Abstract
The Coronavirus disease 2019 (COVID-19) outbreak increased the usage of hand rub disinfectants, resulting in skin damage. The World Health Organization (WHO) proposed hand rubs formulations incorporating glycerine due to its moisturizing effect; nonetheless, glycerine can reduce the antimicrobial activity of alcohol-based hand rubs. Here we demonstrated that incorporating hydroglycolic extract as a substitute for glycerol in hand rub formulations reduces epidermis damage while improving antimicrobial effectiveness. We applied the hydroglycolic extracts from Cucumis sativus L., Fragaria sp. and Calendula officinalis instead of glycerol to modify the formula proposed by the WHO. The antimicrobial activity and the healing skin action were evaluated using the agar diffusion test and a human skin tolerability trial. Furthermore, we assessed the cytotoxicity and wound repair capability of the formulated hydroglycolic extracts against human gingival fibroblasts (HGFs) and the phenotype of murine macrophages. The hand rubs containing Fragaria sp. and C. officinalis hydroglycolic extracts showed improved antimicrobial activity even after six months of fabrication. Moreover, the formulated hand rubs had similar cellular viability behaviour to glycerol. Interestingly, the tolerability and acceptability response were promoted by the C. sativus extract, showing skin protection and increased integrity by up to 14.12%. Our results represent an alternative, ecofriendly and cost-effective option for formulating new potential hand rubs that improve skin protection without compromising the disinfection functionality.
Introduction
Since the health crisis caused by Coronavirus disease 2019 (COVID-19), the population’s lifestyles have considerably changed, as new efforts for personal protection are now practiced among households and institutions. Furthermore, the risk of SARS-CoV-2 spread is highly latent due to human-to-human interactions, close contact with contaminated inanimate surfaces, and microbial persistence, uncovering the hand as the transmission platform. Thus, the development of cleaning protocols at the entrance and exit of homes, schools, and buildings has increased worldwide. However, the disinfection practices that intend to reduce microbial transmission involve excessive cleaning, and usage of disinfection products can severely damage the connective tissue and/or epithelium tissue, such as the skin surface [Citation1,Citation2]. Moreover, since the beginning of the pandemic, the prolonged exposure to irritant chemical products used for disinfectant formulations is now a frequent problem for healthcare workers [Citation34]. Therefore, paying particular attention to the hands’ skin, which is the most sensitive tissue, this damage occurs as redness, cracking, irritation, and in the worst case, severe allergies [Citation5,Citation6].
Worldwide, different alternatives such as cleaning, water temperature, and the formulation of hand-rubs have been chosen to maintain an effective hand hygiene [Citation7]. Interestingly, the WHO has proposed hand rubs formulations to reduce epidermal damage by incorporating glycerine due to its moisturizing effect [Citation8]. However, several studies have shown that glycerine can reduce the antimicrobial activity of alcohol-based hand rubs [Citation9–11]. For instance, Suchomel et al. [Citation9] have attributed that glycerine reduces the antimicrobial activity of hand rubs due to poor drug diffusion resulting in viscosity and sticky agglomerates, which protect bacterial cells and promote bacterial fermentation. Therefore, it is essential to incorporate new active ingredients that can provide skincare while maintaining the antimicrobial activity of hand rubs.
Interestingly, hydroglycolic extracts are obtained by macerating different plant parts, such as leaves, flowers, stems and roots, in non-polar and polar solvents like water. On the other hand, hydroglycolic extracts can provide a vast number of benefits that can decrease the harmful effects of alcohol and active components, meanwhile providing skin protection and antimicrobial effectiveness. Moreover, those extracts present secondary metabolites with important advantages such as regenerative, anti-oxidant and anti-cancer properties [Citation12,Citation13]. Furthermore, the moisturizing and emollient characteristics demanded in hand rubs can be provided by active secondary metabolites such as glycerolipids, sterolipids and saccharolipids present in the extracts [Citation14]. It is essential to highlight that a previous study of Cucumis sativus L. extract showed beneficial skin protection attributes [Citation15]. The authors reported that the extract provided anti-oxidant properties via anti-hyaluronidase, anti-elastase, and reactive oxygen species (ROS) cleavage activity. Similarly, Herman et al. [Citation16] suggested that Calendula officinalis hydroglycolic extracts presented antibacterial activity against gram-positive and gram-negative bacteria for cosmetic applications [Citation16]. Thus far, the above-stated information proposes the potential use of naturally derived hydroglicolic extracts as moisturizing components for skin protection. Nonetheless, incorporating these ingredients has not been extended in disinfectant formulations to promote skin protection while providing antibacterial functionality.
Considering the importance of developing novel formulations for hand rubs disinfectants that do not alter the skin integrity, in the present work, we fabricated and evaluated the antibacterial activity, cytotoxicity, wound healing capability, tolerance and acceptability of hand rubs using commercial cosmetic hydroglycolic extracts (C. sativus and Fragaria sp.) and C. officinalis prepared in our laboratory. These modifications are intended to substitute glycerine in the alcohol-based WHO hand rub formulation. Applying this novel green technology can promote skin protection and bring new qualities of antibacterial activity for the currently demanded hand rubs. Furthermore, the obtained results are expected to guide the fabrication of new hand rubs that will improve the user’s quality of life while maintaining all the safety protocols against the pandemic.
Materials and methods
Formulations
Four hand rub formulations were prepared following the WHO alcohol sanitizer base by modifying the moisturizing agent with the hydroglycolic extracts () [Citation17]. To prepare the ethanol-based hand rub, we used ethanol (96%, JK Servicios, México) at 80% v/v and hydrogen peroxide (3%, Faga Lab, México) at 0.125% v/v as the representative active compounds. On the other hand, to achieve the moisturizing effect, we used glycerol (Natura herbs, México), commercial cosmetic hydroglycolic extracts (C. sativus L. and Fragaria sp.) obtained from Droguería Cosmopolita (Mexico) and C. officinalis hydroglycolic extract prepared in our laboratory. Finally, the components were adjusted in distilled water (to complete 100% v/v), used for the antimicrobial tests, and stored in dark conditions at room temperature for six months of shelf evaluation.
