Abstract
Randomized controlled trials (RCTs) are typically conducted in highly selected patient populations to ideally demonstrate unconfounded clinical efficacy of a drug. In the real world, there may be confounding factors, such as comorbidities or less frequent monitoring. It has therefore become standard practice in many countries to validate findings from RCTs against available real-world datasets. The present analysis investigated a real-world evidence (RWE) cohort of Bulgarian patients treated with ribociclib in combination with letrozole or fulvestrant and compared the clinical benefit rate (CBR, defined as complete remission [CR] or partial remission [PR] or stable disease [SD]) and progression-free survival (PFS) and overall survival (OS) with pivotal RCTs. Data from 812 patients treated between 2018 and 2022 were used. The number of patients at risk was statistically robust to compare PFS and OS during the earlier periods of the real-world data analysis with the corresponding RCTs. Baseline characteristics (age, hormone receptor status, status of newly diagnosed vs. existing, previous treatments, performance status and tumor stage) were largely comparable between the RWE cohort and the RCTs. The RWE cohorts corroborated RCT findings of a CBR benefit of the ribociclib plus letrozole or fulvestrant combination compared to letrozole or fulvestrant alone. In the periods of adequate statistical robustness, PFS and OS were comparable within 95% confidence intervals (CIs) with RCT findings. This analysis found that patients with comparable characteristics use ribociclib in the real-world similarly to what has been investigated in the RCTs. Real-world effectiveness and outcomes of ribociclib combination therapy were comparable with observations from RCTs.
Introduction
Breast cancer is the most prevalent cancer among women and the second leading cause of cancer-related mortality [Citation1,Citation2]. According to the European Network of Cancer Registries, there were 355,457 new breast cancer cases in the EU-27 in 2020, representing 13.3% of all new cancer cases and 28.7% of all new cancers in women; 91,826 affected women died [Citation3]. These numbers are in line with the prevalence in Bulgaria, with 4061 new cases in 2020, representing 11.1% of all new cancers and 25.5% of new cancers in women [Citation4]. Besides the tremendous impact on the lives of the affected women and their families, breast cancer represents a substantial public health challenge. The five-year survival of breast cancer patients in the EU-27 shows a regional difference with highest survival rates in Northern and Western Europe and the lowest in Eastern Europe, including Bulgaria. Differences in healthcare expenditure and resulting quality of diagnosis and treatment were proposed as explanations for the observed differences in survival on a national level [Citation3].
There have been significant advancements in the treatment of breast cancer over the years, largely due to a better understanding of pathological subtypes and the development of a number of corresponding targeted therapies. Cyclin-dependent kinase 4 or 6 (CDK4/6) are mediators of cell cycle transition from G1 to S phase and are thus important for cancer growth and survival [Citation5,Citation6]. The introduction of CDK4/6 inhibitors has improved the prognosis for women with metastatic hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer, leading to enhanced progression-free survival (PFS), overall survival (OS) and clinical benefit rates (CBR) [Citation7]. The European and national guidelines [Citation8,Citation9] therefore recommend the combination of endocrine therapy and CDK4/6 inhibitors as first-line systemic therapy in such patients. Three CDK4/6 inhibitors, palbociclib, ribociclib and abemaciclib, have received approval from the European Medicines Agency (EMA) [Citation10–12] and are available on the Bulgarian pharmaceutical market all CDK4/6 inhibitors have been included in the Bulgarian Positive Drug List (PDL), with a requirement for monitoring their effectiveness in real-world practice. This monitoring requirement is mandated for new medicines with insufficient data on therapeutic effectiveness or unclear cost-effectiveness for the national healthcare system [Citation9].
Randomized, controlled trials (RCTs), such as PALOMA [Citation13,Citation14], MONALEESA [Citation15–22] and MONARCH [Citation23,Citation24], have shown significant PFS and OS benefits in metastatic breast cancer. Complementing RCT evidence, real-world evidence (RWE) provides valuable insights into the utilization and effectiveness of therapies in unselected patient populations in routine clinical practice.
