Abstract
The Coronavirus 2019 (COVID-19) pandemic caused the global healthcare to face one of the most significant challenges since the World Health Organization was founded over 70 years ago. The development and approval of marketing authorization for vaccines with the COVID-19 indication represents an opportunity to address this public health threat. The aim of this study is to analyze the awareness of healthcare professionals (HCPs) on the benefit-risk balance, safety, and risk minimization measures associated with vaccine administration. This is an open-label, prospective, 1-year cross-sectional study (2021–2022). It was conducted using the indirect anonymous survey method by completing an online and paper questionnaire containing open and close-ended questions. The results obtained locally and internationally were validated, systematized, compared, and analyzed. The study included 344 HCPs from 40 countries on six continents. The data show that among HCPs, the highest proportion are pharmacists and doctors in the age group of 21–30 years. А 20% difference in results was observed regarding the statement that vaccines approved for use are of high quality, efficacious, and safe, 68% (on local level) and 88% (on global level) of the respondents agreeing. On the other hand, ∼80% of HCPs both in Bulgaria and abroad felt that they need additional professional data for the vaccines authorized for use. Surveys assessing the awareness and attitudes of HCPs regarding risk management and adverse reactions are key to increasing the level of informed decision-making and can help fighting the world infodemic with data from trusted sources.
Introduction
The Coronavirus disease 2019 (COVID-19) pandemic caused the global healthcare to face one of the most significant challenges the World Health Organization (WHO) was founded over 70 years ago [Citation1]. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was first identified in Wuhan City, China, in December 2019 and is thought to be acquired from a zoonotic origin [Citation2].
Globally, COVID-19 has led to around 759 million confirmed cases and over 6.8 million deaths by March 2023 [Citation3]. Several SARS-CoV-2 variants have evolved. The currently circulating variant of concern is Omicron (B.1.1.529) [Citation4] and is rapidly spreading worldwide. To protect the community from the COVID-19 pandemic, vaccinations appeared to be the key public health intervention and the most successful technique. Vaccines play a critical role in preventing deaths, and hospitalization caused by infectious diseases, and are contributing to controlling the spread of the disease [Citation5], thus protecting populations at higher risk. The global impact of the COVID-19 pandemic has resulted in an unprecedented level of public interest in vaccines. Several vaccines, subject to a conditional marketing authorization (MA), were quite innovative, which necessitates proper and timely information campaigns towards healthcare professionals (HCPs), as they are at the frontline.
Being an obstacle to delivering evidence-based data, the COVID-19 infodemic threatens the protection of public health. This phenomenon is characterized by an overabundance of information—including inaccurate or outdated information—shared digitally, person-to-person, and through other media and channels [Citation6].
We therefore sought to assess and raise awareness among HCPs in Bulgaria and worldwide regarding COVID-19 vaccine safety and to identify potential gaps and ways to improve the existing strategies for raising public awareness.
Materials and methods
An international cross-sectional study was carried out between May 2021 and December 2022, targeting HCPs locally in Bulgaria and worldwide. The study aimed to assess their awareness, willingness to accept, and attitude about COVID-19 vaccination. Data were collected anonymously via an online survey created using the Google® Forms platform. The applied method was an indirect questionnaire consisting of six open-ended questions, which collected sociodemographic data (age, gender, educational level, workplace, and length of service), and 17 closed-ended questions aiming to assess the level of knowledge of the vaccine mechanism of action, awareness of risk minimization measures and sources used for safety information. Only the answers to questions related to the topic of the present study are presented and discussed in the results. The questionnaire was distributed through professional social media groups with a healthcare focus (Facebook and LinkedIn groups) and through WHO’s Collaborating Centre for International Drug Monitoring at Uppsala Monitoring Centre on their Facebook, LinkedIn, and Twitter pages, too.
In the research design, we established the following inclusion criterion: degree in the field of healthcare; and our exclusion criteria were: (i) wrong fillings of the questionnaire and (ii) having lower than Bachelor degree.
All the extracted data was validated, systemized, compared, and analyzed through descriptive statistics in Excel v.2016.
Results
Demographic data
A total of 344 HPCs were reached on a national (n = 228) and global level (n = 116), of whom 183 are pharmacists with a Master’s degree in pharmacy and 161 other HCPs. The mean age was 35 (min. 21 max. 75) years (). The prevailing part of the participants were within the age group 21–30 years (153 HCPs, 45%). The percentage of females, who filled in the questionnaire is 70 while the one of the males is 30. Nearly 70% of the respondents were living and working in the capital city, 13% in a regional city, 12% in a municipality, and only 1% reported working in a village.
