Abstract
The aim of this study was to investigate a possible association between the variations and effects of gene changes in the OX40/OX40L pathway and the risk of developing bladder cancer in a Turkish population. The study included 104 patients with bladder cancer and 97 healthy individuals. The distribution of OX40 (rs17568) and OX40L (rs1234313) polymorphisms was evaluated by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP). The genotype distributions of OX40 rs17568 regions showed non-significant differences between the patient and control groups (p = 0.256). No difference was also found in T2 invasive tumours, high, and low-grade tumours. The genotype distributions of the OX40L rs1234313 region were significantly different between the patient and control groups (p = 0.023). The frequency of the GG genotype and homozygous GG/AA genotypes was higher among the patients than in the control group, and the difference was significant (for GG genotype: OR 0.462; 95% CI 0.255–0.835; p = 0.010; for GG/AA genotype: OR 0.456 (0.257–0.808; p: 0.007). The OX40L A allele (AA/AG) had higher frequency in T2 invasive bladder cancer patients than in those with T1 and Ta (p = 0.028). There was no statistically significant difference in the OX40L genotype distributions among the graded tumour groups (p = 0.689). The results indicate that the rs1234313 A/G gene polymorphism may contribute to the development of bladder cancer in this Turkish population. Further observations in a larger cohort need to confirm this suggestion.
Introduction
Bladder cancer ranks ninth among the most common cancers in the world [Citation1]. Many genetic and molecular changes in both proto-oncogenes, such as the epidermal growth factor receptors (EGFR), and tumour suppressors, such as tumor protein p53 (TP53) and retinoblastoma protein (RB), have a role in the ethiopathogenesis of bladder cancer [Citation2, Citation3].
A marker that will fully show the diagnosis, treatment, and prognosis of bladder tumours has not been defined yet. Recently, molecular epidemiological studies in different populations determined that single nucleotide polymorphisms (SNPs) may modulate the risk of bladder cancer development [Citation4]. Nevertheless, the effects of genetic and epigenetic changes, gene–gene interactions, and signalling pathways in the development of bladder cancer are still not fully determined [Citation5, Citation6]. The main goal of these investigations is to improve the prognosis and decrease the mortality rate and incidence rate of bladder cancer. As it is known, the immunotherapeutic Bacillus Calmette Guérin (BCG) agent has been used in intravesical treatment for a long time. Since most patients are resistant to these kinds of immunotherapeutic treatments, sophisticated research studies have been done for more effective immunotherapeutic agents [Citation7].
Better understanding of the role of immunoregulation in the development and pathogenesis of bladder cancer will be possible with the recognition of new target molecules. One of the largest receptor families controlling the immune system is the tumour necrosis factor receptor super family (TNFRSF). Previous studies have determined that the OX40/OX40 ligand pair from the tumour necrosis factor (TNF) superfamily is involved in T cell activation and increases the survival of T cells and cytokine release. Some genetic variations of TNFRSF in cancer are also currently being extensively studied. The OX40/OX40L pair can initiate T-cell activation and programmed cell death (apoptosis) by binding to the target cells of the immune system. It is known that the participation of the OX40/OX40L pathway in apoptosis plays a role in the development and maintenance of tissue homeostasis and the elimination of cancer cells [Citation8, Citation9].
Many pieces of evidence have indicated that the inflammatory process plays a crucial role in tumour development, tumour initiation, and its progression. T cells play a critical role in this inflammation process. Blocking the OX40-OX40L interaction can provide a different approach to the treatment of many diseases, such as atherosclerosis, diabetes, asthma, and especially cancer [Citation10]. Decreased apoptosis or resistance to apoptosis plays a very important role in carcinogenesis. Therefore, biomarkers that may emerge as a result of research on ligands and genes related to apoptosis may offer new approaches to the diagnosis and treatment of bladder cancer [Citation11]. The aim of this study was to investigate the effects of genetic changes in the genes encoding OX40 and OX40L on the risk and progression parameters of the disease.
Subjects and methods
Ethics statement
The approval of the Medical Ethics Committee of Haydarpasa Training Hospital was obtained for the study (HNEAH-KAEK 2020/128). All participants gave their informed consent for participation in this study.