The objective of this study was to perform a comprehensive analysis of real-world data (RWD) from the Bulgarian population. This analysis involved utilizing deep learning natural language processing algorithms (NLPs) to extract and normalize clinical data from Electronic Health Records (EHRs) of breast cancer patients who were undergoing ribociclib treatment. The study aimed to compare these findings with the primary endpoints of RCTs [Citation25].
Subjects and methods
Ethical considerations
This study was based on secondary usage of anonymized data using a hospital-integrated software. Ethics committee approval or patient informed consent was not required for this type of research as per local Bulgarian regulations.
Data sources
The RWD source for this study consisted of EHRs from 57 university, multispecialty and oncology hospitals in Bulgaria and included both reimbursed and donated therapy prescriptions. Data were extracted from both structured and unstructured free-text via NLP Algorithms. Data analysis was conducted using the Danny Platform (Sqilline Health, Sofia, Bulgaria; https://sqilline.com/products/danny-platform/), an analytic platform that integrates massive amounts of RWD mainly from EHRs with embeded Machine Learning (ML) and NLP algorithms enabeling extraction of free text data from different languages to ensure high data quality.
The retrospective analysis included data from the period of 1 January 2018 to 31 December 2022.
The RCTs used for comparison were MONALEESA-2 [Citation15–17] and MONALEESA-3 [Citation19–21]. Both trials investigated postmenopausal women with hormone receptor-positive and HER2-negative advanced and/or recurrent metastatic breast cancer who had relapsed from initial therapy within 12 months. The MONALEESA-2 trial investigated the efficacy of ribociclib in combination with letrozole vs. letrozole plus placebo. MONALEESA-3 evaluated the efficacy of ribociclib in combination with fulvestrant vs. fulvestrant plus placebo.
Inclusion and exclusion criteria
The present RWE study included patients comparable to MONALEESA-2 and MONALEESA-3. Patients aligning with the MONALEESA-2 cohort received first-line letrozole plus ribociclib therapy or had second-line letrozole plus ribociclib therapy with prior first-line letrozole monotherapy for a duration of less than 6 months. The RWE control group aligning with the MONALEESA-2 placebo group consisted of first-line letrozole monotherapy. Patients aligning with the MONALEESA-3 cohort were those who received first-line ribociclib in combination with fulvestrant or second-line fulvestrant plus ribociclib therapy. The RWE control group comparing with the MONALEESA-3 placebo group consisted of patients receiving first-line fulvestrant monotherapy.
Patients who had received more than one course of ribociclib were excluded from the analysis.
Outcome measures
The present RWE study investigated several endpoints to evaluate treatment outcomes, including PFS (defined as probability of receiving treatment during the period of observation without disease progression or censoring for reason other than death), OS (defined as probability of receiving treatment during the period of observation without censoring for death from any cause), and CBR (defined as complete remission plus partial remission [CR + PR] plus stable disease [SD]).
Statistical considerations
The Wilson score method was utilized to calculate 95% confidence intervals (CIs) for binomial variables. Hazard ratios (HRs) were calculated to estimate the relative effectiveness of the experimental groups (i.e. ribociclib plus letrozole or fulvestrant, respectively) and control groups (i.e. letrozole or fulvestrant monotherapy, respectively). Cox regression models were used to calculate HRs based on RWE, in alignment with the corresponding RCTs, to enable comparison. OS and PFS survival functions were estimated using the Kaplan–Meier (KM; product-limit) estimator, with CIs calculated using Greenwood’s method. To compare treatment outcomes in RWE and RCTs, weighted survival analysis was employed. This analysis aimed at addressing potential biases arising from differences in baseline characteristics between the two groups. The iterative proportional fitting (IPF) algorithm [Citation26] was used to balance selected characteristics before conducting survival analyses. IPF adjusts the underlying distributions based on target distributions, ensuring a balanced comparison. In this study, characteristics, such as ECOG status, ER status, PR status, age, newly diagnosed status, prior chemotherapy (CTx) history, and prior hormonal therapy history were considered for IPF weighting. IPF derives weights for a given characteristic to adjust the underlying distributions in order to match a target distribution.