Table 1. Demographic data summary.
HCPs from 17 countries in Europe, 11 countries in Asia, five countries in South America, three countries in North America, two countries in Africa, and one country from Oceania participated in the international part of the study (). The largest number of respondents came from Europe (55%), with the most participants from Serbia (10%) and the Czech Republic (8%), followed by Asia (30%), with the most participants from India (12%) and Iran (5%).
Table 2. Summary of the countries that participated in the international part of the study.
Role of the pharmacist in public awareness campaigns for COVID-19
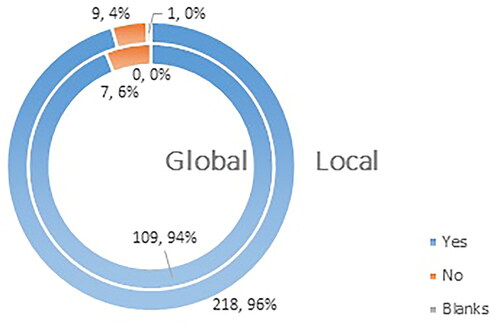
The results from the survey showed that 94% (109 respondents) of international participants and 96% (218 respondents) of the participants on the national level support the idea of pharmacists participating in public awareness campaigns about different vaccines ().
Morbidity and vaccination rates among HCPs
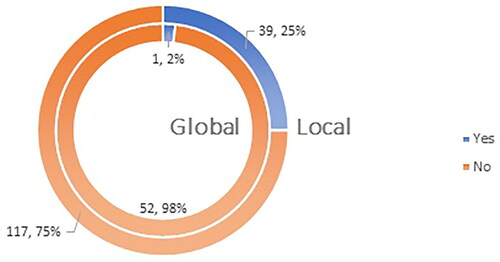
Sixty-four percent (146 respondents) of participants in the local survey and 46% (53 respondents) in the global survey had been diagnosed with COVID-19 (), with 2% (one respondent) globally and 25% (39 respondents) nationally having required hospitalization. The difference in the hospitalization rates in the responding countries could be attributed to differences on the national level in terms of initial diagnosis ().
A significant difference (over 20%) is observed in the vaccination process among HCPs at the global and local level: 93% (108 respondents) globally confirm their vaccination, while 72% (164 respondents) confirm vaccination locally ().
HCPs awareness of safety and risk-minimizing measures for COVID-19 vaccines
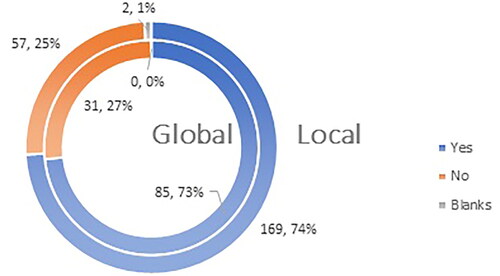
Seventy-four percent (169 respondents) in the local survey and 73% (85 respondents) in the global survey were aware that vaccines initially had a conditional marketing authorization. However, we should take into consideration that some of the participating countries are not part of the European Economic Area and may have a different regulatory framework than that of the European Union and this might affect the result ().
Figure 5. Q5: Are you familiar with the fact that vaccines against COVID-19 have received conditional marketing authorization on the territory of EU for a period of 1 (one) year?
Seventy-seven percent (175 respondents) in the local survey and 97% (112 respondents) in the global survey would recommend vaccination to their patients. Despite the fact that the percentage of positive responses is high both locally and globally, there are 20% more positive responses in the international survey ().
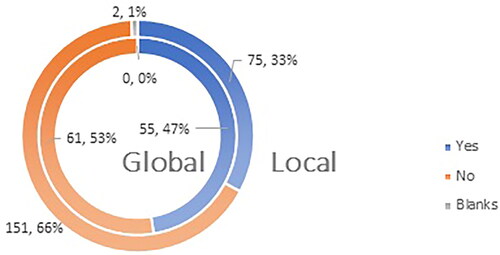
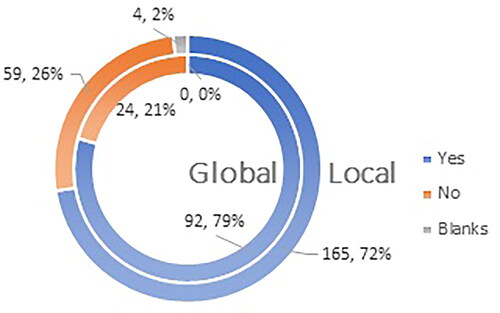
Approximately three-quarters of those surveyed, 72% (165 respondents) on the local level and 79% (92 respondents) on the global level would more confidently recommend vaccination against COVID-19 if vaccines had a standard marketing authorization for a period of 5 years ().