Table 1. Hand rub tolerability evaluation format.
Hydroglycolic extract preparation
Hydroglycolic extract of C. officinalis flower was prepared by macerating 10 g of dried flowers in 90 g of distilled water: propylene glycol (50:50; v/v) mixture for 48 h. We applied 48 h of maceration in order to obtain a higher yield of phytochemical components, which could provide an optimal antimicrobial response and promote cellular migration activity. The preparation was then filtered with a 45 Whatman filter and stored in an amber container at room temperature.
Ethics statement
The Ethical and Research Committee from the Engineering Institute approved the research protocol. According to the Declaration of Helsinki, each participant signed informed consent before the application of the hand rubs.
Tolerability and acceptance test
The tolerability and acceptance of hand rubs were tested as previously described with some modifications [Citation18]. The protocol included 40 participants (male: 19 and female: 21) between the ages of 20 and 60 years, who did not show skin damage symptoms, following a double-blind examination for five days. First, each participant tested all the formulations using an equal amount of each product (pressing the container four times). Next, after applying the hand rub, the participants waited 5 min to evaluate each sanitizer, using the procedure indicated in the hand rub tolerability evaluation format ().
The hand rub tolerability evaluation format was applied before and after the application of each formulation, using the Likert scale with seven values, with 0 being the lowest value and 6 being the highest value for each evaluated aspect. For scales to evaluate skin condition by the observer (objective evaluation), 0 refers to healthy skin, while 5 indicates severely damaged skin. In the self-assessment of skin integrity by the study participant and evaluation of the test product, the value of 0 implies no acceptance or tolerability of the product. In contrast, a value of 6 is the maximum satisfaction. For self-assessment of skin integrity by study participant and evaluation of the test product, the data were treated per item, and considering a total of five days and 40 participants in the study, the maximum total per evaluated aspect was 1200, therefore assigning 0 - 599 as a product not tolerated or nonaccepted: 600 neutral value and from 601 to 1200 as a tolerated and accepted product. This work was conducted in accordance to the ethical principles stipulated in the Declaration of Helsinki, and the evaluation was performed after signing the written informed consent for the application of hand rubs.
Cell culture and cytotoxicity tests
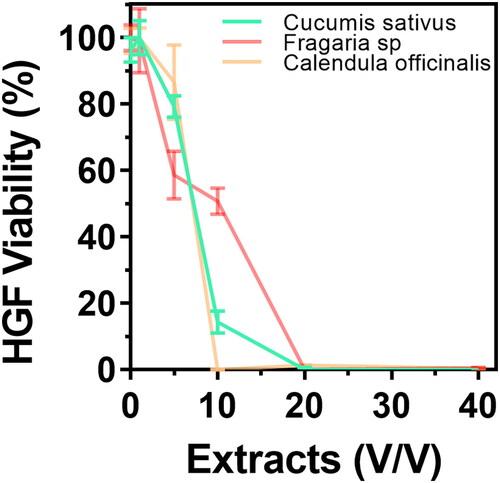
In order to evaluate the cytotoxicity behaviour of the experimental extracts, we applied the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) viability test [Citation19]. For the cytotoxic response tests, we used primary cultures of human gingival fibroblasts (HGFs) isolated as previously described [Citation20]. On the other hand, the RAW 264.7 murine macrophage cell line (ATCC TIB-71) was used to evaluate the immune toxicity and the phenotype alterations by the different treatments. Initially, 1 × 104 cells/mL of HGFs and RAW 264.7 were harvested and cultured, respectively, in Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific, USA) for HGFs and Roswell Park Memorial Institute (RPMI, Thermo Fisher Scientific, USA) supplemented with 10% heat-inactivated foetal bovine serum (SBF, Thermo Fisher Scientific, USA) and 100 units/mL of penicillin-streptomycin (Thermo Fisher Scientific, USA) in separate 96-well flat-bottom polystyrene culture plates (Corning, USA) at 37 °C in a humidified 5% CO2 incubator for 24h. Next, the cells were washed thrice for 5 min with warm 1 × phosphate-buffered saline (PBS). The HGFs were incubated for 24h with the hydroglicolic extracts dilutions (1%, 5%, 10%, 20% and 40% v/v) in complete DMEM. Then the RAW 264.7 cells were exposed for 5 min against each experimental formulation of hand rubs (without dilution) and washed thrice in PBS to eliminate any artefact present during the treatments. The MTT tests were carried out by adding 100 µL of MTT (5 mg/mL, Sigma Aldrich, USA) in DMEM (HGFs) or RPMI (RAW 264.7) to each well of the cultured 96-well plate and incubated at 37 °C in a humidified 5% CO2 incubator for 3h. The resulting formazan crystals were dissolved by eliminating the remaining medium containing MTT. The plate was placed in an orbital shaker at 140 rpm with 200 µL of dimethyl sulfoxide (Sigma Aldrich, USA) for 20 min. Subsequently, the optical density (O.D.) of the dissolved crystals was measured at 590 nm using a microplate reader (Thermoskan, Thermo Fisher Scientific, USA). The baseline controls were measured in a series of culture wells using the hydroglycolic extracts at the experimental dilutions separately and prepared for MTT in the absence of HGFs as described. Moreover, HGFs without any testing extract were selected as a negative control of cytotoxicity. Furthermore, the dose at which 50% of HGFs growth viability is inhibited (IC50) was calculated by means of a nonlinear regression curve fit using GraphPad Prism 7.03.
Cell migration/scratch assay
Initially, HGFs were cultured in individual wells of separate 12-well polystyrene culture plates (Corning, USA) at 25 × 103 cells/well and incubated until a confluent monolayer (100%) was established. Then, the monolayer was washed with warm PBS and wounded using a 20 – 200 µL sterile tip by gliding the tip across the cell surface from a 12-o’clock to 6-o’clock location [Citation21]. Next, the wells were washed with warm PBS to remove any debris and were further cultured in either fresh DMEM (5% SBF, control) or DMEM (5% SBF) supplemented with 1.45% (v/v) of the experimental extracts, and H2O2 (250 µmol/L, negative control). The wound closure was monitored and images were taken every 4h over a 24h period (0, 4, 8, and 12h) with a phase-contrast digital microscope (ZOE, Bio-Rad, USA). The size of the wound of each treatment and the monitored time point was recorded by analysing an average of three random measurements per field.