Results
Patient disposition
Overall, there were 812 patients in the real-world dataset who were treated with ribociclib. The RWE cohort comparable to MONALEESA-2 consisted of 189 patients (n = 177 first-line letrozole plus ribociclib, n = 12 had second-line letrozole plus ribociclib). The RWE control group mirroring the MONALEESA-2 placebo group consisted of 118 patients.
The RWE cohort comparable to MONALEESA-3 consisted of 123 patients (n = 120 first-line fulvestrant plus ribociclib and n = 3 s-line fulvestrant plus ribociclib). The RWE control group equivalent to the MONALEESA-3′ placebo group consisted of 82 patients.
Supplemental Table S1 shows the distribution of patients across the years of the study period.
Patient demographics and disease characteristics
RWE vs. MONALEESA-2
Age and hormone receptor status were comparable between the RWE cohort and the MONALEESA-2 RCT. However, the percentage of newly diagnosed patients was higher in the RWE cohort (47.1%) compared to the RCT (34.1%). The RWE cohort had slightly more severe ECOG status and TNM stages were more broadly distributed. Fewer patients in the RWE cohort had prior adjuvant or neoadjuvant chemotherapy or endocrine therapy (Supplemental Table S2).
RWE vs. MONALEESA-3
Age, hormone receptor status and the ratio of newly diagnosed vs. existing patients were comparable between the RWE cohort and MONALEESA-3. The RWE cohort had slightly more patients with ECOG 1 and TNM stages were more broadly distributed. Fewer patients in the RWE cohort had prior adjuvant or neoadjuvant chemotherapy, while prior endocrine therapy use was similar (Supplemental Table S3).
Clinical benefit rate
RWE vs. MONALEESA-2
In the RWE cohort, 42 patients (22.2%) had no response assessments recorded at the time of data extraction. In MONALEESA-2, 82 patients (24.5%) did not have a response assessment (i.e. were non-CR-non-progressive disease [NCRNPD] or unknown). Of the patients with response assessments, the CBR was 90.5% (95% CI: 84.7 − 94.2%) in the RWE cohort compared with 92.1% (95% CI: 88.1 − 94.8%) in MONALEESA-2.
RWE vs. MONALEESA-3
In the RWE cohort, 37 patients (30.1%) had no response assessment, whereas in MONALEESA-3, 118 patients (24.4%) had no response assessment (i.e. were NCRNPD or unknown). Of the patients with a response assessment, the CBR was 82.6% (95% CI: 73.2 − 89.1%) in the RWE cohort and 86.9% (95% CI: 83.0 − 90.0%) in MONALEESA-3.
Progression-free survival
RWE vs. MONALEESA-2
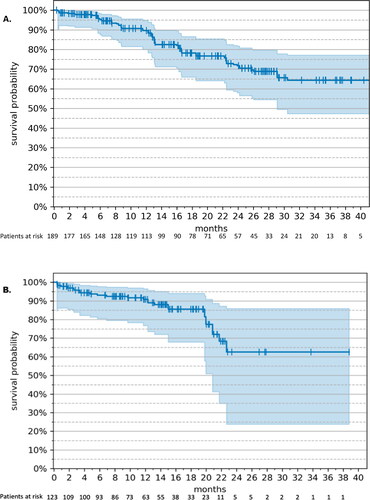
In the RWE cohort, the median PFS was 20.4 (95% CI: 12.1 – not estimable [NE]) months () at a median duration of ribociclib treatment of 6.8 months (207 d). PFS rates over time are shown in Supplemental Table S4(A). In MONALEESA-2, the median PFS was 25.3 (95% CI: 23.0–30.3) months at a median duration of follow-up of 26.4 months. During the period of observation, 64 patients (33.9%) progressed in the RWE cohort, whereas 140 patients (41.9%) progressed in MONALEESA-2.
Figure 1. Progression-free survival – RWE cohort vs. MONALEESA-2 (17). (A) Kaplan–Meier estimates. The tick marks represent censored patients; the shaded area represents 95% CI. (B) Weight-adjusted hazard ratio, ribociclib + letrozole (n = 189) vs. letrozole monotherapy (n = 118). Drug: ribocilib + letrozole; Baseline: letrozole monotherapy; ECOG: Eastern Cooperative Oncology Group; ER: estrogen receptor; PR: progesterone receptor; +: positive; −: negative.