Subjects
The study was designed as a prospective case-control study and was conducted in July–October 2020 in the Urology Clinic of Haydarpasa Numune Training and Research Hospital. It was conducted with a total of 201 subjects, 74 women (36.8%), and 127 men (63.2%), aged between 44 and 92 years. The mean age of the patients was 68.5 ± 8.886 years, and the mean age of the control (clinically healthy) subjects was 63.32 ± 14.636 years. The subjects were evaluated in two age-matched groups: ‘Case’ (n = 104) and ‘Control’ (n = 97). The Case group patients were selected from patients with a diagnosis of bladder cancer and patients over 18 years of age. Patients who received chemotherapy and radiotherapy due to bladder cancer, those who were diagnosed with other cancers, and those with acute infections and immunosuppressive diseases were excluded from the study. All patients underwent surgery when their urine cultures were found to be sterile. If urinary infection was found, antibiotic therapy was prescribed before surgery.
Laboratory analyses
Laboratory analyses were carried out in the Department of Molecular Medicine, Aziz Sancar Institute of Experimental Medicine, Istanbul University, Turkey.
DNA extraction
The blood samples of the participants were taken into EDTA tubes after obtaining informed consent. DNA isolation from blood samples was performed by the spin-column method using the Invitrogen PureLink™ Genomic DNA MiniKit (Cat. No: K182002, Thermo Fisher Scientific).
Genotyping assay
The target DNA region was amplified by polymerase chain reaction (PCR) using specific primers. Specific primers were designed, checked by the researchers using online tools, and specially synthesized. The primer sequences, restriction enzymes, and proper conditions for detection of each genotype are given in .
Table 1. Using primers and restriction enzymes for the detection of OX40 and Ox40L genotypes.
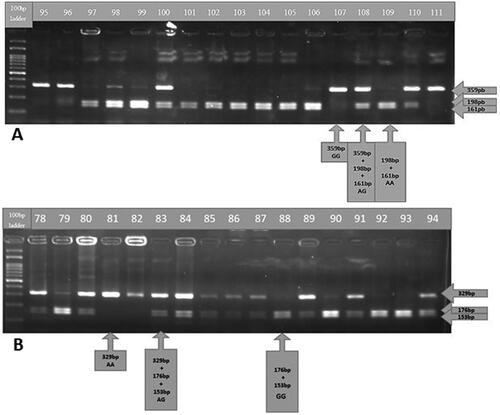
Genotyping was performed via the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. Allele and genotype distributions were determined according to fragment lengths. Samples were run on 2% agarose gel at 100 V for 40 min. We detected and visualized PCR products and restriction fragments via ethydium bromide staining and a UV light imaging system. A representative gel image of BsmAI restriction enzyme-cut PCR products belonging to the selected gene regions (OX40 rs17568 A/G polymorphism) is given in .
Data analysis
Values are means with a standard deviation (±SD). The SPSS20 package program was used for statistical analysis. Differences were considered statistically significant at the p < 0.05 level. Chi-square test (χ2) and Fisher’s exact test were used to determine the frequency of genotypes and alleles, differences between groups, and possible relationships between genotypes and clinical parameters. The Student’s T test was also used for statistics on numerical values. The gender difference between the groups was equalized after logistic regression analysis.
Results
Participants were divided into two groups: the case group (104, 82 males, 22 females) and the control group (97, 44 males, 53 females). There was a significant difference between the sexes in the two groups (p < 0.001). This was taken into consideration via logistic regression analysis. The average age of the patient group was 68.05 ± 8.886 years, while the average age of the control group was 63.32 ± 14.636 years (p = 0.053).
OX40 rs17568 A/G genotypes were detected after restriction with the BsmAI enzyme (). The genotype distributions of the OX40 polymorphic region of interest were compared between the case and control groups. There was no significant difference in the genotype distribution between the two groups (p = 0.256).
The OX40L genotype distributions were significantly different between patient and control groups (p = 0.023) (). In addition, the frequency of the GG genotype (OR 0.462; 95% CI 0.255–0.835; p = 0.010) and homozygous GG/AA OR 0.456 (0.257–0.808) in the patient group was higher than that in the control group, and the difference was statistically significant. The risk status of carrying the GG genotype in the patient group was also preserved when the gender difference between the groups was equalized after logistic regression analysis (OR = 2.34; 95% CI 1.239–4.440; p = 0.009).