Cellular phenotype characterization
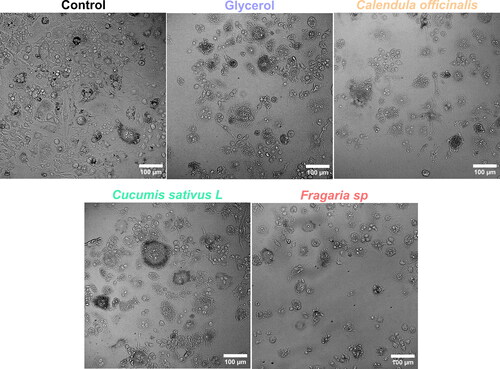
For the structural and phenotype alterations stimulated by the hand rubs and the hydroglicolic extracts, the HFGs and RAW 264.7 cells were cultured in individual wells of separate six-well polystyrene culture plates (Corning, USA) at a density of 25 × 104 cells/well using complete DMEM (HGFs) or RPMI (RAW 264.7) for 24h. Afterward, the wells were washed thrice with warm PBS for 5 min, and the HGFs were treated with the experimental extracts at 37 °C in a humidified 5% CO2 incubator for 24h. Similarly, the RAW 264.7 cells were washed and treated with the hand rubs at 37 °C in a humidified 5% CO2 incubator for 5 min, to assess an acute response. To end the corresponding treatments, the wells were washed with warm PBS. Finally, each cell culture of the six-well culture plates was analysed and imaged using a phase-contrast digital microscope (ZOE, Bio-Rad, USA).
Bacterial cultures
Model pathogens associated with bacterial diseases in humans were selected for antibacterial activity assays, particularly pathogenic bacteria detected on contaminated hands and related to secondary infections after COVID-19 [Citation22,Citation23]. Escherichia coli (E. coli, ATCC 25922), Klebsiella pneumoniae (K. pneumoniae, ATCC 13883) and Pseudomonas aeruginosa (P. aeruginosa, ATCC 27853) were selected as gram-negative models. The gram-positive bacteria were Staphylococcus aureus (S. aureus, ATCC 25923) and methicillin-resistant Staphylococcus aureus (MRSA, ATCC 33591). To prepare the working microbial cultures, freshly grown cells were individually inoculated in Tryptic soy broth (TSB; Becton Dickinson, Sparks, MD, USA) and incubated under aerobic conditions at 37 °C for 24 h. Then, each inoculum in new culture broth was diluted to approximately 1 × 107 CFU/mL for the antimicrobial assays.
Agar diffusion test
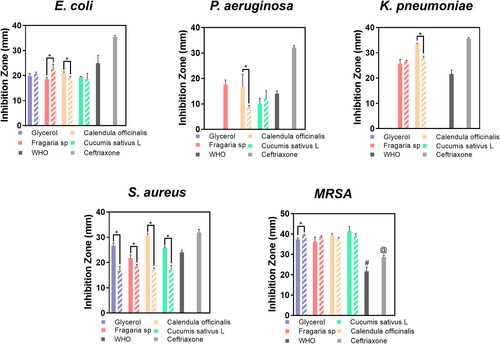
The antibacterial activity was evaluated using the agar diffusion test [Citation22] immediately after the fabrication of the disinfectant hand rubs and after storage for six months in dark conditions at room temperature. Initially, 0.1 mL of different freshly prepared working microorganisms were cultured on tryptic soy agar (25 mL of liquid per 100 mm petri dish). After 30 min, we generated 4 holes of 7 mm diameter and 4 mm depth using a sterile metallic cylinder per dish. Then, 50 µL of the corresponding hand rub, freshly fabricated and after six months of shelf storage, were individually loaded for each bacterial culture and incubated at 37 °C for 24h. Moreover, ceftriaxone (50 µg/mL) was used as a positive control of antibacterial activity. The resulting inhibition zones were measured using an electronic digital calliper and digitalized on a dark field colony counter (Reichert, NY, NY, USA).
Statistical analysis
The numerical data were analysed after three independent studies performed each in triplicate. The results are expressed as the mean values as standard deviation (±SD) and evaluated using GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA). The significance of differences was tested using the Student’s t-test or One-way analysis of variance (ANOVA) followed by Tuckeýs multiple comparison test when needed. Differences were considered statistically significant at the p < .05 level.
Results
Skin conditions followed by tolerability and acceptance
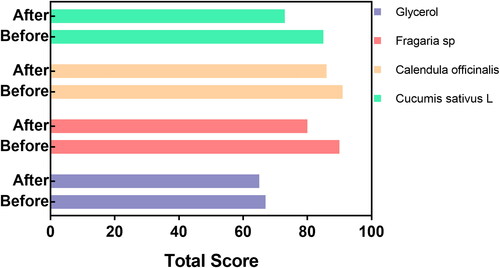
We carried out the general integrity evaluation, and the hand skincare was documented before and after applying each experimental hand rub. In , we presented the results of five-day follow-up analysis using a group of 40 volunteers. The test was performed by comparing the reported value before and after the hand rubs applications, monitoring each day, and considering the initial skin condition as the control. The volunteers reported improved skin integrity after the application of the novel formulated hand rubs, remarking on the improvement following C. sativa L. > Fragaria sp. > C. officinalis > Glycerol (). Importantly, the hydroglicolic extracts modulated the skin condition enhancing the total quality scores after the treatment (). Therefore, our results suggested that the extended use of the fine-tuned disinfectants effectively increased skin quality compared to the simple WHO-based glycerine formulation.
Table 2. Improved skin integrity after the hand rubs trial.
