Abstract
Bulgaria, like many other countries, has experienced the far-reaching consequences of the Coronavirus disease 2019 (COVID-19) pandemic. Seroprevalence studies serve as a crucial method for assessing the exposure and immunity levels within a population. In this serosurvey, which is the first of this kind conducted in Bulgaria, we enrolled 104 healthcare workers (HCWs) from one front-line Bulgarian hospital and 237 individuals from the general population. Serum samples were collected in December 2021 from the general population and in February 2023 from HCWs and the general population. The presence of four anti-SARS-CoV-2 antibodies was evaluated: anti-Spike 1-IgG, anti-Nucleoprotein-IgG, anti-Spike1-IgA and anti-Recombinant binding domain neutralizing antibodies. Our results showed high percentages of seropositivity in both the HCWs and the general population (99% and 78%, respectively) in February 2023. HCWs had significantly higher mean IgA and mean anti-S1-IgG antibody levels compared to the general population. The seropositivity in the general population in December 2021 was 79%. The mean levels of IgA and anti-NCP-IgG were significantly higher, whereas the mean anti-S1-IgG levels were significantly lower in February 2023 compared to December 2021. We found a strong correlation between neutralizing antibodies and anti-S1-IgA and anti-S1-IgG antibodies for all tested groups. It is necessary to perform large-scale serosurveys to provide analysis of the seroprevalence in a larger population and its dynamics over time and to facilitate evidence-based strategies that will safeguard the health and well-being of the Bulgarian population and contribute to the global efforts in combating the COVID-19 pandemic.
Introduction
The Coronavirus disease 2019 (COVID-19) pandemic has profoundly impacted societies worldwide [Citation1–3], necessitating rigorous research to understand its dynamics and devise effective control measures. Bulgaria, like many other countries, has experienced the far-reaching consequences of the virus [Citation4, Citation5]. To better comprehend the extent of COVID-19 infection within the population, conducting serosurveys has emerged as a valuable tool.
The COVID-19 seroprevalence study serves as a crucial method for assessing the exposure and immunity levels within a population. By detecting the presence of antibodies against the Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), these surveys provide insights into the overall infection rates and help to identify the vulnerable populations who may require additional protection. Healthcare workers (HCWs), at the forefront of the battle against the pandemic, face an elevated risk of exposure [Citation6–8], necessitating a deeper understanding of their seroprevalence rates and immune response.
The general population, encompassing individuals from various backgrounds and age groups, represents a broader picture of COVID-19 transmission within a country. Assessing the seroprevalence and immune status of this population can aid in assessing the effectiveness of public health interventions, identifying potential risk factors, and guiding future mitigation strategies.
Variable results have been published from seroprevalence investigations conducted in different countries and regions around the world [Citation9–12]. However, the time and the current state of the pandemic at a local level are essential factors in the interpretation of the data. Nevertheless, the overall seroprevalence rates worldwide are increasing due to infection, vaccination or both [Citation6, Citation13, Citation14].
In this study, we conducted a serosurvey encompassing both healthcare workers and individuals from the general population in Bulgaria. This paper aims to contribute to the existing body of knowledge on COVID-19 by providing valuable insights into the seroprevalence and immune response among the studied cohorts. By contributing to the ongoing attempts to unravel the complexities of this unprecedented crisis, we hope to facilitate evidence-based strategies that will safeguard the health and well-being of the Bulgarian population and contribute to the global efforts in combating the COVID-19 pandemic.
Subjects and methods
Ethics statement
Ethical approval for this study was obtained from the Institutional Review Board at NCIPD (approval number 4/17.02.2021). Written informed consent was obtained from all patients before the study.
Participant recruitment and sample collection
The study was performed in Bulgaria and enrolled 104 healthcare workers (HCWs) from one front-line hospital and 237 healthy individuals from the general population in Sofia. The eligibility requirements included the capacity of HCWs to provide informed consent. Individuals who refused to give informed consent or had contraindications to venipuncture were excluded from the study. Serum samples from the general population were collected randomly from persons visiting laboratories in primary healthcare centers for routine prophylactic check-ups in Sofia. Samples from HCWs were collected from February 1, 2023 to March 1, 2023. Samples from the general population were collected during two time periods: from December 1, 2021 to January 1, 2022 (124 individuals) and from February 1, 2023 to March 1, 2023 (113 individuals). The HCWs population included 12 male and 92 female participants, mean age was 50 years (SD 10.4). The study included 114 male and 123 female participants from the general population; the mean age was 47 years (SD 15.3). Serum samples were collected in accordance with the national sample collection guidelines.
