?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Mumps is an acute, contagious, viral vaccine-preventable disease caused by mumps virus (MuV). The measles, mumps, rubella (MMR) vaccine that is used in many countries is considered highly effective with decreased MuV incidence, but suboptimal MuV long-term immunity. This study assessed the MuV seropositivity and antibody titre among vaccinated children in Bulgaria to provide evidence for a better understanding of MuV circulation and immunity in Bulgaria. The samples from 734 immunized children (369 females and 365 males) aged 1 to 16, divided into four age groups (≥1, 2–6, 7–11, and 12–16) were tested. Qualitative and quantitative indirect enzyme-linked immunosorbent assay (Anti-Mumps IgG ELISA, Euroimmun, Germany) was performed to determine the mumps IgG antibody levels in sera. Among all participants, protective MuV immunity was identified in 93%. MuV IgG antibody positivity ranged between 87% in the age group between 1 and 2 years and 96% in the age group 12–16 years, but no statistically significant difference was found among age groups. At the same time, there was a statistically significant difference (p = 0.01) between seropositivity in male and female participants, with male participants having an overall seropositivity of 90% and female 95%. Among the antibody-positive samples, the quantitative measurements of median anti-MuV IgG concentrations showed that titres decreased with increasing age. A slightly waning immunity following vaccination was observed, but positivity remained high among vaccinated children over the years. Similar studies show that maintaining high immunity is crucial to prevent mumps outbreaks.
Keywords:
Introduction
Mumps (epidemic parotitis) is an acute human infectious disease caused by an enveloped single-stranded RNA virus (mumps virus, MuV) belonging to the Paramyxoviridae family, with only a single serotype. The MuV infection has an incubation period of 2 to 4 weeks, average 21 days. Clinical symptoms include an acute febrile disease with nonspecific prodromal symptoms followed by painful swelling of one or both parotid salivary glands. Normally mumps is a mild, self-limiting disease and disappears without sequelae. However, complications (aseptic meningitis, mastitis, encephalitis, orchitis and oophoritis, sensorineural deafness) may occur. Vaccination with a live attenuated mumps-containing vaccine is the most effective way to prevent mumps, and the incidence of mumps has significantly decreased in countries where large-scale MuV vaccination has been carried out [Citation1–4].
In Bulgaria, MuV immunization was initiated in 1971. In 1986–1987, temporary suspension of vaccination led to a large epidemic with 102241 cases, and another large outbreak took place in 1997–1999 (40662 cases). Since 2002, in Bulgaria, like in many of the countries on the European continent, mumps immunization has been carried out solely with a three-component vaccine (MMR - measles, mumps and rubella) in two doses—at 13 months of age and at 12 years of age. The mumps component of the MMR vaccine is about 88% (range: 32%–95%) effective when a person gets two doses; one dose is about 78% (range: 49%–92%) effective [Citation5–7]. Since 2002, the last large outbreak in the country was in 2007–2009 (11992 cases) [Citation8], and since then, circulation has continued at lower intensity [Citation9].
According to national data, 94 cases of mumps have been laboratory confirmed in the country in the last five years (2018–2022). Among them, 51 cases (54% of all cases) were reported among children aged 1 to 16 years, some of whom, were immunized [Citation9]. This is an expected phenomenon, considering that a small percentage of immunized children may not develop immunity, as the mumps component of the MMR vaccine is 78% effective after a single dose and 88% effective after two doses [Citation4]. Of note, 31 cases (33% of all cases) were registered in the group of 5–9 year olds [Citation9]. The identification of MuV risk groups is extremely important because it is recommended to carry out MMR booster vaccination in some countries, in case of frequent outbreaks [Citation10].
This study was carried out in 2021–2022, with the aim to assess the seropositivity and antibody titre among vaccinated children in Bulgaria, in order to provide evidence for a better understanding of MuV circulation and immunity against MuV among immunized children in the country.
Subjects and methods
Ethics statement
This study was approved by the Ethical Committee at the National Centre of Infectious and Parasitic Diseases, Sofia, Bulgaria (International Ethical Committee; no. 00006384/2021). The clinical samples used were residual material. The studies are in line with ethical norms with respect for confidentiality and safety rules and it is not required to collect informed consent from the studied patients or their parents and legal guardians.