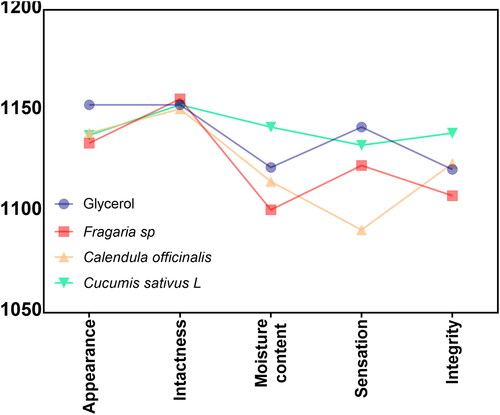
Skin integrity trial
In , we reported the results of skin integrity behaviour manifested after concluding the evaluation trial concerning the tissue appearance and sensibility to the experimental disinfectants. Interestingly, the volunteers manifested similar outcomes of appearance and intactness between the analysed disinfectants. Moreover, the moisture content behaviour, as well as the sensation, showed comparable results for the glycerol, C. offcinalis, and Fragaria sp. Nonetheless, the C. sativus extract increased the sense of satisfaction after five days. On the other hand, the considered integrity observed after the trial revealed similar values for the C. officinalis and the glycerol, lower perception for the Fragaria sp., and the highest integrity option was reported for the Cuccumis sativus L preparation. It is important to highlight that the formulated solutions accumulated a score significantly higher than 1000. Thus, substantially indicating that the disinfectants are well-tolerated and accepted in terms of skin appearance, cracking, dryness and sensation, collectively benefiting the skin integrity.
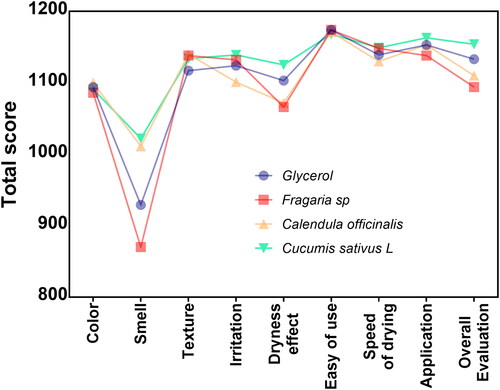
Formulation acceptability
Far more important are the cosmetic and functional parameters required for the acceptance of new commercially available disinfectant products that were tested and reported (). Initially, the formulated disinfectants showed similar colour, texture and irritation acceptability outcomes. Moreover, the volunteers’ susceptibility to easiness for usage, the dryness velocity, as well as the applicability were similar among the experimental hand rubs (disinfectant formulations, ). However, the way the glycerol and Fragaria sp. solution smell were indicated as the most unacceptable, showing score values lower than 1000. Taking together the clinical assessments and the overall results, the volunteers preferred the cosmetic and skin-resulting perception of the C. satuvis L-derived disinfectants ().
Table 3. Ingredients and content of evaluated hand rubs.
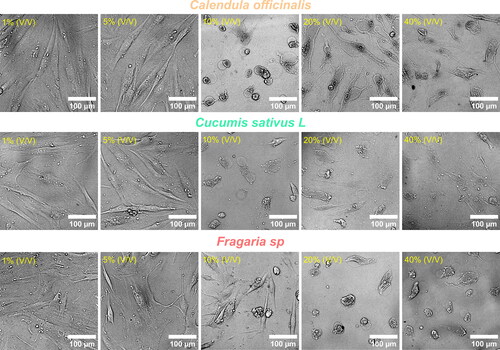
HGF cytotoxicity of the extracts
We studied the cellular morphology alterations after 24h of exposure to the experimental hydroglycolic extracts. The results suggested that concentrations below 10% did not generate critical phenotype alterations (). Interestingly, the morphology showed cellular extension, intercellular junctions, well-defined nuclei, and fine-tuning sharped filopodia formation, outcomes of cell proliferation. Consequently, these results indicate retained HGFs adhesion, properties needed for connective tissue integrity. On the other hand, doses over 20% could cause cellular aberrations, nuclei disintegration and altered cell architecture with decreased adhesion, which was principally detected for the Fragaria sp. derived extract. The MTT cytotoxicity test () showed similar outcomes of metabolic activity behaviour for the extracts at concentrations below ≈ 8%. The IC50 values of cytotoxic dysfunction for HGFs were Fragaria sp. (6.06 ± 1.2%) < C. sativus (5.25 ± 1.4%) < C. officinalis (5 ± 1.7%), which were closely similar. On the contrary, the elevated concentrations evidenced that HGFs lost cellular activity, which aligns with the transformed morphology presented in . The cumulative end results further supported that the hydroglycolic extracts used in this work presented a similar viability trend in the optimal concentrations for the formulated skin disinfectants.
Wound repair of HGFs monolayer with hydroglycolic extracts
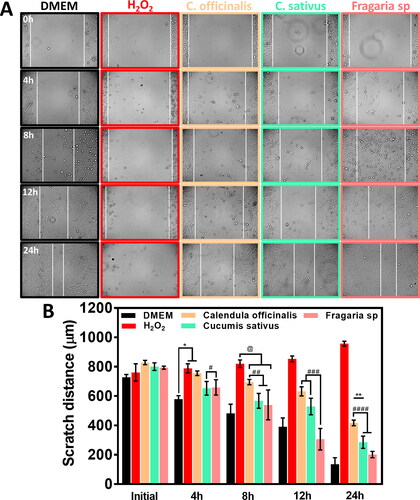
We evaluated the HGFs migration behaviour stimulated by the hydroglycolic treatments (1.45% v/v) for 24h. It is important to highlight that the experimental dosages did not inhibit wound closure (). Nonetheless, the H2O2 (negative control) disrupts cell migration, thus avoiding the cellular confluence and concluding with disrupting the initial peripheral cell layer as expected. On the other hand, the measurement of the scratch width () showed that C. sativus and Fragaria sp. followed a similar initial (4h) closure behaviour to the control and continued this trend after 8h of incubation. There was consistent wound healing achieved by treatment with the Fragaria sp. extract at 12h and 24h, illustrating similar behaviour of cell migration and wound closure rate as the positive control. However, the C. officinalis extract did not reach a significant wound closure, suggesting a possible disruption of metabolic activity and subsequent cell growth, avoiding the fast cell migration process over each evaluation point.
