. 2006. Comparative studies on in vitro and in vivo multiplication of guava (Psidium guajava L.) [doctoral dissertation]. Hisar: Chaudhary Charan Singh Haryana Agricultural University.
- Coser SM, da Silva Ferreira MF, Ferreira A, et al. Assessment of genetic diversity in Psidium guajava L. using different approaches. Sci Hortic. 2012;148:223–229.
- Kumar C, Kumar R, Singh SK, et al. Development of novel g-SSR markers in guava (Psidium guajava L.) cv. Allahabad safeda and their application in genetic diversity, population structure and cross species transferability studies. PLoS One. 2020;15(8):e0237538.
- Usman M, Samad WA, Fatima B, et al. Pollen parent enhances fruit size and quality in intervarietal crosses in guava (Psidium guajava). Int J Agric and Biol. 2013;15(1):125–129.
- Kalia RK, Rai MK, Kalia S, et al. Microsatellite markers: an overview of the recent progress in plants. Euphytica. 2011;177(3):309–334.
- Liu SR, Li WY, Long D, et al. Development and characterization of genomic and expressed SSRs in citrus by genome-wide analysis. PLoS One. 2013;8(10):e75149.
- Rai MK, Phulwaria M, Gupta AK, et al. Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tiss Organ Cult. 2012;111(2):259–264.
- Mehmood A, Jaskani MJ, Khan IA, et al. Genetic diversity of pakistani guava (Psidium guajava L.) germplasm and its implications for conservation and breeding. Sci. Hortic. 2014;172:221–232.
- Wang S, Zhou RB, Pan QP, et al. Genetic diversity analysis of Lonicera macranthoides hand.-mazz. germplasm by ISSR markers. Pharm. Biotechnol. 2009;16:149–152.
- Ning L, Chen HG, He J, et al. Genetic relationship among guava germplasms accessed by using ISSR markers. Fujian J Agric Sci. 2017;32(2):138–143.
- Ma Z, Liu S, Liang Z, et al. Analysis of genetic diversity of 45 guava germplasm evaluated using SSR markers. Int J Fruit Sci. 2020;20(3):385–393. .
- Diaz-Garcia L, Padilla-Ramírez JS. Development of single nucleotide polymorphism markers and genetic diversity in guava (Psidium guajava L.). Plants People Planet. 2023;5(1):58–69.