Abstract
The impact of COVID-19 vaccination on hospitalizations in individuals with solid malignancies remains uncertain. This nationwide retrospective study aims to clarify this issue. We analyzed data from 1,126,946 confirmed COVID-19 cases between March 2020 and June 2022, obtained from the Ministry of Health’s United Information Portal. Outcomes were compared between fully vaccinated and non-vaccinated cohorts, stratified by sex, age and viral variant. Among the 1,126,946 confirmed COVID-19 cases, 0.53% (n = 6025) had solid malignancies and comorbidities. Of these, 31.3% (n = 1797) had sole solid malignancies. In this subgroup, 40% (n = 764) required hospitalization, with a median age of 63 years. Unadjusted univariate analysis showed a significant reduction in hospitalization rates among COVID-19 vaccinated patients with isolated solid malignancies [OR 0.2776 (95% CI 0.1621 to 0.4753); p < 0.0001], especially in those aged over 63 [OR 0.5981 (95% CI 0.4244 to 0.8429); p = 0.0033]. Lower ICU admission rates favored vaccinated cohorts [OR 0.3418 (95% CI 0.2023 to 0.5777); p = 0.0001]. These reductions were observed exclusively with mRNA-based vaccines [OR 0.5845 (95% CI 0.4333 to 0.7884); p = 0.0004]. Statistically significant vaccination benefits were found only within the omicron cohort [OR 0.2307 (95% CI 0.13687 to 0.3890); p < 0.0001]. The results from the analysis suggest that individuals with sole solid malignancies face an increased risk of hospitalization and ICU admission. However, these risks can be mitigated through vaccination, particularly with an mRNA-based vaccine, especially in those aged 63 years or older.
Introduction
There is a prevailing uncertainty surrounding the connection between cancer and COVID-19 outcomes. Although there is a general consensus that cancer patients face an elevated risk, the available studies have presented inconsistent findings, particularly in terms of hospitalization rates. These discrepancies warrant further exploration and analyses to provide clarity on this critical issue [Citation1,Citation2].
These discrepancies in understanding the relationship between cancer and COVID-19 outcomes can be attributed to a multitude of factors. Variations in study design, the size of the participant groups, the specific demographics of the patients involved, and the diverse types of cancer being studied all play pivotal roles in shaping these differing conclusions [Citation3,Citation4]. Furthermore, the influence of various cancer treatments on the outcomes of COVID-19 has added to the complexity, with conflicting evidence stemming from research – some studies indicate an elevated risk associated with certain treatments, while others fail to establish a significant correlation. This intricate web of factors underscores the need for further investigations to unravel the precise dynamics between cancer, its treatments, and COVID-19 outcomes [Citation2,Citation5–7].
In order to shed light on this complex issue, an extensive nationwide retrospective analysis was conducted in this study. This analysis was purposefully focused on a subset of patients who had a solid malignancy as their exclusive chronic comorbidity. The significance of uncovering these findings extends beyond the realm of research; they have the potential to substantially influence clinical decision-making and the formulation of public health strategies for effectively managing COVID-19 in this specific patient demographic [Citation8]. The insights gleaned from this study hold promise for enhancing the quality of care and safeguarding the well-being of individuals grappling with both cancer and the challenges posed by the ongoing pandemic.
Subjects and methods
Ethics statement
Approval for this retrospective analysis was granted by the Bulgarian Ministry of Health (document number 94-4750 from 09.11.2022).
Patient cohort criteria
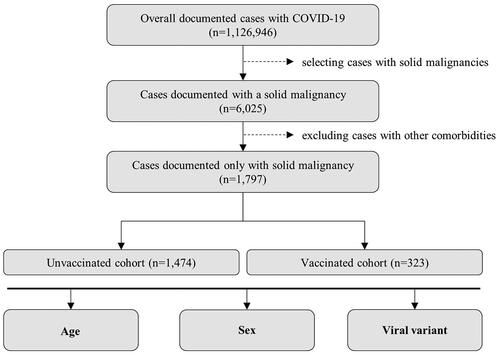
This retrospective multicenter real-world study involved an initial cohort of 1,126,946 individuals confirmed as COVID-19 patients between March 2020 and June 2022. Medical professionals conducted disease confirmation using polymerase chain reaction (PCR) or antigen testing, both in ambulatory and hospital settings. To gather the necessary data for our analysis, a formal request for access was meticulously submitted to the United Information Portal of the Bulgarian Ministry of Health [Citation9]. This portal served as the repository of information, aggregating data from all 28 provinces within Bulgaria.