Figure 6. Scratch assay showing the wound healing behaviour of HGFs at different periods. (A) Micrographs depicting the cellular migration reached at specific culture times. The horizontal white lines highlight the width of wound reduction during cell growth with the experimental treatments. (B) Quantitative wound coverage evaluation of scratch distance analysis during 24 h of hydroglycolic treatments. The *, **, @, #, ##, ### and #### showed significant differences.
RAW 264.7 cell behaviour after disinfectants exposure
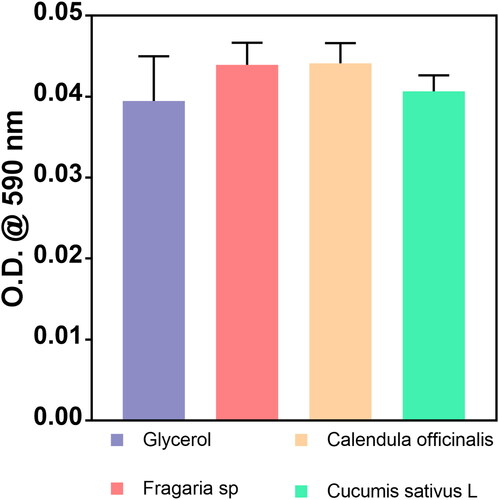
In order to analyse the macrophage phenotype change that occurred in response to the treatments (), we applied acute doses of complete disinfectants (containing glycerol and plant extracts) to simulate contact with the skin. Initially, we detected that the glycerol, C. officinalis and Fragaria sp. treatment were associated with morphology alterations suggesting M1 differentiation. On the other hand, the cellular phenotype in the control and in the treatment with C. sativus L. disinfectant was closely similar, suggesting that macrophages could reach M2 differentiation. It is essential to highlight that the exposure time was only 5 min (acute dose), which is not a predominant factor in establishing a complete cellular induction process. However, these initial outcomes of phenotype transformation indicated reduced acute inflammatory response by the C. sativus extract during the skin treatment. Therefore, considering the analysis of cellular morphology, we evaluated the cytotoxic potential of the disinfectants after 5 min of treatment (). The MTT assay showed similar outcomes of metabolic activity between the formulated green disinfectants and the WHO base with glycerol. Interestingly, these analyses hypothesize that hand rubs conduct macrophage alterations due to the possible activation of different surface receptor factors without decreasing the cell viability, as evidenced by our experiments.
Antibacterial performance
The main goal of disinfectant technologies is to achieve efficient antibacterial action after a prolonged storage period. Therefore, we evaluated the antibacterial properties of freshly prepared disinfectant formulations as well as after six months of shelf storage against gram-positive and gram-negative bacterial models (). The experimental disinfectants inhibited the growth of the E. coli strain efficiently. E. coli growth was significantly inhibited by the application of Fragaria sp. and C. sativus, and the effect was maintained even after six months of shelf storage and antibacterial reevaluation. Nonetheless, the antibacterial activity of the C. sativus disinfectant against S. aureus decreased after prolonged shelf storage, while the other formulations retained their antibacterial activity. Moreover, the glycerol formulation did not show antibacterial response against P. aeruginosa and K. pneumoniae, suggesting that the incorporation of the hydroglycolic extracts improved the broad-spectrum antibacterial action. On the other hand, the tested fresh formulations orchestrated a critically inhibitory effect on the gram-positive S. aureus and MRSA models; the green-based disinfectants and the WHO recommended formulation exerted and maintained comparable antibacterial activity to that of the positive control (ceftriaxone). Our evidence of antibacterial efficacy suggests that the mucoid bacterial cells (K. pneumoniae and P. aeruginosa) will require prolonged disinfectant treatments (including by the formulations recommended by the WHO) to achieve consistent effectiveness.
Discussion
The new protocols established after the COVID-19 pandemic disease have resulted in safer and cleaner procedures reducing microbial transmission and reusing different medical protective equipment [Citation22]. Nevertheless, several drawbacks have been reported due to the components present in commercially available disinfectant products. On the other hand, the skin damage caused by disinfectants, showing protein denaturation, cellular alterations, skin inflammation, and the removal of transepidermal water, leading to severe skin dryness [Citation24]. Therefore, in order to reduce those critical side effects, it is necessary to use a moisturizing agent to protect the stratum corneum without compromising the antibacterial activity. Interestingly, the WHO proposed an alcohol-based hand disinfectant formulation using glycerine as the emollient and moisturizing reagent. For this reason, here we hypothesized that incorporating a moisturizing and greener agent with dermatological protection (such as hydroglycolic extracts) instead of glycerol should increase the skin surface tolerability (). The three hydroxyl groups of the aliphatic chain in glycerol provide beneficial hygroscopic and effective moisturizing properties, particularly favouring humidified skin integrity. However, in the WHO formula, glycerol can intercalate in the skin’s lipidic structure avoiding, on the one hand, the loss of water and acting as a moisture reservoir for the lipids and proteins of the stratum corneum [Citation11]. On the other hand, previous work has suggested that glycerine can critically compromise the antibacterial performance of skin antiseptics [Citation9]. The study concluded that glycerine reduces the diffusion of alcohol and active components, promoting the formation of agglomerates that can direct biofilm formation and subsequent microbial fermentation. Nonetheless, alcohol-based disinfectants containing glycerol showed an overall acceptable skin sensitivity, texture, and sensation, providing wide acceptance among the volunteers. Thus, new moisturizing agents are required to substitute the beneficial properties of glycerine in classic sanitizers.