Figure 7. Q12: Would you recommend vaccination to your patients more confidently if vaccines have marketing authorization valid for 5 (five) years?
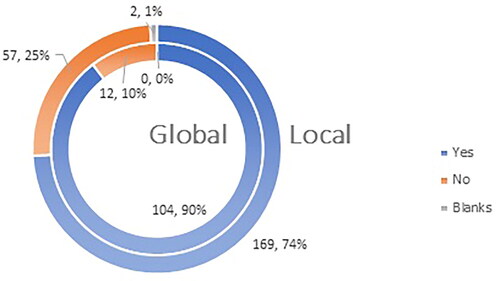
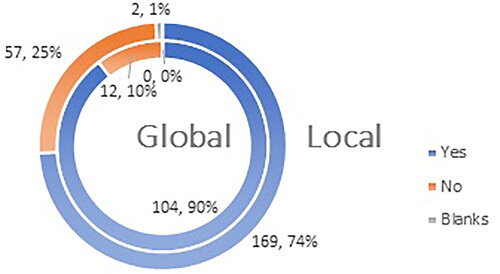
Ninety percent (104 of the participants) in the global study feel that they are sufficiently aware of the most common side effects after vaccination against COVID-19, while 74% (169 participants) of the participants from the local Bulgarian study share this opinion ().
Figure 8. Q11: Do you consider yourself familiar enough with the most frequent ADRs (adverse drug reactions) after administration of a COVID-19 vaccine?
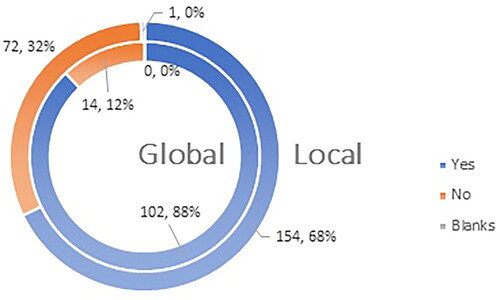
Of the respondents, 68% (154 HCPs on the local level) and 88% (102 HCPs on the global level) agree that vaccines approved for use in the EU are safe, but 78% (on local and global levels) think that they need additional professional data for the vaccines authorized for use. There is a 20% difference in the level of support for the statement that vaccines are ‘of high quality, efficacious and safe’ nationally and globally: 68% of Bulgarian HCPs and 88% of global HCPs ( and ).
Figure 9. Q16: Do you agree with the statement that the vaccines authorized for use in EU are ‘of high quality, efficacious and safe’?
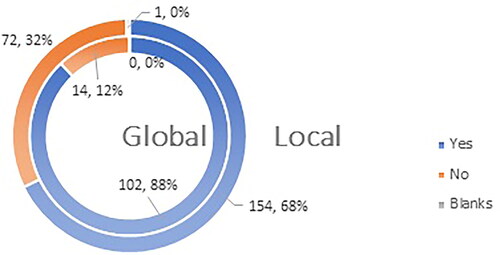
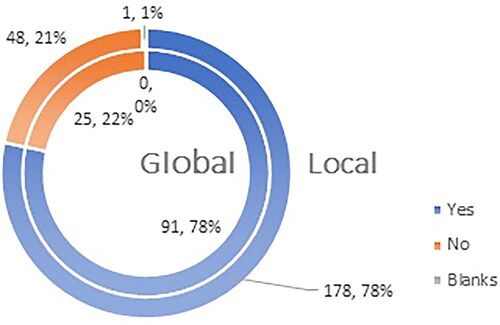
Figure 10. Q14: Do you consider that you need additional professional data for the vaccines authorized for use in EU?
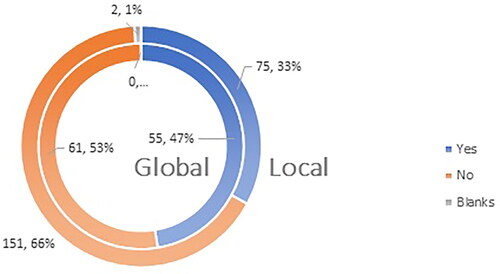
Less than half of the HCPs who completed the questionnaire (at a local and global level) are familiar with the content of ‘Risk Minimization Plan’ of the vaccines authorized for use: 33% of the participants in the national and 47% of the participants in the global survey ().