The weight-adjusted HR of PFS in the RWE group receiving ribociclib plus letrozole vs. letrozole monotherapy () was 0.52 (95% CI: 0.35–0.77). In MONALEESA-2, the HR for PFS of ribociclib plus letrozole vs. letrozole plus placebo was 0.57 (95% CI: 0.46–0.70). In the RWE cohort, all subgroups of age, ECOG, and hormone receptor status tended to favor ribociclib-based treatment. The 95% CIs for ECOG 0 and estrogen/progesterone receptor-negative status spanned 1.0 and the HR was therefore not statistically significant.
RWE vs. MONALEESA-3
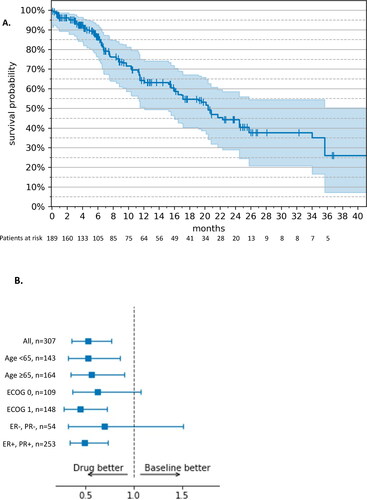
In the RWE cohort, the median PFS was NE (95% CI: 9.0 months – NE) () at a median duration of ribociclib treatment of 5.9 months (179 d). The PFS rates over time are shown in Supplemental Table S4(B). In MONALEESA-3, the median PFS was 20.5 months (95% CI: 18.5 − 23.5) at a median duration of study drug exposure of 15.8 months. Disease progression was observed in 33 patients (26.8%) in the RWE cohort and 210 patients (43.38%) in MONALEESA-3.
Figure 2. Progression-free survival – RWE cohort vs. MONALEESA-3 (19). (A) Kaplan–Meier estimates. The tick marks represent censored patients; the shaded area represents 95% CI. (B) Weight-adjusted hazard ratio, ribociclib + fulvestrant (n = 123) vs. fulvestrant monotherapy (n = 82). Drug: ribocilib + fulvestrant; Baseline: fulvestrant monotherapy; ECOG: Eastern Cooperative Oncology Group; ER: estrogen receptor; PR: progesterone receptor; +: positive; −: negative.
The weight-adjusted HR of PFS in the RWE group receiving ribociclib plus fulvestrant vs. fulvestrant monotherapy () was 0.64 (95% CI: 0.35 − 1.18). In MONALEESA-3, the HR for PFS of ribociclib plus fulvestrant vs. fulvestrant plus placebo was 0.73 (95% CI: 0.59 − 0.90) at a median of 56.3 months of follow-up. In the RWE cohort, all subgroups of age, ECOG and hormone receptor status (except ECOG − 1) tended to favor ribociclib-based treatment. For all but two subgroups (age < 65 years, ECOG 0), the 95% CIs spanned 1.0 and the HR was, therefore not statistically significant.
Overall survival
RWE vs. MONALEESA-2
In the RWE cohort, OS was NE (95% CI: 29.1 months – NE) () at a median duration of follow-up of 15.2 months. The OS rates over time are shown in Supplemental Table S5(A). In MONALEESA-2 the median OS was 63.9 (52.4 − 71.0) months at a median follow-up of 80 months. During the period of observation, 40 patients (21.2%) died in the RWE cohort, whereas 181 patients (54.2%) died in MONALEESA-2.