It is noteworthy to highlight that hydroglycolic extracts contain secondary metabolites and a wide number of polar phytocompounds that can increase wetting behaviour [Citation25]. At the end of the skin application trial, the volunteers indicated that the hydroglycolic-derived formulations showed overall positive acceptance of skin appearance, texture, and irritation protection. Meanwhile, the C. officinalis and C. sativus components additionally presented outstanding organoleptic and satisfactory properties, even higher than those approved by the WHO. Moreover, this compelling behaviour could be explained due to the given fragrant and moisturizing saponins, terpenoids, flavonoids, glycosides and glycerolipids, quercetins, retinoids, squalene and polar aromatic metabolites in the extracts [Citation26]. Furthermore, previous studies demonstrated that cucumber-derived products provided phytosterols, phenolic acids, fatty acids, and cucurbitacins, improving skin regeneration [Citation15,Citation27]. Further contributing to the wetting and cosmetic requirements demanded in skin-designed formulations that are absent when using pure glycerol [Citation28], as evidenced here. Interestingly, the hydroglycolic extracts protect against UV-A and UV-B rays, mainly by acting as an adjuvant in inhibiting skin’s deterioration due to DNA damage and lipid peroxidation [Citation29,Citation30].
In order to explain the beneficial effects reported by the volunteers’ observations, we evaluated the viability behaviour of human gingival fibroblasts treated with hydroglycolic extracts. The MTT assay showed that the hydroglycolic working concentration required to generate the moisturizing properties in the disinfectants was five-fold lower than the IC50 values. Moreover, we detected that 10% (v/v) overdoses induced slight cellular alterations mainly characteristic of the Fragaria sp. and C. officinalis extracts. The volunteers’ observations of improved skin care were in line with the obtained in vitro evidence, supporting the potential of the tested formulations to improve the skin care. The tissue regeneration process requires optimal viable fibroblasts to tailor the wound healing process. Thus, fibroblasts growing functionality may orchestrate the development of extracellular matrix and collagen deposition guiding the characteristic cell-cell junction, filopodia interconnection, and cell extension [Citation31], as observed here (). Furthermore, wound tissue repair is an essential complex process that demands a fast and sustained fibroblast migration process, as facilitated by the Fragaria sp. and C. sativus extracts. Those interesting observations could be explained by the antioxidants’ protecting and modulatory effects, the flavonoids’ growth factor response, and the proliferating stimulus guided by the terpenoids provided by the extracts [Citation32] that were included instead of glycerol.
On the other hand, macrophages are essential in modulating the inflammatory response, cell clearance, and participating in the tissue healing process [Citation33]. Therefore, it is important to highlight that the acute treatments with C. officinalis, Fragaria sp., and glycerol-based formulations promoted macrophage M1 phenotype configuration. As reported in previous works, the treatments resulted in irregular, spindle-shaped, and extended cell-cell interconnecting filopodia [Citation34]. The M1 polarized subpopulations are characterized as potent pro-inflammatory cells overexpressing cytokines, interleukins and chemokines [Citation35]. The C. sativus extract promoted a flattened, expanded shape, with irregular macrophage cell bodies and short bonding lamellipodia, suggesting M2 turnover [Citation34]. Hence, the M2 polarization state orchestrated non-inflammatory and pro-wound healing conditions that could exacerbate the expression of anti-inflammatory cell-surface receptors and interleukins secretion [Citation36]. The MTT cytotoxicity assays showed that the acute doses of the experimental disinfectants did not disrupt the macrophage viability, further supporting the potential beneficial effects of the developed antiseptics.
The antimicrobial performance of novel green and eco-friendly sanitizer technologies determines the effectiveness against hands-transmitted SARS-CoV-2 associated opportunistic microorganisms [Citation37], such as those studied here. In this work, the occurring secondary metabolites incorporated with the hydroglycolic extracts did not block the antimicrobial performance of the antiseptic compounds recommended by the WHO. Nonetheless, we detected that the experimental disinfectants promoted the antibacterial action against E. coli and the gram-positive models, even after six months of shelf storage. The phytocompounds that act as antimicrobial plant defence (e.g. phytoalexins and phytoanticipins) can explain the improved wide-spectrum of the observed antibacterial action of the extracts [Citation38–40]. These important phytochemicals are considered part of the plant’s ‘immune system’ regulatory mechanisms for defence/stress response [Citation38]. These low molecular weight natural broad-active antibacterial products include glucosinolates, cyanogenic glucosides, saponins, phenylpropanoid derivates, alkaloids, flavonoids, terpenes and polyketides. In addition, the presence of quercetins, reserpine and tannins is also of importance, as they are recognized as potent microbial inhibitory phytochemicals in the studied extracts [Citation38,Citation41,Citation42]. On the other hand, the common active ingredients of the evaluated hand rubs are ethanol and hydrogen peroxide, supporting a sustained antimicrobial effect, especially against gram-positive bacteria. However, our results showed that K. pneumoniae and P. aeruginosa showed resistance the WHO disinfectant (). A previous study suggested that gram-negative mucoid bacteria could resist those compounds due to the non-permeable, thick and dense cell wall coating. Moreover, the cell-membrane drug-resistance pumps can extrude toxins and restrict the internalization of considerable compounds [Citation43]. Furthermore, culture variants of K. pneumoniae have shown extensive resistance to common disinfectants such as alcohol, chlorhexidine and iodophor [Citation44]. Several pieces of evidence demonstrated that gram-negative bacteria are broadly resistant to chemical and biochemical disinfectants and responsible for many deaths associated with nosocomial infections worldwide [Citation45,Citation46]. Therefore, our present work opens a new road for the development of greener and more effective antiseptic technologies with improved antimicrobial activity and reduced human cytotoxicity. However, more analytical studies are recommended to elucidate those findings.