The principal objective of this study was to perform a comparative analysis of outcomes among distinct cohorts, with a specific focus on individuals who presented with a solid malignancy as their sole chronic comorbidity. In order to provide such an assessment, we employed stratification techniques based on key factors, including sex, age, vaccination status and viral variant (). Patients were classified as fully vaccinated when they had received either two doses of an mRNA-based vaccine (i.e. BNT162b2 or CX-024414), two doses of ChAdOx1-SARS-COV-2, or one dose of Ad26.COV2-S. Since most patients included in this analysis had received a single type of vaccine, our study did not specifically investigate differences in vaccination regimens within the vaccinated cohort (Supplementary Table S1).
Figure 1. The patient cohort selection process was standardized across both groups. The isolated patient groups were required to have a solid malignancy as their only documented pathology. Any duplicate cases, if identified, were excluded from the study. These cohorts were then further stratified based on criteria such as sex, age and viral variant.
Data analysis
For the statistical analysis, we employed SPSS version 27.0 (SPSS, Chicago, IL, USA) as the chosen analytical tool. To examine the relationships between variables, we applied Pearson’s Chi-Square test. To quantify the association between COVID-19 vaccination and mortality, while accounting for multiple factors, we calculated the odds ratio (OR). This statistical measure served as a robust indicator of the strength and direction of this association. In our interpretation of the results, we used a p-value framework to determine statistical significance. Specifically, a p-value of p ≤ 0.05 indicated statistical significance, p ≤ 0.01 denoted a high level of statistical significance, and p ≤ 0.001 represented a very high degree of statistical significance. These thresholds were instrumental in our assessment of the significance of the findings in the context of the study.
Results
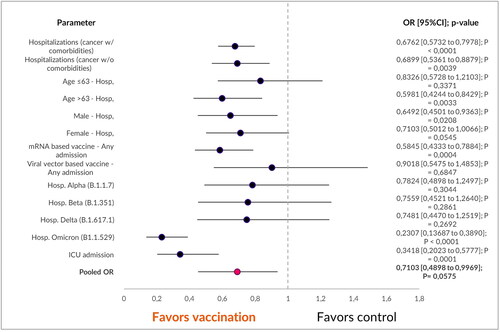
Out of the total 1,126,946 confirmed COVID-19 patients, 0.53% (n = 6025) were diagnosed with both a solid malignancy and at least one additional comorbidity, encompassing cardiovascular, metabolic, chronic pulmonary and immune-related disorders. The unadjusted univariate statistical analysis () demonstrated a substantial reduction in hospitalizations among COVID-19 vaccinated patients (unvaccinated n = 1630/3305; vaccinated n = 281/708) [OR 0.6762 (95%CI 0.5732 to 0.7978); p < 0.0001].
Table 1. Confirmed COVID-19 cases in cancer patients by vaccination status and hospitalizations indexed between March 2020 and June 2022.
Within this subset, 31.3% (n = 1797) had a solid malignancy as their only documented pathology, with a median age of 63 years. Among these individuals, COVID-19 vaccinated patients with an isolated solid malignancy exhibited a significant reduction in hospitalizations [OR 0.2776 (95% CI 0.1621 to 0.4753); p < 0.0001]. This reduction was most pronounced in the cohort aged over 63 years (unvaccinated n = 390/803; vaccinated n = 61/169) [OR 0.5981 (95% CI 0.4244 to 0.8429); p = 0.0033].
Both males (unvaccinated n = 305/647; vaccinated n = 55/149) [OR 0.6492 (95%CI 0.4501–0.9363); p = 0.0208] and females (unvaccinated n = 326/827; vaccinated n = 55/174) [OR 0.7103 (95%CI 0.5012–1.0066); p = 0.0545] benefited unequally from vaccination, demonstrating reduced hospitalization rates. Intensive-care unit admissions were lower in the vaccinated cohort (unvaccinated n = 195/1474; vaccinated n = 16/323) [OR 0.2307 (0.13687–0.3890); p < 0.0001]
When comparing the main vaccine platforms and hospitalization rates, vector-based (unvaccinated n = 631/1474; vaccinated n = 27/67) and mRNA-based (unvaccinated n = 631/1474; vaccinated n = 70/230), statistically significant reductions in hospitalizations were observed exclusively with mRNA-based vaccines [OR 0.5845 (95%CI 0.4333–0.7884); p = 0.0004].
In the examination of different viral variants, our analysis unveiled vaccination advantages, which were statistically significant exclusively within the omicron variant cohort [OR 0.2307 (95% CI 0.13687–0.3890); p < 0.0001]. Moreover, when we conducted a pooled analysis, encompassing all factors, it demonstrated an overall benefit that favored the vaccinated group. This benefit manifested as a significant reduction in hospitalization rates among patients grappling with solid malignancies [OR 0.7103 (95% CI 0.4898–0.9969); p < 0.05] ().