Discussion
This study analyses the awareness of safety and risk management measures (RMMs) of vaccines, during the COVID-19 pandemic among a sample of HCPs from all around the globe. To the best of our knowledge, this is the first study so far to assess the knowledge of HCPs about vaccines, related safety, and RMMs of the COVID-19 vaccines on the national level and worldwide [Citation7].
The results showed that the HCPs had an overall satisfactory level of knowledge about authorized vaccines against COVID-19. It is well recognized that HCPs have a central role in vaccinations, and they must have acceptable knowledge to appropriately inform and update the general population, as well as the most fragile and vulnerable patients [Citation8].
A cross-sectional study was conducted recently among community pharmacists in Albania, Bulgaria, Romania, and Serbia [Citation9]. The study analyses their perception towards vaccination services and willingness to administer COVID-19 vaccines to patients and their role in vaccination campaigns. The results show that pharmacists are willing to participate but some barriers should be overcome in terms of limited access to vaccination training and lack of national regulation and guidelines defining vaccination activities among pharmacists [Citation9].
Another study conducted among pharmacy, medical, and dental students in Bulgaria shows that there is still a need for a more detailed approach concerning the role of HCPs in raising public awareness about vaccination and this approach should start at the university level [Citation10]. Our study showed that there is overall awareness about the safety of COVID-19 vaccines and acceptance of pharmacists to take part in increasing the awareness among the public and HCPs about vaccines and that they can help fight the world infodemic with data from trusted sources.
Few studies, however, have attempted to estimate the intention rate of HCPs to get vaccinated against COVID-19. A cross-sectional study similar to ours but conducted in France aimed to determine the COVID-19 vaccine acceptance rate in HCPs on a national level [Citation11] The survey based on a questionnaire was proposed to individuals via social networks, shared by e-mail which matches up with the dissemination methods of our study. In the French study, 76.9% of the respondents declared that they would intend to get vaccinated against COVID-19 if a vaccine was currently available. In our study, 93% (108) of respondents worldwide confirmed their vaccination. The rate is higher than the reported intentions in France, but this is most likely due to the fact that access to vaccines was limited during the French study because it was conducted earlier than ours. In our study, 72%, or 164 respondents, confirmed vaccination locally. The lower percentage compared to that of the global survey, despite their roughly the same period of implementation, can be explained by the fact that access to vaccines was delayed in some Eastern European countries. Regarding the demographic characteristics, the ratio of females/males is comparable to that in the international part of our survey: females prevailed over males (74%—French study; 72%—our study). Pharmacists also represented a great proportion of the respondents [Citation11].
Another survey conducted at Catania University Hospital in Southern Italy [Citation8] showed that the gaps in information and safety concerns were the main barriers to COVID-19 vaccine and flu vaccine acceptance. Research conducted in Israel also proved that HCPs’ main concerns regarding vaccines are safety, efficacy, and quality [Citation12]. In our study, we found that approximately three quarters of respondents would recommend vaccination against COVID-19 more confidently if vaccines had a standard marketing authorization for a period of 5 years, which is directly related to the collection of more data on quality, efficacy, and safety of vaccines. These two studies reveal the need for vaccine awareness campaigns to be organized to ensure high vaccination rates among HCPs. Furthermore, workers in the healthcare sector must be well informed to recommend vaccination to their patients. In our study, 95% оr 327 respondents supported the idea of pharmacists participating in public awareness campaigns about different vaccines, and only 5% (16 respondents) were opposed to it. The opinion on this issue was similar at the national and global levels. The survey in Italy also found that vaccination hesitancy was lower among workers in the COVID-19 area of the hospital, which could be because of dealing with severe cases of COVID-19. In Bulgaria, despite the higher level of hospitalization compared to that worldwide according to the results of our study (25% in Bulgaria vs. 2% worldwide), the level of vaccination was lower compared to that worldwide (72% in Bulgaria vs. 92% worldwide).
In contrast to the results in France and Italy, in the Democratic Republic of Congo [Citation13] only 27.7% of HCPs agreed to get a COVID-19 vaccination. The reported willingness of Congolese healthcare workers to get vaccinated against SARS-CoV-2 is very low. The authors attribute this result to the role of social networks and the spread of misinformation [Citation13]. It is widely recognized that the COVID-19 infodemic has spread across traditional media and social media since the first COVID-19 cases.