Table 2. Genotype frequencies of Ox40 rs17568 and Ox40L rs1234313 gene variants in patients with bladder cancer and controls.
The distribution of genotypes was also examined in groups divided according to their invasiveness, including high- and low-grade tumours. The genotype frequencies within these groups are given in . There was no statistical difference in the genotype distributions between high-grade and low-grade tumour groups (p = 0.689). However, the A allele frequency in T2 invasive bladder cancer patients was higher than in those with pT1 and pTa, and the difference was statistically significant (p = 0.028) (). The differences between the case and control groups in terms of genotype distribution, invasiveness, and high- and low-grade tumour status were non-significant (p = 0.256).
Table 3. Distribution of Ox40 and Ox40L genotypes according to tumour histopathological characteristics.
Table 4. OX40 rs17568 /OX40L rs1234313 allele frequencies and bladder cancer patient’s histopathological characteristics.
The final statistical evaluation of genotype data revealed that the genotype distributions of the rs17568 A/G polymorphisms were compatible with the Hardy-Weinberg equilibrium in both the case (χ2 = 0.0683, p = 0.7937) and control group (χ2 = 1.6733, p = 0.1958). The genotypes of the rs1234314 A/G polymorphism were also compatible with the Hardy-Weinberg balance in the patient group (χ2 = 1.251 p = 0.2633), but did not comply with the Hardy-Weinberg equilibrium in the control group (χ2 = 15.497, p = 0.000083). The distributions of the genotypic combinations of OX40 rs17568 and OX40L rs1234313 were grouped as AAAG/other genotype combinations and AGAG/other genotype combinations for statistical evaluation. There was no statistically significant difference in these groups (p = 0.528; p = 0.202, respectively) ().
Table 5. OX40 rs17568 and OX40L rs1234313 combined genotyping results.
Discussion
Non-muscle invasive bladder cancer (NMIBC) is typically treated with transurethral resection (TUR), followed in some cases by intravesical therapy, to reduce the risk of tumour recurrence and progression [Citation12, Citation13]. Bacillus Calmette-Guérin (BCG) is the most popular intravesical agent for prophylaxis against recurrence of carcinoma in situ and high-grade bladder cancer [Citation12]. However, intravesical gene transduction and immune modulation represent promising future targeted therapies [Citation13]. Still, many patients remain at risk of tumour recurrence and progression to muscle-invasive bladder cancer (MIBC). Nevertheless, cystectomy is the gold standard treatment in MIBC. As pointed out before [Citation14], epidemiological studies suggest that the cooperative effect of polymorphisms in detoxification mechanisms with environmental factors is much more significant than the effects of polymorphisms alone.
Epidemiological studies have reported the GSTM1 gene as a risk factor for bladder cancer, with the deletion of this gene being associated with a lower level of mutations in suppressor genes, such as TP53. Bladder cancer is commonly caused by smoking and exposure to arylamines, both of which are well-established factors in the aetiology of the disease. Öztürk et al. [Citation14] suggest that the combination of the CYP1A1 Ile/Ile genotype with the GSTM1 null genotype might be associated with an increased risk of bladder cancer. Although combinations of the CYP1A1 Val genotype with the GSTM1 null genotype are not directly associated with the risk of bladder cancer, they might be associated with higher grade tumour in patients. It has been shown in several studies that GSTM1 null genotype carriers have a higher risk for tobacco-related bladder cancer [Citation14, Citation15].