Serological testing
Assessment of the serological profile of the participants involved semiquantitative measurement by enzyme-linked immunosorbent assay (ELISA) of IgG and IgA antibodies against Spike (S) protein and IgG antibodies against Nucleoprotein (NCP) in the serum. The test kits utilized were provided by EUROIMMUN Medizinische Labordiagnostika AG, Germany (Cat. No. EI 2606-9601 A, Cat. No. EI 2606-9601 G), and the samples were processed in accordance with the manufacturer’s instructions. Results were calculated as a ratio of the extinction of the test sample over the extinction of the calibrator. Interpretation of the results was as follows: positive if ratio ≥1.1 and negative if ratio <1.1.
Anti-RBD neutralizing antibodies (NAbs) were determined with cPass SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript Diagnostic Technology Co., Ltd., Cat. No. L00847-C) according to the manufacturer’s instructions. Results were calculated as a percentage of the signal inhibition and were interpreted as follows: positive if percentage inhibition ≥ 30% and negative if inhibition <30%.
Statistical analysis
The statistical analysis and graphics were done with GraphPad Prism 8.0 Software. Statistical analysis of the data was performed using independent samples Kruskal–Wallis test and differences were considered statistically significant at the level of p < 0.05.
Results
Seropositivity in the studied groups
Among the HCWs, anti-S1-IgG was found in 99% (103/104) of the participants, anti-NCP-IgG in 56% (58/104), anti-S1-IgA in 99% (103/104) and anti-RBD NAbs in 98% (102/104). In the general population during the first time period (December 2021), 70% (87/124) were positive for anti-S1-IgG, 10% (13/124) for anti-NCP-IgG, 79% (82/124) for anti-S1-IgA and 68% (84/124) for anti-RBD NAbs. During the second time period (February 2023) 78% (88/113) of the individuals were positive for anti-S1-IgG, 48% (54/113) for anti-NCP-IgG, 88% (99/113) for anti-S1-IgA and 88% (99/113) for anti-RBD NAbs. The results are presented in , giving the seropositivity in each group with a 95% Confidence interval (CI).
Table 1. Seropositivity levels of healthcare workers and the general population.
Comparison of the specific antibody levels between HCWs and the general population (February 2023)
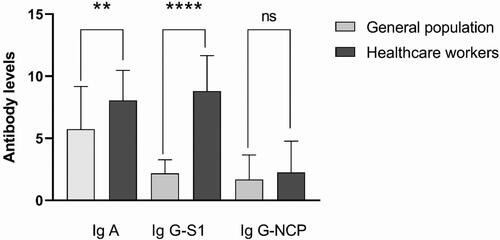
To better understand the occupational risk of COVID-19 transmission we compared the mean antibody levels of HCWs and the general population during the period from February 1, 2023 to March 1, 2023 (). As described in the previous section, we established high levels of seropositivity, which reached 99% for anti-S1-IgG in the HCWs group. We found a significant difference in mean IgA (8.06 ± 2.40 vs. 5.73 ± 3.43) and mean anti-S1-IgG (2.18 ± 1.09 vs. 8.80 ± 2.85) antibody levels between HCWs and the general population, while the differences between the mean anti-NCP-IgG antibody levels of the two groups were not significant (1.70 ± 1.95 vs. 2.52 ± 2.52).
Comparison of the specific antibody levels among the general population in December 2021 and February 2023
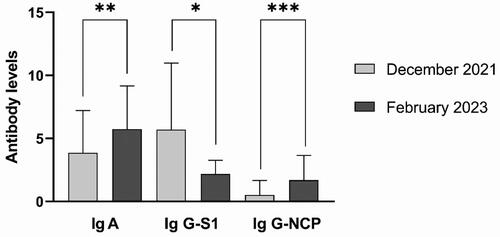
To improve our comprehension of how the immune status of the general population against COVID-19 evolves over time, we compared the specific antibodies measured in December 2021 and February 2023. The first survey coincides with the spread of the Delta SARS-CoV-2 variant and its peak in Bulgaria. Despite that, we detected anti-NCP-IgG antibodies, which are involved in the innate immune response after infection with SARS-CoV-2, only in 10% of the studied cohort. The percentages of seropositivity for the other antibodies were significantly higher: 70% for anti-S1-IgG and 78% for anti-S1-IgA. During the second study, was expected a rise in the number of vaccinated and/or infected persons in the general population. Almost half (48%) of tested participants were seropositive for anti-NCP-IgG antibodies. The increase in seropositivity for anti-spike antibodies was not so significant: 78% for anti-S1-IgG and 88% for anti-S1-IgA.