RWE vs. MONALEESA-3
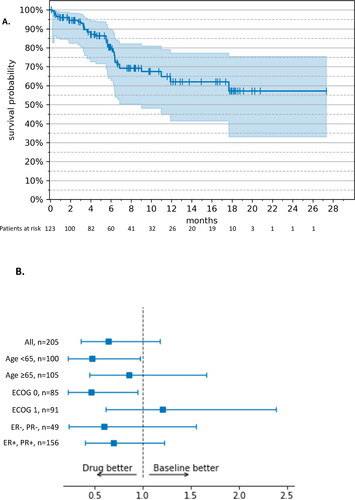
In the RWE cohort, the median OS was NE (95% CI: 20.8 months – NE) () at a median duration of follow-up of 12.3 months. The OS rates over time are shown in Supplemental Table S5(B). In MONALEESA-3, the median OS was 53.7 (95% CI: 46.9 – not reached [NR]) months at a median follow-up of 56.3 months. In the RWE cohort, 20 patients (16.3%) died compared with 222 (45.9%) in MONALEESA-3.
Discussion
This analysis compared the outcomes of patients receiving ribociclib in combination with letrozole or fulvestrant in Bulgarian clinical practice with the respective RCTs MONALEESA-2 [Citation15–17] and MONALEESA-3 [Citation19–21], overall and by subgroup. This analysis found adequate effectiveness of ribociclib combination therapy in the unselected Bulgarian real-world population.
For the combination of ribociclib with letrozole, the CBR was similar in the RWE cohort compared to the RCT (90.5 vs. 92.1%). The KM PFS curves were comparable (i.e. the median PFS of the RCT resided within the CI of the PFS of the RWE cohort) up to month 16 (60% vs. approx. 68%), after which time the number of patients at risk in the RCT became very small. The KM OS curves were similar up to month 6 (95% vs. approx. 98%), after which timepoint the KM curve of the RWE cohort steadily declined to below the placebo group of the RCT.
For the combination of ribociclib with fulvestrant, the CBR was somewhat lower than in the RCT (82.6 vs. 86.9%). The KM curve for PFS in the RWE cohort was similar to the MONALEESA-3 RCT throughout the period of observation, but the number of patients at risk in the RWE cohort steadily decreased and fell below 20 at 16 months. The KM curve for OS in the RWE cohort was similar to the RCT up to month 16 (86 vs. 89%), and steeply dropped thereafter due to the low number of patients at risk for OS in the RWE cohort. The proportion of progressed (26.8% vs. 43.4%) or deceased (16.3 vs. 45.9%) patients was lower in the RWE cohort compared to the RCT. This is probably due to the vast difference in the duration of follow-up, which allowed a substantially longer time in the RCT for events to occur, compared to the RWE cohort.
Studies investigating the effectiveness of ribociclib in routine clinical practice are available from Brazil [Citation27], Spain [Citation28,Citation29] and Greece [Citation30]. These real-world studies consistently found that ribociclib was effective in routine clinical practice. PFS and OS data vary strongly between studies; however, OS data were not provided for the Brazilian and Spanish studies, and the duration of treatment and follow-up was mostly not provided for the real-world studies and tended to be very long in the RCTs.
The Brazilian study was conducted in patients with hormone receptor-positive, HER2-negative metastatic breast cancer who received a CDK4/6 inhibitor [Citation27]. The patients receiving ribociclib (n = 42), mostly received a combination with letrozole (n = 29, 69.0%) or fulvestrant (n = 11, 26.2%), mostly in first metastatic treatment line (n = 33, 78.6%). In this setting, the patients receiving a ribociclib-based regimen, had a CBR of 95.2% and a median PFS of 28 (95% CI: 13.1 − 42.9) months. OS was not analysed in the Brazilian study.
Data from Spain were reported by Fernández-Cuerva et al. [Citation29] and García‑Trevijano Cabetas et al. [Citation28]. Fernández-Cuerva et al. [Citation29] primarily investigated the ribociclib safety in 53 patients with hormone receptor-positive, HER2-negative metastatic breast cancer. They found a median PFS, defined as the time from start of treatment to the date of last contact, death or start of the subsequent treatment) of 27.3 (95% CI: 20.8 − 71.8) months; OS was not assessed. García‑Trevijano Cabetas et al. [Citation28] assessed the effectiveness of CDK4/6 inhibitors in hormone receptor-positive metastatic breast cancer patients. The 28 patients receiving ribociclib mostly received the drug in first-line (67.9%) where it was administered in combination with letrozole (n = 24), fulvestrant (n = 3) and exemestane (n = 1). During the period of observation of that study, the median PFS was NR in patients receiving ribociclib. The PFS rates were 78.6% and 68.9% among the patients receiving ribociclib at 12 and 18 months. OS was not mature at the time of the data cut.