The therapy options for advanced MIBC are currently limited to systemic therapy with chemo- and immunotherapeutics. Some promising results of recent immunotherapeutic agents in cancer treatment are reported. To better understand the mechanisms of resistance to immunotherapy, it has been very popular in recent years to investigate the expression dynamics of various molecules that target immune checkpoints or play a role in the anti-tumour response against the tumour. SNPs are one of the mechanisms affecting gene expression, so related studies are critical for this purpose [Citation16]. In addition, programmed death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) immune control points have been studied a lot in recent years, as well as have control points such as OX40, CD40, and CD27, which act as co-activators and co-inhibitors [Citation5]. Zaini et al. [Citation17] found that the maturation of dendritic cells stimulated by TNF-α was accompanied by increased expression of OX40L in mice. They also reported that dendritic cells could not generate a cellular anti-tumour immune response in the absence of OX40L expression [Citation17]. Studies have suggested that OX40L gene polymorphisms are also associated with other diseases such as systemic lupus erythematosus, atherosclerosis, coronary heart disease, asthma, Behçet’s disease, and myocardial infarction, as well as different types of cancer [Citation18–21]. In another study, the risk of developing squamous cell carcinoma in the nose and paranasal sinuses of individuals with the GG genotype in the rs17568 region was increased more than twice [Citation22].
There is a synonymous SNP located in exon5, rs17568 A/G, in OX40. The conversion of the adenine base to guanine leads to a functional result, which is the replacement of the glutamine codon with another codon for this amino acid. Despite this being a synonymous SNP, some studies have reported that this SNP in the OX40 gene may directly affect its expression [Citation23, Citation24]. In our study, when bladder cancer patients and the control group were compared in terms of risk, there was no significant difference in the rs17568 A/G genotype distribution or allele frequencies. No significant difference was also found between genotypic and disease grading in this polymorphic region.
Another study by Chen et al. [Citation25] reported that the rs17568 A/G SNP can affect the serum level of HDL-C (high density lipoprotein cholesterol). The rs17568 A/G was evaluated together with age, gender, diabetes, hypertension, and lipid levels in the carriers of the G allele, and it was reported that the risk of acute heart disease was significantly higher in this population [Citation25]. Similar results found that the rs17568 polymorphism affected serum HDL levels and that individuals carrying the A allele in this region had higher LDL and total cholesterol levels than the G allele carriers [Citation24]. In a further study conducted with large artery atherosclerosis and small vessel disease, the AA genotype of the rs1234313 SNP was related to a high risk of carotid plaque calcification in patients with ischemic stroke [Citation26]. In another study, rs1234313 was associated with Behçet’s disease, suggesting that it played a role in susceptibility to this disease [Citation22]. In a Chinese population [Citation27], there was no significant difference between the allele and genotype distributions of rs1234313, rs1234314, and rs17568 in patients with atherosclerotic cerebral infarction (ACI) (n = 450) and control individuals (n = 378). In the case-control study of Chen et al. [Citation28] with coronary heart disease, five SNPs, including rs1234314 G/A, were evaluated, but they could not find a significant result.
There are limited reports in the literature regarding rs1234313 in various cancer types. Significant associations between rs385064, rs844648, and rs10912580 and breast cancer were identified. However, they could not find a significant relationship with rs1234313 [Citation29]. The OX40L A allele (AA/AG) had a higher frequency in T2 invasive bladder cancer patients than in those with T1 and Ta (p = 0.028). In our study, the genotype and allele distributions were significantly different between bladder cancer patients and control groups. In addition, carrying the rs1234313 GG genotype was 1.7 times higher in the patient group, and carrying the homozygous GG/AA genotype was 1.6 times higher than the control group, and the difference was statistically significant. The high-risk status of carrying the GG genotype in the patient group remained stable after the logistic regression analysis, when the gender difference between the groups was equalized. Genotype distributions were also examined in groups divided according to the status of high- and low-grade tumours, and there was no relationship between rs17568 A/G and rs1234313. However, the frequency of carrying the OX40L rs1234313 A allele (AA/AG) in T2 invasive bladder cancer patients was statistically significantly higher than in pT1 and pTa bladder cancer patients.
When we identified Ox40L rs1234313 AA and GG genotypes as high-risk genotypes, carrying the A allele was an important finding in terms of pathological staging. The number of T2 invasive patients who have the A allele was higher than those with pT1 and pTa. When we analyzed the combined genotypes, we could not detect any association between the OX40/OX40L combined genotypes and the risk of bladder cancer. Our study presents the first data evaluating the rs17568 A/G and rs1234313 A/G gene polymorphisms in patients with bladder cancer in a Turkish population.