Samples
In 2021–2022, residual material from serum samples from patients testing for pertussis-unrelated health conditions was collected by diagnostic laboratories both within and outside hospitals across the whole country. Sample collection was organized by the Regional health authority of each region, under instruction from the Ministry of Health, with the aim to obtain a representative sample of patient sera, stratified by region and age group, in order to assess measles and rubella seroprevalence in the country. Limited case-based data was collected from patients providing the samples, including data on age, sex, and MMR vaccination status. The present study used residual materials from specimens collected with regard to this national seroepidemic study. Inclusion criteria for this study were: sufficient material to carry out additional laboratory tests; age group 1–16; data on vaccination status indicating that the patient had been vaccinated with one or two doses of MMR. The patients whose samples did not meet these conditions were excluded from the study.
The serum samples were stored at 2 °C–8 °C and transported in a cold chain to the National Reference Laboratory (NRL) of Measles, Mumps, Rubella, Department of Virology at the National Centre of Infectious and Parasitic Diseases (NCIPD), Sofia. For a long period of time, specimens were stored at −20 °C (freezing).
Laboratory methods
The laboratory samples were tested at the NRL of Measles, Mumps, Rubella. Qualitative and quantitative indirect enzyme-linked immunosorbent assay (Anti-Mumps IgG enzyme-linked immunosorbent assay (ELISA), Euroimmun, Germany, cat. no. EI 2630-9601 G, 96 break-off wells test kit format) was performed to determine the mumps IgG antibody levels in sera. The ELISA plates were incubated in an Memmert incubator (Germany) and automatic plate washing was done using Rayto ELISA Microplate Washer RT-3100, Labexchange, Germany.
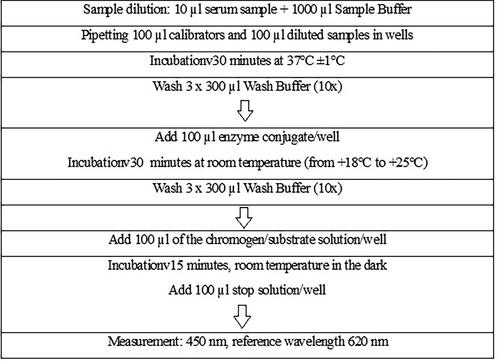
The tests and results interpretation were performed in accordance with the manufacturer’s instructions () and also based on previous studies and validated methodology [Citation11, Citation12].
Figure 1. Test procedure for detection of anti-MuV IgG antibodies in human serum by ELISA, Euroimmun, Germany.
The absorbance values (optical density, O.D.; 450 nm, reference wavelength 620 nm) measurement was performed using a multimode reader (EPOCH, BioTek Instruments, Inc, USA).
The O.D. values of the tested samples were divided by the mean absorbance values of the cut-off (calibrator) and the results were interpreted qualitatively as positive, negative, or equivocal.
Ratio (R) <0,8: Negative
Ratio (R) ≥0,8 to 1,1: Equivocal
Ratio (R) ≥1,1: Positive
For antibody-positive samples, quantitative analysis was also performed and the level of protective antibodies in relative units per millilitre (RU/mL) by plotting a standard curve with the extinction of the patient sera and the three calibrators included in the kit was calculated.
<16 RU/mL: Negative
≥16 to <22 RU/mL: Equivocal
≥RU/mL: Positive
The assay specificity was 100% and sensitivity 99.3%, according to the manufacturer.
Data analysis
Participants were stratified into four age groups: ≥1 (n = 23), 2–6 (n = 316), 7–11 (n = 243), and 12–16 (n = 152). We calculated overall and group-specific percent seropositivity and 95% confidence intervals (Wilson score intervals by online program Proportion Confidence Interval Calculator). To compare seropositivity among the different groups under investigation, we used the Fisher’s exact test by online calculator (Easy Fisher Exact Test Calculator) and considered differences as significant at the level of p ≤ 0.05.