The Greek study [Citation30] reported data from 64 patients with advanced hormone receptor-positive, HER2-negative breast cancer who received a CDK4/6 inhibitor in combination with endocrine therapy (the distribution of letrozole and fulvestrant is not detailed by type of CDK4/6 inhibitor), at any line of treatment. They found a median PFS of 13.5 (95% CI: 11.1 − 18.1) months and a median OS of 29.9 (95% CI: 23.0–NR) months.
The comparison of RWE and RCTs in the present real-world setting in Bulgaria has some limitations. The duration of treatment and duration of follow-up in the RWE cohort was limited by the window of observation of the study. The RWE cohort observed patients with differing durations of treatment and follow-up, depending on the timepoint of treatment start and availability for observation of each individual patient during the observed period of 1 January 2018 to 31 December 2022 (see Supplemental Table S1). The duration of follow-up in the RWE cohort was therefore substantially shorter than in the corresponding RCT populations, which has a large impact on the analysis of PFS, OS and death rates. In Bulgarian real-world clinical practice, routine follow-up and outcomes assessment is often not conducted at the frequency (routinely approximately every 6 months) and level of accuracy which is standard for RCTs. Therefore, there may be a discrepancy in the documented outcome and partial remissions may be classified as SDs or vice versa and best response may not be adequately captured. In our analysis, we observed similar CBRs in the RWE as compared to MONALEESA-2 and MONALEESA-3, but the partial remission rates were consistently lower and the SD rates were consistently higher than reported for the RCTs (data not shown). It is assumed that these differences arise from the quality of imaging available to doctors in the real-world, or may be consistent with a clinical goal of disease stabilization in the advanced/metastatic setting. A certain degree of selection bias might have been introduced by excluding all patient records that had missing data. However, these numbers were small (of 209 eligible patients for comparison with MONALEESA-2, 189 were included; of 146 patients eligible for comparison with MONALEESA-3, 123 were included) and are not considered to impact the overall results or our study.
Our patient cohort was retrieved from 57 university, multispecialty and oncology hospitals in Bulgaria. This breadth of data sources suggests a high degree of generalizability of our findings to the wider Bulgarian clinical practice.
Conclusions
Patients receiving ribociclib in combination with letrozole or fulvestrant in the real-world clinical practice seem to be able to attain acceptable CBR, PFS and OS, while noting that available real-world data is heterogeneous in terms of patient population and outcomes. The shorter duration of treatment and follow-up observation in the RWE setting, the smaller number of patients at risk beyond certain time points, and some discrepancies in the way outcome parameters are assessed in clinical practice, limit the robustness of some comparisons. Further research and validation are needed to better understand these differences and ensure the reliability of RWE in evaluating treatment outcomes.
Clinical trial registry
Not applicable.
Author contributions
MM, AS, BZ, DP: Conceptualization, Writing – Original draft preparation, Reviewing; DP, MH – Methodology, Software, Writing – Original draft preparation; JA, RM, AD, MV, AS, DA – Validation, Writing – Reviewing and Editing; ST, MV, AS – Supervision, Validation, Writing – Reviewing and Editing. All authors have read and approved the final version of the manuscript.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Qualified researchers may obtain anonymized data from this study from the corresponding author upon reasonable request.
Additional information
Funding
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1–9. doi: 10.1016/j.ejca.2012.12.027.
- Surveillance E, and End Results Program. Cancer stat facts: breast. 2023. Available from: https://seer.cancer.gov/statfacts/html/breast.html
- European Commission. Breast cancer burden in EU-27. 2020. Available from: https://ecis.jrc.ec.europa.eu/pdf/Breast_cancer_factsheet-Oct_2020.pdf
- The Global Cancer Observatory. GLOBOCAN - cancer fact sheet: Bulgaria. 2021. Available from: https://gco.iarc.fr/today/data/factsheets/populations/100-bulgaria-fact-sheets.pdf
- He Y, Liu Z, Qiao C, et al. Expression and significance of WNT signaling components and their target genes in breast carcinoma. Mol Med Rep. 2014;9(1):137–143. doi: 10.3892/mmr.2013.1774.