Discussion
To the best of our knowledge, this study stands as the inaugural real-world investigation that delves specifically into the outcomes of COVID-19 among patients who have a solid malignancy as their exclusive coexisting health condition. In essence, our research adds a vital piece to the existing mosaic of scientific literature by offering substantial insights into the repercussions of COVID-19 on individuals confronting the challenges of a solid malignancy as their sole comorbidity. It is imperative to acknowledge the existence of conflicting evidence within this field of study, underscoring the complexity of the issue. Our findings should be interpreted within the context of these disparities, as the body of scientific knowledge regarding this intersection of COVID-19 and solid malignancies is continually evolving.
When interpreting our findings, it is crucial to take into account the considerable variability in study design, sample sizes, patient demographics, and the diverse spectrum of cancer types. These variables can significantly influence how our results compare to the existing conflicting evidence. The disparities observed in the literature may, in part, stem from differences in research methodologies and the characteristics of the patient populations under investigation.
To address this potential source of variability, our study took a deliberate approach by focusing exclusively on patients who had a solid malignancy as their sole comorbidity. This stringent criterion aimed to homogenize the patient cohort, reducing the impact of confounding factors and making our findings more directly comparable.
Our research revealed a noteworthy trend of increased hospitalization rates among patients with a solid malignancy, lending support to the notion that this particular population faces an elevated risk when it comes to COVID-19. Importantly, these observations align closely with the trends documented in a significant amount of published literature, reinforcing the credibility and clinical relevance of the outcomes of our study [Citation10–14].
Patients receiving palliative treatment exhibited higher mortality rates, suggesting the influence of underlying health status, disease stage and limited treatment options on COVID-19 outcomes [Citation13,Citation15]. Furthermore, patients who had received chemotherapy within the last 30 days showed a decrease in mortality rates, emphasizing the potential impact of recent treatment on immune status and susceptibility to severe COVID-19 and the development of the often fatal immune phase of the disease in both vaccinated and unvaccinated patients [Citation16–18]. Some studies have shown that patients undergoing active chemotherapy experienced no discernible effect on mortality rates [Citation7,Citation19].
Our study unveils a significant safeguarding impact of vaccination in reducing hospitalizations during infections attributed to the omicron variants of SARS-CoV-2. Notably, the emergence of the omicron variant marked a discernible overall decrease in the virus’s pathogenicity [Citation20]. Intriguingly, this period of interest synchronously aligned with the commencement of booster vaccinations, which served to reinforce and fortify the observed favorable outcomes linked to vaccination in the context of mitigating admissions attributed to the omicron variant [Citation21].
Limitations
Our study is characterized by certain inherent limitations that warrant consideration. First, the retrospective design we adopted introduces constraints related to data collection and the potential for biases. Our reliance on pre-existing medical records and databases may have inadvertently introduced selection biases, as the available data might not comprehensively encompass all relevant variables or outcomes. To enhance the robustness of our evidence, it is imperative to underscore the necessity of prospective studies equipped with meticulously crafted protocols. Such studies are poised to provide more resilient and dependable insights.
However, it is crucial to mention the dynamic and ever-evolving nature of SARS-CoV-2 and the persistent challenges posed by an ongoing pandemic. The virus’s rapid evolution, the emergence of new variants, and the perpetually shifting epidemiological landscape collectively pose obstacles to conducting prospective studies in real-time.
Second, our study did not categorize patients based on cancer stages or tumor type. It is essential to acknowledge that these factors can exert a profound influence on their overall health status and treatment outcomes. The absence of this stratification represents a limitation in our capacity to offer a more nuanced analysis of the impact of these factors.
Finally, the choice to pursue univariate analysis was driven by specific constraints that influenced our decision-making process. The dataset at our disposal exhibited certain limitations, chiefly related to the variables at our disposal and their completeness. These constraints limited our ability to execute multivariate analysis with optimal effectiveness. Furthermore, it is worth noting that in datasets with a limited number of events, engaging in multivariate analysis may introduce the risk of overfitting, potentially undermining the overall reliability of our findings.
Recognizing these limitations, we advocate for future research endeavors to address these complexities and enhance our understanding of the intricate interplay between COVID-19, cancer, and their respective treatments.
Conclusions
Individuals with sole solid malignancies face elevated risks of hospitalization and ICU admission, particularly benefiting from vaccination with mRNA-based vaccines, especially if aged 63 or older. Patients receiving palliative care exhibited higher mortality, while the impact of recent chemotherapy varied. Despite the retrospective nature of our study due to challenges in conducting prospective analyses, our research contributes a valuable piece to the complex relationship between COVID-19 and cancer, underscoring the importance of ongoing research and adaptability in this ever-evolving landscape.