We compared the mean levels of antibodies between the first and the second survey and all differences were statistically significant (). The mean levels of anti-S1-IgA (3.85 ± 3.36 vs. 5.73 ± 3.43), and anti-NCP-IgG (0.52 ± 1.15 vs. 1.70 ± 1.95) in February 2023 were higher in comparison with December 2021 (p < 0.005 for IgA and p < 0.0005 for anti-NCP antibodies). In contrast, the levels of anti-S1-IgG antibodies were lower, p < 0.05, (5.71 ± 5.27 vs. 2.18 ± 1.09).
Specific virus-neutralizing antibodies
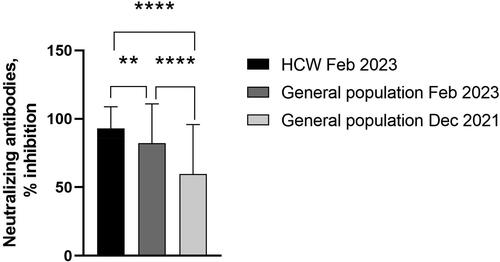
We found a strong correlation between neutralizing antibodies and anti-Spike IgA and IgG antibodies for all tested groups. Seropositivity for each group and statistical differences are presented in .
Discussion
In this study, we evaluated the presence of specific COVID-19 antibodies among HCWs in one front-line hospital in February 2023 and the Bulgarian general population in December 2021 and February 2023 and compared the results obtained from the different cohorts.
Overall, our study demonstrated high percentages of seropositivity in both the HCWs and the general population (99% and 78%, respectively) in February 2023. We consider that the higher levels of anti-S1 antibodies (both IgA and IgG) among healthcare workers are due to frequent exposure to SARS-CoV-2, higher vaccination coverage among this group, or both. Some of the first seroprevalence studies also report significantly higher seroprevalence in HCWs groups in comparison with the general population [Citation15, Citation16].
The observation that anti-NCP seropositivity was increased in the general population during the second survey, while anti-S IgG levels remained the same, may indicate that the immunity in the group is achieved mainly after infection with SARS-CoV-2. The higher levels of IgA antibodies compared to IgG are probably related to the milder course of COVID-19 at the time of the study, which may have resulted in a predominantly mucosal immune response without IgG production [Citation17]. Interestingly, the difference in mean anti-NCP antibody levels in both groups is not statistically significant, despite the slightly higher seropositivity in the HCWs group.
The correlation between RBD-NAbs and anti-S1 IgA and IgG was expected, and the similar levels of seropositivity in the studied cohorts are reflective of the duration of anti-SARS-CoV-2 immunity. It is known that NAbs against the common coronaviruses last for years, and parallel testing of both neutralizing and total antibodies may be useful for further evaluation of their involvement in the COVID-19 immune response [Citation18].
To date, there is a lack of available reports on seroprevalence rates from Bulgaria. Several serological surveys have been conducted in the neighbouring countries. A study from Romania conducted between July and September 2021 revealed seroprevalence of 41.04% among blood donors [Citation19]. From Greece, a report on seroprevalence among the rural population of Deskati conducted in January 2021 described a prevalence rate of 45% [Citation20]. Another study from Greece was conducted between March and June 2021 among children and their parents and reported seroprevalence rates of 13.8% and 16.9%, respectively [Citation21]. A serological study from Serbia among healthcare workers was conducted between March and June 2022 and reported 92.96% overall seroprevalence [Citation22].
Limitations
As a limitation of this study, we note the relatively small number of participants. The survey represents data only within one settlement.
Conclusions
COVID-19 serological studies provide critical information on infection prevalence, immunity, vaccine effectiveness, transmission dynamics, and the impact of public health interventions. This is the first study of this kind conducted in Bulgaria. We found high levels of specific anti-SARS-CoV-2 antibodies in two studied groups, healthcare workers (99%) and the general population (79%). In addition, it is necessary to perform large-scale serosurveys to provide an analysis of the seroprevalence in a larger population and its dynamics over time.
Authors’ contributions
Conceptualization: I.C. and I.T.; experimental work: T.G. and V.I.; writing—original draft preparation: I.T. and K.N.; visualization: I.T. and K.N.; collection of biological material: M.K.; data analysis: I.T., K.N., M.K. I.C.; writing—review and editing: I.T., K.N., I.C. and V.M.; acquisition of funding: I.C.; supervision: I. C, V.M. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors report no potential conflict of interest.
Data availability statement
The data that support the findings of this study are available on request from the corresponding authors.
Additional information
Funding
References
- WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/.
- Shreffler J, Petrey J, Huecker M. The impact of COVID-19 on healthcare worker wellness: a scoping review. West J Emerg Med. 2020; 21(5):1–5. doi: 10.5811/westjem.2020.7.48684.
- Hiscott J, Alexandridi M, Muscolini M, et al. The global impact of the coronavirus pandemic. Cytokine Growth Factor Rev. 2020;53:1–9. doi: 10.1016/j.cytogfr.2020.05.010.