- Lamb R, Lehn S, Rogerson L, et al. Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle. 2013;12(15):2384–2394. doi: 10.4161/cc.25403.
- Shimoi T, Sagara Y, Hara F, et al. First-line endocrine therapy for postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and meta-analysis. Breast Cancer. 2020;27(3):340–346. doi: 10.1007/s12282-020-01054-7.
- Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019.
- Republic of Bulgaria. National council on prices and reimbursement of medicinal products. 2023. Available from: https://www.ncpr.bg/en/
- EMA. Ibrance: EPAR - product information [internet]. Amsterdam, Netherlands: EuropeanMedicines Agency; 2016. Last updated: 06/06/2023. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/ibrance
- EMA. Kisqali: EPAR - product information [internet]. Amsterdam, Netherlands: European Medicines Agency; 2017. Last updated: 25/08/2023. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/kisqali
- EMA. Verzenios: EPAR - product information [internet]. Amsterdam, Netherlands: European Medicines Agency; 2018. Last updated: 12/07/2023. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/verzenios
- Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. doi: 10.1056/NEJMoa1810527.
- Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373(3):209–219. doi: 10.1056/NEJMoa1505270.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709.
- Hortobagyi GN. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res. 2018;20(1):123. doi: 10.1186/s13058-018-1050-7.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386(10):942–950. doi: 10.1056/NEJMoa2114663.
- Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-Negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi: 10.1200/JCO.2018.78.9909.
- Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021;32(8):1015–1024. doi: 10.1016/j.annonc.2021.05.353.
- Slamon DJ, Neven P, Chia SKL, et al. Updated overall survival (OS) results from the phase III MONALEESA-3 trial of postmenopausal patients (pts) with HR+/HER2- advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB). J Clin Oncol. 2021;39(15):1001–1001. doi: 10.1200/JCO.2021.39.15_suppl.1001.
- Tripathy D, Im S-A, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi: 10.1016/S1470-2045(18)30292-4.
- Sledge GW, Jr., Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- Advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. doi: 10.1200/JCO.2017.73.7585.
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5(1):5. doi: 10.1038/s41523-018-0097-z.
- Boyang Z. Clinical data extraction and normalization of cyrillic electronic health records via deep-learning natural language processing. J Clin Oncol Clin Cancer Informatics. 2019;3:1–9.
- El-Khorazaty JA, Koch G, Preisser J. The iterative proportional fitting algorithm for adjusted agreement in a non-inferiority diagnostic clinical trial. Pharm Stat. 2014;13(3):173–178. doi: 10.1002/pst.1614.
- Queiroz MM, Sacardo KP, Ribeiro MF, et al. Real-world treatment outcomes in HR + HER2- metastatic breast cancer patients treated with CDK4/6 inhibitors: results from a reference center in Brazil. Cancer Treat Res Commun. 2023;35:100683. doi: 10.1016/j.ctarc.2023.100683.
- García-Trevijano Cabetas M, Lucena Martínez P, Jiménez Nácher I, et al. Real-world experience of palbociclib and ribociclib: novel oral therapy in metastatic breast cancer. Int J Clin Pharm. 2021;43(4):893–899. doi: 10.1007/s11096-020-01193-z.
- Fernández-Cuerva C, Chinchilla-Alarcón T, Alcaraz-Sánchez JJ. Real-world effectiveness of ribociclib in metastatic breast cancer patients: does dose affect survival? J Oncol Pharm Pract. 2022;10781552221144280. doi: 10.1177/10781552221144280.
- Fountzilas E, Koliou GA, Vozikis A, et al. Real-world clinical outcome and toxicity data and economic aspects in patients with advanced breast cancer treated with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors combined with endocrine therapy: the experience of the hellenic cooperative oncology group. ESMO Open. 2020;5(4):e000774. doi: 10.1136/esmoopen-2020-000774.