Ethics approval
Approval for this retrospective analysis was granted by the Bulgarian Ministry of Health – document number 94-4750 from 09.11.2022.
Authors’ contributions
GD and TV designed, implemented the study, analyzed the data and wrote the manuscript. RA verified the validity of the study. All authors have read and approved the final version of the manuscript.
Supplemental Material
Download PDF (596.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available upon formal request from the Ministry of Health of the Republic of Bulgaria. Access to these data is subject to restrictions and was obtained with permission for this specific study. For data access, please visit https://coronavirus.bg/ in compliance with the Ministry of Health’s policies and guidelines.
Additional information
Funding
References
- Khoury E, Nevitt S, Madsen WR, et al. Differences in outcomes and factors associated with mortality among patients with SARS-CoV-2 infection and cancer compared with those without cancer: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):1. doi: 10.1001/jamanetworkopen.2022.10880.
- Chavez-MacGregor M, Lei X, Zhao H, et al. Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. JAMA Oncol. 2022;8(1):69–6. doi: 10.1001/jamaoncol.2021.5148.
- Horn L, Whisenant JG, Torri V, et al. Thoracic cancers international COVID-19 collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival. J Clin Oncol. 2020;38(18_suppl):LBA111–LBA111. doi: 10.1200/JCO.2020.38.18_suppl.LBA111.
- Manzano J-GM, Muthu M, Kheder E, et al. Hospitalization characteristics and outcomes of patients with cancer and COVID-19 at a comprehensive cancer center. Support Care Cancer. 2022;30(9):7783–7788. doi: 10.1007/s00520-022-07209-w.
- Wu Q, Luo S, Xie X. The impact of anti-tumor approaches on the outcomes of cancer patients with COVID-19: a meta-analysis based on 52 cohorts incorporating 9231 participants. BMC Cancer. 2022;22(1):241. doi: 10.1186/s12885-022-09320-x.
- Sengar M, Chinnaswamy G, Ranganathan P, et al. Outcomes of COVID-19 and risk factors in patients with cancer. Nat Cancer. 2022;3(5):547–551. doi: 10.1038/s43018-022-00363-4.
- Aboueshia M, Hussein MH, Attia AS, et al. Cancer and COVID-19: analysis of patient outcomes. Future Oncol. 2021;17(26):3499–3510. doi: 10.2217/fon-2021-0121.
- Ali JK, Riches JC. The impact of the COVID-19 pandemic on oncology care and clinical trials. Cancers. 2021;13(23):5924. doi: 10.3390/cancers13235924.
- Health BMo. United Informatio Portal 2023. [2023 Feb 09]. Available from: https://coronavirus.bg/.
- Lei H, Yang Y, Zhou W, et al. Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis. Lung Cancer. 2021;157:60–65. doi: 10.1016/j.lungcan.2021.05.002.
- Kathuria-Prakash N, Antrim L, Hornstein N, et al. Factors associated with hospitalization among breast cancer patients with COVID-19: a diverse multi-center Los Angeles cohort study. Clin Breast Cancer. 2022;22(4):e558–e66. doi: 10.1016/j.clbc.2021.12.005.
- Abuhelwa Z, Alsughayer A, Abuhelwa AY, et al. In-hospital mortality and morbidity in cancer patients with COVID-19: a nationwide analysis from the United States. Cancers. 2022;15(1):222. doi: 10.3390/cancers15010222.
- de Azambuja E, Brandão M, Wildiers H, et al. Impact of solid cancer on in-hospital mortality overall and among different subgroups of patients with COVID-19: a nationwide, population-based analysis. ESMO Open. 2020;5(5):e000947. doi: 10.1136/esmoopen-2020-000947.
- Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. doi: 10.1016/S1470-2045(20)30442-3.
- Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011.
- Lee LYW, Starkey T, Ionescu MC, et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 2022;23(6):748–757. doi: 10.1016/S1470-2045(22)00202-9.
- Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022;19(1):92. doi: 10.1186/s12985-022-01814-1.
- Monari C, Sagnelli C, Maggi P, et al. More severe COVID-19 in patients with active cancer: results of a multicenter cohort study. Front Oncol. 2021;11:662746. doi: 10.3389/fonc.2021.662746.
- Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/S0140-6736(20)31173-9.
- Shuai H, Chan JF-W, Hu B, et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 omicron. Nature. 2022;603(7902):693–699. doi: 10.1038/s41586-022-04442-5.
- Chalkias S, Harper C, Vrbicky K, et al. A bivalent omicron-containing booster vaccine against covid-19. N Engl J Med. 2022;387(14):1279–1291. doi: 10.1056/NEJMoa2208343.