Conclusions
The present study provides evidence for hydroglycolic extracts used as glycerine substitutes in alcohol-based hand rubs designed according to the WHO recommendations. According to the volunteers’ trial, the new formulations hallmarked a substantial overall acceptance (14.12%), showing promoted tolerability, moisturizing, and dermatological properties as compared to the glycerine-based alternative. These results were in part explained by conserved fibroblast morphology, the cytotoxic protection, the high tolerance and wound healing capability of the extracts. The modulated macrophage behaviour suggested M1 pro-inflammatory polarization stimulated by the glycerol, C. officinalis and Fragaria sp.-based formulations. On the contrary, the C. sativus L disinfectant influenced the M2 phenotype differentiation, thus far suggesting a non-inflammatory and pro-wound healing process, in agreement with the volunteers’ skin-beneficial outcomes. The antibacterial tests revealed that the developed hand rubs further supported the broad-antimicrobial action even after six months of shelf storage. Our findings proposed that the antimicrobial phytochemicals naturally occurring in the hydroglycolic extracts improved the bactericidal performance as compared to the glycerine-containing formulation. However, we recommend paying particular attention to mucoid gram-negative bacteria due to the tailored disinfectant resistance detected here. The positive outcomes of dermal protectiveness and the fine-tuning of antibacterial effectiveness shed light on developing novel green disinfectant technologies for pandemic and nosocomial protection.
Authors’ contributions
Conceptualization, T.R.-M, B.V.-S. and E.B.-P.; experimental procedure, T.R.-M., B.V.-S., J.S.-C., M.S., R.Z. and E.B.-P.; writing – original draft preparation, T.R.-M., B.V.-S., M.S., R.Z. and E.B.-P.; writing, review and editing, B.V.-S., M.S. and E.B.-P.; supervision, B.V.-S. and E.B.-P., funding acquisition B.V.-S. and E.B.-P. All authors have read and agreed to the final version of the manuscript, with the exception of R.Z. who passed away during the conclusion of the original draft.
Acknowledgements
The authors wish to thank M.Sc. Claudia Gutierrez (Instituto de Ingeniería at Universidad Autónoma de Baja California) for her technical support in the preparation of the scratch assays.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data that supports the findings reported in this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Larson E, Girard R, Pessoa-Silva CL, et al. Skin reactions related to hand hygiene and selection of hand hygiene products. Am J Infect Control. 2006;34(10):1–14. doi: 10.1016/j.ajic.2006.05.289.
- Polecka A, Owsianko N, Awchimkow A, et al. Questionnaire-based study evaluating the hand hygiene practices and the impact of disinfection in the COVID-19 pandemic on hand skin conditions in Poland. J Clin Med. 2022;12(1):195. doi: 10.3390/jcm12010195.
- Girard R, Bousquet E, Carré E, et al. Tolerance and acceptability of 14 surgical and hygienic alcohol-based hand rubs. J Hosp Infect. 2006;63(3):281–288. doi: 10.1016/j.jhin.2006.01.017.
- Zhang B, Zhai R, Ma L. 2019 Novel coronavirus disease epidemic: skin protection for healthcare workers must not be ignored. J Eur Acad Dermatol Venereol. 2020;34(9):e434–e435. doi: 10.1111/jdv.16573.
- Kampf G, Muscatiello M, Häntschel D, et al. Dermal tolerance and effect on skin hydration of a new ethanol-based hand gel. J Hosp Infect. 2002;52(4):297–301. doi: 10.1053/jhin.2002.1311.
- Li Y, Lu Y, Wang Y, et al. Investigation on the effectiveness of ventilation dilution on mitigating COVID-19 patients’ secondary airway damage due to exposure to disinfectants. Build Environ. 2023;228:109787. doi: 10.1016/j.buildenv.2022.109787.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO guidelines on hand hygiene in health care: first global patient safety challenge clean care is safer care. Geneva: World Health Organization; 2009.
- Menegueti MG, Laus AM, Ciol MA, et al. Glycerol content within the WHO ethanol-based handrub formulation: balancing tolerability with antimicrobial efficacy. Antimicrob Resist Infect Control. 2019;8(1):109. doi: 10.1186/s13756-019-0553-z.
- Suchomel M, Rotter M, Weinlich M, et al. Glycerol significantly decreases the three hour efficacy of alcohol-based surgical hand rubs. J Hosp Infect. 2013;83(4):284–287. doi: 10.1016/j.jhin.2012.11.030.
- Suchomel M, Kundi M, Allegranzi B, et al. Testing of the World Health Organization-recommended formulations for surgical hand preparation and proposals for increased efficacy. J Hosp Infect. 2011;79(2):115–118. doi: 10.1016/j.jhin.2011.05.005.
- Suchomel M, Steinmann J, Kampf G. Efficacies of the original and modified World Health Organization-recommended hand-rub formulations. J Hosp Infect. 2020;106(2):264–270. doi: 10.1016/j.jhin.2020.08.006.
- Barbieri R, Coppo E, Marchese A, et al. Phytochemicals for human disease: an update on plant-derived compounds antibacterial activity. Microbiol Res. 2017;196:44–68. doi: 10.1016/j.micres.2016.12.003.
- Gaweł-Bęben K, Strzępek-Gomółka M, Czop M, et al. Achillea millefolium L. and Achillea biebersteinii Afan. Hydroglycolic extracts–bioactive ingredients for cosmetic use. Molecules. 2020;25(15):3368. doi: 10.3390/molecules25153368.
- Macabuhay A, Arsova B, Walker R, et al. Modulators or facilitators? Roles of lipids in plant root–microbe interactions. Trends Plant Sci. 2022;27(2):180–190. doi: 10.1016/j.tplants.2021.08.004.
- Nema NK, Maity N, Sarkar B, et al. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch Dermatol Res. 2011;303(4):247–252. doi: 10.1007/s00403-010-1103-y.
- Herman A, Herman AP, Domagalska BW, et al. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J Microbiol. 2013;53(2):232–237. doi: 10.1007/s12088-012-0329-0.
- World Health Organization. Guide to local production of WHO-recommended handrub formulations. Geneva: World Health Organization; 2009.
- World Health Organization. Protocol for evaluation of tolerability and acceptability of alcohol-based handrub in use or planned to be introduced: method 2. Geneva: World Health Organization: 2009.
- Valdez-Salas B, Beltrán-Partida E, Zlatev R, et al. Structure-activity relationship of diameter controlled Ag@Cu nanoparticles in broad-spectrum antibacterial mechanism. Mater Sci Eng C Mater Biol Appl. 2021;119:111501. doi: 10.1016/j.msec.2020.111501.