- Peneva PA. Novel coronavirus—a challenge in diagnosing and timely treatment, and its impact on population health status. Folia Med (Plovdiv). 2021; 63(3):315–320. doi: 10.3897/folmed.63.e56228.
- Marinov GK, Mladenov M, Rangachev A, et al. SARS-CoV-2 reinfections during the first three major COVID-19 waves in Bulgaria. PLoS One. 2022;17(9):e0274509. doi: 10.1371/journal.pone.0274509.
- Bergeri I, Whelan MG, Ware H, et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: a systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022;19(11):e1004107. doi: 10.1371/journal.pmed.1004107.
- Chou R, Dana T, Buckley DI, et al. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173(2):120–136. doi: 10.7326/M20-1632.
- Tabah A, Ramanan M, Laupland KB, PPE-SAFE contributors., et al. Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): an international survey. J Crit Care. 2020;59:70–75. doi: 10.1016/j.jcrc.2020.06.005.
- Müller SA, Wood RR, Hanefeld J, et al. Seroprevalence and risk factors of COVID-19 in healthcare workers from 11 African countries: a scoping review and appraisal of existing evidence. Health Policy Plan. 2022;37(4):505–513. doi: 10.1093/heapol/czab133.
- Grant R, Dub T, Andrianou X, et al. SARS-CoV-2 population-based seroprevalence studies in Europe: a scoping review. BMJ Open. 2021;11(4):e045425. doi: 10.1136/bmjopen-2020-045425.
- Rostami A, Sepidarkish M, Fazlzadeh A, et al. Update on SARS-CoV-2 seroprevalence: regional and worldwide. Clin Microbiol Infect. 2021;27(12):1762–1771. doi: 10.1016/j.cmi.2021.09.019.
- Ahmad AM, Shahzad K, Masood M, et al. COVID-19 seroprevalence in Pakistan: a cross-sectional study. BMJ Open. 2022;12(4):e055381. doi: 10.1136/bmjopen-2021-055381.
- Breedon AME, Saldanha RJ, Salisbury RL, et al. COVID-19 seroprevalence and active infection in an asymptomatic population. Front Med (Lausanne). 2021;8:749732. doi: 10.3389/fmed.2021.749732.
- Koelle K, Martin MA, Antia R, et al. The changing epidemiology of SARS-CoV-2. Science. 2022;375(6585):1116–1121. doi: 10.1126/science.abm4915.
- Madureira R, Ferreira SA, Marion MAL, et al. Seroprevalence of SARS-CoV-2 in emergency department healthcare workers at Sírio-Libanês hospital, Brazil. Health Secur. 2022;20(5):359–367. doi: 10.1089/hs.2022.0045.
- Sims MD, Maine GN, Childers KL, et al. Coronavirus disease 2019 (COVID-19) seropositivity and asymptomatic rates in healthcare workers are associated with job function and masking. Clin Infect Dis. 2021;73(Suppl 2):S154–S162. doi: 10.1093/cid/ciaa1684.
- Cordova E, Bacelar B, Nieto F, et al. SARS-CoV-2 IgG response in symptomatic and asymptomatic COVID-19-infected healthcare workers. Occup Med (Lond). 2021;71(4-5):215–218. doi: 10.1093/occmed/kqab061.
- Movsisyan M, Chopikyan A, Kasparova I, et al. Kinetics of anti-nucleocapsid IgG response in COVID-19 immunocompetent convalescent patients. Sci Rep. 2022;12(1):12403. doi: 10.1038/s41598-022-16402-0.
- Olariu TR, Lighezan R, Ursoniu S, et al. High SARS-CoV-2 seroprevalence in blood donors from Romania after the third COVID-19 pandemic wave. Infect Dis (Lond). 2022;54(7):534–537. doi: 10.1080/23744235.2022.2036811.
- Papagiannis D, Kotsiou OS, Fradelos EC, et al. Work place and prevalence of COVID-19 in a rural population in Greece. Rural Remote Health. 2022;22(3):6751. doi: 10.22605/RRH6751.
- Dimopoulou D, Kyritsi M, Dadouli K, et al. Seroprevalence of anti‑SARS‑CoV‑2 antibodies among children and their parents in Greece. Eur J Pediatr. 2023;182(1):439–449. doi: 10.1007/s00431-022-04681-8.
- Ristić M, Vuković V, Patić A, et al. Seroepidemiology of SARS-CoV-2 virus in healthcare workers before circulation of the omicron sublineages BA.4/BA.5 in Vojvodina, Serbia. Vaccines (Basel). 2022;10(12):2168. doi: 10.3390/vaccines10122168.