Similar to the study in the Democratic Republic of Congo, a survey with HCPs in New Mexico [Citation14] showed comparable results with our survey. Most participants were younger than 40 years of age (54%), female (75%), completed a bachelor’s degree or higher (80%), and indicated urban area as a workplace. This research has revealed that about 36% of the respondents were willing to take a COVID-19 vaccine as soon as it became available at the time of the survey. The majority of the HCPs (56%) were not sure or would wait to review safety data before being vaccinated. Only 8% of respondents were unwilling to take the vaccine at all [Citation14]. The results from our study correlate with the above-mentioned studies, as a higher rate of the participants confirmed that they need additional professional data for the authorized vaccines. This may be explained by the enormous amount of data unsupported by scientific facts. Our findings and the findings from similar studies could serve as a basis to increase the awareness of healthcare professionals on professional data sources and to develop indicators for the regulators to detect and control unsupported and untrusted data and tools for academia to increase the knowledge of future HCPs.
Conclusions
COVID-19 vaccines are well accepted among healthcare workers. Most healthcare professionals would recommend to their patients to get vaccinated. Although the majority of healthcare professionals are aware of the ADRs from administering the COVID-19 vaccine and consider these products of high quality, safe, and efficacious, more than half of the participants are not familiar with the Risk Minimization Plan and still need more professional information. Institutions and professionals must find the most appropriate way to raise awareness about the safety of vaccines and the Risk Minimization Plan but still refer to reliable sources of information.
Author contributions
DP and VGK created the questionnaire and collected data. DP analyzed the data and drafted the manuscript. MD, IG, and AS reviewed the results and participated in drafting the manuscript and its final revision. All authors have read and approved the final version of the manuscript.
Acknowledgements
The authors also want to thank the WHO’s Collaborating Centre for International Drug Monitoring at Uppsala Monitoring Centre for their support in the dissemination of the questionnaire on the international level.
Data availability statement
The data that support the findings reported in this study are available from the corresponding author [MD] upon reasonable request.
Additional information
Funding
References
- The COVID-19 pandemic and continuing challenges to global health [Internet]. [cited 2023 Mar 12]. Available from: https://www.who.int/about/funding/invest-in-who/investment-case-2.0/challenges
- Dong Y, Dai T, Wei Y, et al. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1):1. doi: 10.1038/s41392-020-00352-y.
- WHO coronavirus (COVID-19) dashboard [Internet]. [cited 2023 Mar 12]. Available from: https://covid19.who.int
- Tracking SARS-CoV-2 variants [Internet]. [cited 2023 Mar 12]. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants
- Statement for healthcare professionals. How COVID-19 vaccines are regulated for safety and effectiveness (revised March 2022) [Internet]. [cited 2023 Mar 12]. Available from: https://www.who.int/news/item/17-05-2022-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness?fbclid=IwAR2Myj5jxrUrK4owQ-LWsfyKTHj3iHc6mwZ8ymk6PfoDt7FG34LqDKdfNZw
- WHO. WHO policy brief: COVID-19 infodemic management, 14 September 2022 [Internet]. Geneva: WHO. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Policy_Brief-Infodemic-2022.1
- Panayotova D. Study of the healthcare professionals awareness on safety and risk minimization measures related to the approved Covid-19 vaccines. Annual Academic Meetings of the Health & Medical Sciences Division; 10th Annual International Conference on Health & Medical Sciences; 2022 May 2–5; Athens, Greece. Available from: https://www.atiner.gr/2022hsc-pro
- Ledda C, Costantino C, Cuccia M, et al. Attitudes of healthcare personnel towards vaccinations before and during the COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(5):2703. Mar 8doi: 10.3390/ijerph18052703.
- Turcu-Stiolica A, Kamusheva M, Bogdan M, et al. Pharmacist’s perspectives on administering a COVID-19 vaccine in community pharmacies in four Balkan countries. Front Public Health. 2021;9:766146. doi: 10.3389/fpubh.2021.766146.
- Serbezova A, Mangelov M, Zaykova K, et al. Knowledge and attitude toward COVID-19 vaccines amongst medical, dental and pharmacy students. A cross-sectional study from Bulgaria. Biotechnol Biotechnol Equip. 2021;35(1):2024–7. 1doi: 10.1080/13102818.2022.2041097.
- Gagneux-Brunon A, Detoc M, Bruel S, et al. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect. 2021;108:168–173. doi: 10.1016/j.jhin.2020.11.020.
- Dror AA, Eisenbach N, Taiber S, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–779. doi: 10.1007/s10654-020-00671-y.
- Kabamba Nzaji M, Kabamba Ngombe L, Ngoie Mwamba G, et al. Acceptability of vaccination against COVID-19 among healthcare workers in the democratic republic of the Congo. Pragmat Obs Res. 2020;11:103–109. doi: 10.2147/POR.S271096.
- Shekhar R, Sheikh AB, Upadhyay S, et al. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. 2021;9(2):119. doi: 10.3390/vaccines9020119.