The allele distribution frequencies of OX40 rs17568 A (69.07%) and G (30.92%) genotypes in the Turkish population were closer to those of the European population with rs17568 A (79%) and G (21%) [Citation30]. The frequencies of the A allele (39.69%) and G allele (60.3%) that we determined for OX40L rs1234313 are also similar to the European population values, respectively, of the A allele (31%), and G allele (69%) [Citation31]. The identification and use of de novo molecular markers in urine, tissue, or blood will offer opportunities to identify bladder cancer earlier, stratify the risk of the disease, and assist local or systemic chemotherapy or immunotherapy in treatment.
Further studies investigating OX40/OX40L gene variants in a larger population size are needed to elucidate the molecular mechanisms of bladder cancer. The present study has several limitations, such as a small sample size and ethnic differences.
Conclusions
The results of rs17568 A/G and Rs1234313 A/G gene polymorphisms in patients with bladder cancer reported here suggest that rs1234313 A/G gene polymorphism could be a valuable parameter in terms of bladder cancer risk. This parameter may be a potential future predictor of bladder cancer recurrence, progression, and survival in both non-invasive cancer patients and patients with advanced bladder cancer. Further studies are needed to explore the OX40/OX40L gene variants and the underlying mechanisms of bladder cancer in larger cohorts.
Author contributions
All authors contributed to the study conception and design. MTH, US, DS and IY: manuscript preparation, data collection and analysis. OB, LV, ÇT, OY: clinical evaluations of the patients. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [LV], upon reasonable request.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–8. doi: 10.3322/caac.21492.
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374(9685):239–249. doi: 10.1016/S0140-6736(09)60491-8.
- Junttila TT, Laato M, Vahlberg T, et al. Identification of patients with transitional cell carcinoma of the bladder overexpressing ErbB2, ErbB3, or specific ErbB4 isoforms: real-time reserve transcription-PCR analysis in estimation of ErbB receptor status from cancer patients. Clin Cancer Res. 2003;9(14):5346–5357.
- Wigner P, Grębowski R, Bijak M, et al. The interplay between oxidative stress, inflammation and angiogenesis in bladder cancer development. Int J Mol Sci. 2021;22(9):4483. doi: 10.3390/ijms22094483.
- Deng J, Zhao S, Zhang X, et al. OX40 (CD134) and OX40 ligand, important immune checkpoints in cancer. OncoTargets Ther. 2019;12:7347–7353. doi: 10.2147/OTT.S214211.
- Figueroa JD, Ye Y, Siddiq A, et al. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Hum Mol Genet. 2014;23(5):1387–1398. doi: 10.1093/hmg/ddt519.
- Lenis AT, Lec PM, Chamie K, et al. Bladder cancer: a review. JAMA. 2020;324(19):1980–1991. doi: 10.1001/jama.2020.17598.
- Lubrano di Ricco M, Ronin E, Collares D, et al. Tumour necrosis factor receptor family co-stimulation increases regulatory T-cell activation and function via NF-κB. Eur J Immunol. 2020;50(7):972–985. doi: 10.1002/eji.201948393.
- Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T cell activation and survival. Crit Rev Immunol. 2009;29(3):187–201. doi: 10.1615/critrevimmunol.v29.i3.10.
- Vakil Monfared R, Mashayekhi F. OX40L gene polymorphism and breast cancer in Iranian population. Exp Oncol. 2018;40(2):132–135. doi: 10.31768/2312-8852.2018.40(2):132-135.
- Balaji S, Terrero D, Tiwari AK, et al. Alternative approaches to overcome chemoresistance to apoptosis in cancer. Adv Protein Chem Struct Biol. 2021;126:91–122.
- Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19(3):573–580. doi: 10.1016/S0094-0143(21)00424-9.
- Narayan VM, Dinney CPN. Intravesical gene therapy. Urol Clin North Am. 2020;47(1):93–101. doi: 10.1016/j.ucl.2019.09.011.
- Ozturk T, Kahraman OT, Toptaş B, et al. The effect of CYP1A1 and GSTM1 gene polymorphisms in bladder cancer development in a Turkish population. In Vivo. 2011;25(4):663–668.
- Avirmed S, Khuanbai Y, Sanjaajamts A, et al. Modifying effect of smoking on GSTM1 and NAT2 in relation to the risk of bladder cancer in Mongolian population: a case-control study. Asian Pac J Cancer Prev. 2021;22(8):2479–2485. doi: 10.31557/APJCP.2021.22.8.2479.
- Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother. 2020;69(1):3–14. doi: 10.1007/s00262-019-02443-4.
- Zaini J, Andarini S, Tahara M, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumour immunity in mice. J Clin Invest. 2007;117(11):3330–3338. doi: 10.1172/JCI32693.
- Gupta V, Kumar S, Pratap A, et al. Association of ITGAM, TNFSF4, TNFAIP3 and STAT4 gene polymorphisms with risk of systemic lupus erythematosus in a North Indian population. Lupus. 2018;27(12):1973–1979. doi: 10.1177/0961203318786432.
- Wang X, Luan Y, Zhang C. A meta-analysis on correlations of OX40L variants with atherosclerotic disorders. J Cell Biochem. 2019;120(6):9624–9630. doi: 10.1002/jcb.28240.
- Maalhagh M, Shojaei M, Erfanian S, et al. Lack of association between rs17568 polymorphism in ox40 gene and myocardial infarction, Southern of Iran. Glob J Health Sci. 2015;8(6):41–46. doi: 10.5539/gjhs.v8n6p41.
- Jiang Y, Cheng L, Li X, et al. Associations between TNFSF4, TNFSF8 and TNFSF15 and Behçet’s disease but not VKH syndrome in Han Chinese. Oncotarget. 2017;8(62):105037–105046. doi: 10.18632/oncotarget.22064.
- Faghih Z, Abtahi S, Khademi B, et al. Association of OX40 gene polymorphisms (rs17568G/a and rs229811A/C) with head and neck squamous cell carcinoma. Mol Biol Rep. 2019;46(3):2609–2616. doi: 10.1007/s11033-019-04602-3.
- Faghih Z, Taherifard E, Daneshmand A, et al. OX40 genetic variations in patients with breast cancer: a case-control study. Br J Biomed Sci. 2021;78(1):44–46. doi: 10.1080/09674845.2020.1776587.
- Ria M, Eriksson P, Boquist S, et al. Human genetic evidence that OX40 is implicated in myocardial infarction. Biochem Biophys Res Commun. 2006;339(3):1001–1006. doi: 10.1016/j.bbrc.2005.11.092.
- Chen Y, Zhang L, Huang H, et al. Association of OX40 and OX40L gene polymorphisms with acute coronary syndrome in a Han Chinese population. DNA Cell Biol. 2011;30(8):597–602. doi: 10.1089/dna.2010.1201.
- Jiang Y, Liu X, Du Y, et al. Rs1234313 and rs45454293 are risk factors of cerebral arterial thrombosis, large artery atherosclerosis, and carotid plaque in the Han Chinese population: a case-control study. BMC Neurol. 2019;19(1):31–40. doi: 10.1186/s12883-019-1259-9.
- Huang Q, Yang OI, Tan XL, et al. Absence of association between atherosclerotic cerebral infarction and TNFSF4/TNFRSF4 single nucleotide polymorphisms rs1234313, rs1234314 and rs17568 in a Chinese population. J Int Med Res. 2014;42(2):436–443. doi: 10.1177/0300060514521154.
- Cheng G, Wang H, Chen M, et al. Lack of evidence to support the association of polymorphisms within the TNFSF4 gene and coronary heart disease in a Chinese Han population. Exp Ther Med. 2011;2(2):275–280. doi: 10.3892/etm.2010.188.
- Weiguang Y, Dalin X, Lidan X, et al. Association of OX40L polymorphisms with sporadic breast cancer in northeast Chinese Han population. PLoS One. 2012;7(8):e41277. doi: 10.1371/journal.pone.0041277.
- Ensembl.org [Internet]. Variation of rs17568 in human population. [cited 2022 Dec 1]. Available from: http://www.ensembl.org/Homo_sapiens/Variation/Population?db=core;r=1:1211542-1212542;v=rs17568;vdb=variation;vf=18479.
- Ensembl.org [Internet]. Variation of rs1234313 in human population. [cited 2022 Dec 1]. Available from: http://www.ensembl.org/Homo_sapiens/Variation/Population?db=core;r=1:173196608-173197608;v=rs1234313;vdb=variation;vf=798897.