Results
The study measured samples from a total of 734 immunized children, including 369 (50.3%) female and 365 (49.7%) male. The mean age of the participants was 7.35 (StDev 4.03 years, range 1–16 years). Based on the data provided for the participants, those in the age groups ≥1, 2–6 and 7–11 years of age had received a single MMR dose, and those aged 12 years and over had received two MMR doses. Overall, 93% (95% CI 91%–94%) of participants had antibodies against MuV. Antibody positivity ranged between 87% (95%CI 68%–95%) in the age group of ≥1 years, and 96% (95%CI 92–98) in the age group of 12–16 years. Positivity increased between the age groups ≥1 and 2–6 and decreased after 7 years of age, only to increase again after the second dose at 12 years of age, but no statistically significant difference was found among age groups through the Fisher’s exact test. At the same time, there was a statistically significant difference (p = 0.01) between seropositivity in male and female participants, with male participants having an overall seropositivity of 90% (95%CI 87%–93%) and female participants having an overall seropositivity of 95% (95%CI 92%–97%) ().
Table 1. Demographic characteristics of the study participants (n = 734) and their vaccination status.
To probe the difference between male and female seropositivity further, we analysed the seropositivity by age group separately among males and females. In both males and females, the seropositivity by age group behaved like in the overall sample, decreasing slightly in the age group 7–11 y and increasing again after the second dose in the age group 12–16 y (). Once again, no statistically significant differences were found among age groups (data not shown). Male and female seropositivity did not differ in any of the specific age groups (data not shown). The difference between male and female seropositivity was statistically significant only in the overall sample ().
Table 2. Seropositivity by age group among male and female study participants.
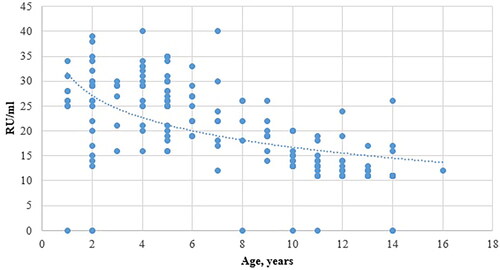
Among the antibody-positive samples (n = 680), the quantitative measurements of the median anti-MuV IgG concentrations showed that with increasing age of children and lengthening of the period since the first dose of MMR vaccine, the concentration of anti-MuV IgG followed a consistently decreasing trend, even after the second dose was administered at age 12 ().
Discussion
At the beginning of 2000, after the introduction of mumps vaccination via two-dose MMR in routine childhood immunization schedules, in all EU/EEA Member States there was a significant reduction in the reported MuV cases compared to the period of campaign immunizations. The reported EU cases in 2017 represent a 7-fold decrease compared to 2000 (92 000 cases) and a 16-fold decrease compared to the peak in 2004 (215 000 cases) [Citation13]. The current circulation of MuV in Europe is due to a combination of incomplete vaccine coverage and waning of post-vaccination immunity over the years among those vaccinated in the absence of a natural boost [Citation14].
The present study was focused on determination of the level of protective immunity against mumps among children in Bulgaria and determination of potential risk groups by age or gender. The estimated total anti-MuV IgG seroprevalence among the 734 children tested was 92.64%.