- Beltrán-Partida E, Valdez-Salas B, Curiel-Álvarez M, et al. Enhanced antifungal activity by disinfected titanium dioxide nanotubes via reduced nano-adhesion bonds. Mater Sci Eng C Mater Biol Appl. 2017;76:59–65. doi: 10.1016/j.msec.2017.02.153.
- Pinto BI, Cruz ND, Lujan OR, et al. In vitro scratch assay to demonstrate effects of arsenic on skin cell migration. J Vis Exp. 2019;(144):e58838. doi: 10.3791/58838.
- Valdez-Salas B, Beltran-Partida E, Cheng N, et al. Promotion of surgical masks antimicrobial activity by disinfection and impregnation with disinfectant silver nanoparticles. Int J Nanomed. 2021;16:2689–2702. doi: 10.2147/IJN.S301212.
- Edmonds-Wilson SL, Nurinova NI, Zapka CA, et al. Review of human hand microbiome research. J Dermatol Sci. 2015;80(1):3–12. doi: 10.1016/j.jdermsci.2015.07.006.
- Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A2 together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39(2):188–196. doi: 10.1111/ics.12364.
- Vyumvuhore R, Tfayli A, Manfait M, et al. Vibrational spectroscopy coupled to classical least square analysis, a new approach for determination of skin moisturizing agents’ mechanisms. Skin Res Technol. 2014;20(3):282–292. doi: 10.1111/srt.12117.
- Fiorentino M, Gravina C, Piccolella S, et al. Calendula arvensis (Vaill.) L.: a systematic plant analysis of the polar extracts from its organs by UHPLC-HRMS. Foods. 2022;11(3):247. doi: 10.3390/foods11030247.
- Salehi B, Quispe C, Sharifi-Rad J, et al. Antioxidant potential of family Cucurbitaceae with special emphasis on Cucurbita genus: a key to alleviate oxidative stress-mediated disorders. Phytother Res. 2021;35(7):3533–3557. doi: 10.1002/ptr.7045.
- Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol. 2008;159(1):23–34. doi: 10.1111/j.1365-2133.2008.08643.x.
- Fierascu RC, Temocico G, Fierascu I, et al. Fragaria genus: chemical composition and biological activities. Molecules. 2020;25(3):498. doi: 10.3390/molecules25030498.
- Gasparrini M, Forbes-Hernandez TY, Afrin S, et al. Strawberry-based cosmetic formulations protect human dermal fibroblasts against UVA-induced damage. Nutrients. 2017;9(6):605. doi: 10.3390/nu9060605.
- Fronza M, Heinzmann B, Hamburger M, et al. Determination of the wound healing effect of calendula extracts using the scratch assay with 3T3 fibroblasts. J Ethnopharmacol. 2009;126(3):463–467. doi: 10.1016/j.jep.2009.09.014.
- Ratanachamnong P, Chunchaowarit Y, Namchaiw P, et al. HPLC analysis and in vitro antioxidant mediated through cell migration effect of C. hystrix water extract on human keratinocytes and fibroblasts. Heliyon. 2023;9(2):e13068. doi: 10.1016/j.heliyon.2023.e13068.
- Yang QQ, Zhang L, Zhou YL, et al. Morphological changes of macrophages and their potential contribution to tendon healing. Colloids Surf B Biointerfaces. 2022;209(Pt 1):112145. doi: 10.1016/j.colsurfb.2021.112145.
- Rostam HM, Reynolds PM, Alexander MR, et al. Image based machine learning for identification of macrophage subsets. Sci Rep. 2017;7(1):3521. doi: 10.1038/s41598-017-03780-z.
- Agarwal H, Nakara A, Shanmugam VK. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: a review. Biomed Pharmacother. 2019;109:2561–2572. doi: 10.1016/j.biopha.2018.11.116.
- Zhai T, Sun Y, Li H, et al. Unique immunomodulatory effect of paeoniflorin on type I and II macrophages activities. J Pharmacol Sci. 2016;130(3):143–150. doi: 10.1016/j.jphs.2015.12.007.
- Marteinson SC, Lawrence MJ, Taranu ZE, et al. Increased use of sanitizers and disinfectants during the COVID-19 pandemic: identification of antimicrobial chemicals and considerations for aquatic environmental contamination. Environ Rev. 2023;31(1):76–94. doi: 10.1139/er-2022-0035.
- Simões M, Bennett RN, Rosa EAS. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep. 2009;26(6):746–757. doi: 10.1039/b821648g.
- Tiku AR. Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In: Mérillon J-M, Ramawat KG, editors. Co-evolution of secondary metabolites. Cham: Springer International Publishing; 2020. p. 845–868.
- Wink M. Current understanding of modes of action of multicomponent bioactive phytochemicals: potential for nutraceuticals and antimicrobials. Annu Rev Food Sci Technol. 2022;13(1):337–359. doi: 10.1146/annurev-food-052720-100326.
- Halberstein RA. Medicinal plants: historical and cross-cultural usage patterns. Ann Epidemiol. 2005;15(9):686–699. doi: 10.1016/j.annepidem.2005.02.004.
- Patel MK, Kumar M, Li W, et al. Enhancing salt tolerance of plants: from metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells. 2020;9(11):2492. doi: 10.3390/cells9112492.
- Tegos G, Stermitz FR, Lomovskaya O, et al. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002;46(10):3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002.
- Guo W, Shan K, Xu B, et al. Determining the resistance of carbapenem-resistant Klebsiella pneumoniae to common disinfectants and elucidating the underlying resistance mechanisms. Pathog Glob Health. 2015;109(4):184–192. doi: 10.1179/2047773215Y.0000000022.
- Lambert PA. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med. 2002;95(Suppl 41):22–26.
- Marchi AP, Farrel Côrtes M, Vásconez Noguera S, et al. Chlorhexidine susceptibility and Eagle effect in planktonic cells and biofilm of nosocomial isolates. Eur J Clin Microbiol Infect Dis. 2023;42(6):787–792. doi: 10.1007/s10096-023-04594-w.