The lowest percentage of anti-MuV IgG seropositive persons was found in the groups of ≥1 years and 7–11 years, 87% and 91% respectively, but no statistically significant difference was found compared to the total number of tested cases. A small proportion of children in this age groups (as indicated above) have data on one dose MMR vaccine, but not proven anti-MuV IgG antibodies. This can be explained by collection of the samples in a period before the development of full-fledged post-vaccination immunity for the first group and a decrease of the anti-MuV immunity in the group of 7–11 years. Davidkin and Julkunen [Citation15] studied the dynamics of anti-MuV antibody titre after the first and second administration of MMP vaccine. They reported a rise in titres immediately after the first and second doses of MMR vaccine and a rapid decline within a year after the first dose. This corresponds to the data of other authors, who report the weakening of vaccine-induced protective immunity over time, especially in children receiving the first dose of MMR, and raises the issue of administering an additional booster dose of MuV-containing vaccine between 1 and 12 years of age [Citation10]. According to the reports of the European Centre for Disease Prevention and Control (ECDC), Bulgaria, Estonia, Germany, and Poland reported the highest notification rate of mumps cases among those aged five to nine years [Citation13, Citation14]. The national data for a five years’ period showed cumulative incidence per 100 000 population (based on the population in 2022) between 2018 and 2022 of 1.8 in the age group ≥1 y, 9.2 in the age group of 2–6 y, 6.6 in the age group of 7–11 y and 0.9 in the age group of 12–16 y [Citation9]. Over the past five years, ongoing MuV outbreaks among vaccinated adults and children have been reported in both Europe and the United States [Citation16, Citation17]. This could be due to, on one hand, transmission in kindergartens and primary schools and, on the other, decreased humoral immunity against mumps which has been demonstrated to take place some years after the first dose [Citation18]. The resurgence of mumps among vaccinated individuals may be due to both waning immunity and a potential lack of immunity to the circulating wild-type strain. A number of studies have reported the presence of amino acid variations between the circulating MuV wild-type strains and vaccine strains. This suggests a difference in cross-reactivity which is caused by the MuV vaccine strain (Jeryl Lynn strain, MuV genotype A) [Citation19, Citation20]. Mossong et al. [Citation21] developed mathematical models according to which the immunity formed by the post-vaccination protective MuV IgG antibodies progressively decreases in the population not infected with wild MuV strains.
Regarding gender, 5% of the tested females were anti-MuV IgG seronegative, compared to 10% anti-MuV IgG seronegative males, with a statistically significant difference (p = 0.01) between seropositivity in male and female participants. These data are similar to those reported by Mahallawi et al. [Citation22], who calculated a higher risk of being seronegative for men compared with women (OR = 1.629; 95% CI: 0.705–3.764). In the analysis of the national reports about mumps confirmed cases in the period of 2018–2022, the cumulative incidence among males from 1 to 16 years of age was 6.1, compared to 4.2 among females [Citation9].
This study is a pilot for Bulgaria regarding the evolution of protective immunity against mumps over time among vaccinated children.
Conclusions
The present study showed that there was high seropositivity in terms of protection against mumps among vaccinated children from different regions of the country. However, the study identified a potentially important mumps immunity gap in the adolescent male population. Maintaining high immunity and conducting national serological surveys are crucial to prevent mumps outbreaks, reduce disease severity, and achieve measles and rubella elimination goals.
Author contributions
SK guarantees the integrity of the entire study, conceptualized and designed the study, interpreted data, supervised, wrote and reviewed the manuscript; RS performed the experimental studies and interpreted data; SS and KP edited the manuscript, designed the study and analysed the data; PGK performed the clinical studies, edited the manuscript and performed literature research. All authors have read and approved the final version of the manuscript.
Disclosure statement
All co-authors reported no conflict of interest.
Data availability statement
The anonymized data that support the findings from this study are available from the corresponding author [SK] upon reasonable request.
Additional information
Funding
References
- Hviid A, Rubin S, Muhlemann K. Mumps. Lancet. 2008;371(9616):1–6. doi: 10.1016/S0140-6736(08)60419-5.
- Sun X, Tang F, Hu Y, et al. High risk of mumps infection in children who received one dose of mumps-containing vaccine: waning immunity to mumps in children aged 2–5 years from kindergartens in Jiangsu province, China. Hum Vaccin Immunother. 2020;16(7):1738–1742. doi: 10.1080/21645515.2019.1708162.
- Better Health Channel. Mumps [Internet]. Australia: department of Health, State Government of Victoria. Last Reviewed 08-11-2018; Available from: https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/mumps.
- Lam E, Rosen JB, Zucker JR. Mumps: an update on outbreaks, vaccine efficacy, and genomic diversity. Clin Microbiol Rev. 2020; 33(2):e00151–19. doi: 10.1128/CMR.00151-19.
- Centers for Disease Control and Prevention. Mumps. Vaccine preventable diseases. Page last reviewed: 26.01.2021. Available from: https://www.cdc.gov/vaccines/vpd/mumps/index.html.
- Centers for Disease Control and Prevention. Epidemiology and prevention of Vaccine-Preventable diseases. Hamborsky J, Kroger A, Wolfe S, Eds. 13th ed. Washington D.C: Public Health Foundation, 2015. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/mumps.pdf
- Latner DR, McGrew M, Williams NJ, et al. Estimates of mumps seroprevalence may be influenced by antibody specificity and serologic method. Clin Vaccine Immunol. 2014;21(3):286–297. doi: 10.1128/CVI.00621-13.
- Kojouharova M, Kurchatova A, Marinova L, et al. Mumps outbreak in Bulgaria, 2007: a preliminary report. Euro Surveill. 2007;12(12):3162. doi: 10.2807/esw.12.12.03162-en.
- National Measles, Mumps, Rubella Surveillance System in Bulgaria. Ministry of Health. National Center of Infectious and Parasitic Diseases. Available from: https://mmr.gateway.bg/disease/par.php.
- Marlow MA, Marin M, Moore K, et al. CDC guidance for use of a third dose of MMR vaccine during outbreaks. J Public Health Manag Pract. 2020;26(2):109–115. doi: 10.1097/PHH.0000000000000962.
- Krumova S, Andonova I, Stoitsova S, et al. Prevalence of measles IgG antibodies among healthcare workers in Bulgaria. Probl Infect Parasit Dis. 2021;49(2):14–19. doi: 10.58395/pipd.v49i2.64.
- Stefanova R, Genova-Kalou P, Andonova I, et al. Healthcare workers in Bulgaria – are they protected from vaccine-preventeble infections? Probl. Inf. Parasit. Dis. 2022;50, 3:19–23.
- Mumps Annual Epidemiological Report on communicable Diseases in Europe [accessed on 18 May 2021]; Available online: https://www.ecdc.europa.eu/en/publications-data/mumps-annual-epidemiological-report-2017.
- https://www.ecdc.europa.eu/sites/default/files/documents/mumps-annual-epidemiological-report-2018.pdf.
- Davidkin I, Valle M, Julkunen I. Persistence of anti-mumps virus antibodies after a two-dose MMR vaccination: a nine-year follow-up. Vaccine. 1995;13(16):1617–1622. doi: 10.1016/0264-410x(95)00064-8.
- Ferenczi A, Gee S, Cotter S, et al. Ongoing mumps outbreak among adolescents and young adults, Ireland, august 2018 to january 2020. Euro Surveill. 2020;25(4):2000047. doi: 10.2807/1560-7917.ES.2020.25.4.2000047.
- Moncla LH, Black A, Debolt C, et al. Repeated introductions and intensive community transmission fueled a mumps virus outbreak in Washington state. Elife. 2021;10:e66448. doi: 10.7554/eLife.66448.
- Rasheed MAU, Hickman CJ, Mcgrew M, et al. Decreased humoral immunity to mumps in young adults immunized with MMR vaccine in childhood. Proc Natl Acad Sci U S A. 2019;116(38):19071–19076. doi: 10.1073/pnas.1905570116.
- Frost JR, Shaikh S, Severini A. Exploring the mumps virus glycoproteins: a review. Viruses. 2022; 14(6):1335. doi: 10.3390/v14061335.
- Won H, Kim AR, Yoo JS, et al. Cross-neutralization between vaccine and circulating Wild-Type mumps viruses in korea. Vaccine. 2021;39(13):1870–1876. doi: 10.1016/j.vaccine.2021.01.039.
- Mossong J, Nokes DJ, Edmunds WJ, et al. Modeling the impact of subclinical measles transmission in vaccinated populations with waning immunity. Am J Epidemiol. 1999;150(11):1238–1249. doi: 10.1093/oxfordjournals.aje.a009951.
- Mahallawi WH, Kurdi MM, Ibrahim NA. Serostatus of IgG antibody against mumps virus in adult population of Al Madinah Al munawarah, Saudi Arabia. Saudi Med J. 2021; 42(8):862–868. doi: 10.15537/smj.2021.42.8.20210228.