Abstract
The studies of rejuvenation are important for promoting health and longevity, preventing age-related diseases, reducing economic burdens, improving quality of life and addressing the challenges posed by an aging global population. This review explores the intersection of aging, regeneration and bioelectric fields examining the emerging role of membrane potential in processes like cell proliferation, differentiation, limb regeneration and potentially aging. Manipulation of membrane potential opens a novel dimension to the rejuvenation landscape offering an alternative or complementary approach to partial reprograming method presenting exciting possibilities for therapeutic interventions targeting age-related cellular changes.
Background on aging and hallmarks of aging
Aging is a complex biological process that affects most multicellular organisms, including humans. It is characterized by a gradual decline in physiological functions, increased vulnerability to age-related diseases and heightened risk of mortality [Citation1]. Understanding the mechanisms underlying aging has been a longstanding pursuit in biology and medicine. In 2013, a paper by López-Otín and colleagues [Citation2] introduced the concept of the ‘hallmarks of aging’. These hallmarks represent key processes and features associated with aging, serving as a framework for comprehending the multifaceted nature of this phenomenon (). The original hallmarks of aging, as described in the paper, included genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion and altered intercellular communication [Citation2].
Figure 1. The hallmarks of aging. These key factors represent a framework proposed to comprehensively describe the complex processes that contribute to aging at the cellular and molecular levels. The hallmarks include: Loss of proteostasis (decline in the cellular machinery responsible for maintaining protein homeostasis); Mitochondrial dysfunction (deterioration in mitochondrial function, including reduced energy production and increased generation of reactive oxygen species); Stem cell exhaustion (reduced regenerative capacity due to the depletion or dysfunction of stem cells); Cellular senescence (accumulation of cells that have ceased dividing and often secreting pro-inflammatory molecules); Altered intercellular communication (changes in the communication between cells, leading to the chronic low-grade inflammation known as inflammaging); Genomic instability (accumulation of DNA damage over time, leading to mutations); Telomere attrition (shortening of telomeres with each cell division); Deregulated nutrient sensing (impaired metabolic function as a result of dysregulation of nutrient-sensing signaling pathways). According to the prevailing opinion, one of the identified hallmarks of aging - Еpigenetic changes (alterations in epigenetic marks, such as DNA methylation and histone modifications) most likely has a leading role and controls the aging processes. Modified from López-Otín et al. [Citation2].
![Figure 1. The hallmarks of aging. These key factors represent a framework proposed to comprehensively describe the complex processes that contribute to aging at the cellular and molecular levels. The hallmarks include: Loss of proteostasis (decline in the cellular machinery responsible for maintaining protein homeostasis); Mitochondrial dysfunction (deterioration in mitochondrial function, including reduced energy production and increased generation of reactive oxygen species); Stem cell exhaustion (reduced regenerative capacity due to the depletion or dysfunction of stem cells); Cellular senescence (accumulation of cells that have ceased dividing and often secreting pro-inflammatory molecules); Altered intercellular communication (changes in the communication between cells, leading to the chronic low-grade inflammation known as inflammaging); Genomic instability (accumulation of DNA damage over time, leading to mutations); Telomere attrition (shortening of telomeres with each cell division); Deregulated nutrient sensing (impaired metabolic function as a result of dysregulation of nutrient-sensing signaling pathways). According to the prevailing opinion, one of the identified hallmarks of aging - Еpigenetic changes (alterations in epigenetic marks, such as DNA methylation and histone modifications) most likely has a leading role and controls the aging processes. Modified from López-Otín et al. [Citation2].](/cms/asset/2e3fef19-ed1e-4b9c-a137-fabbc2c1ce9a/tbeq_a_2358999_f0001_c.jpg)
Maintaining the integrity of the genome is crucial for an organism’s survival and well-being. Yet, the genome is constantly under assault from various internal and external factors. Internal factors include normal cellular processes such as DNA replication and recombination, which can introduce errors [Citation3]. External factors encompass exposure to environmental stressors like ionizing radiation, chemical toxins, and reactive oxygen species (ROS) generated during metabolic processes [Citation4]. These stressors can inflict various types of damage on DNA, including single-strand breaks, double-strand breaks, and chemical alterations to the bases [Citation5,Citation6]. Genomic instability is a hallmark of aging that stems from the cumulative effect of these DNA-damaging processes over time. While cells possess a remarkable repertoire of DNA repair mechanisms to correct these damages, the efficiency of these processes can diminish with age. Cells may struggle to repair damage accurately, leading to an increased likelihood of mutations or unrepaired DNA lesions. Genomic instability does not merely affect the genome; it also reverberates throughout cellular function. Dysfunctional genes and regulatory elements resulting from DNA damage and mutations can lead to errors in protein synthesis, disrupted signaling pathways, and impaired cellular processes. This can, in turn, contribute to cellular dysfunction and compromise the overall health of tissues and organs.
Genomic instability is closely intertwined with another hallmark of aging—telomere attrition. Telomeres are specialized structures located at the ends of linear chromosomes in eukaryotic cells. They serve a vital function: to protect the genetic information contained within the chromosome. Telomeres consist of repetitive DNA sequences (TTAGGG in humans) and associated proteins, forming a protective cap akin on the ends of chromosomes [Citation7]. Telomeres act as a kind of ‘molecular clock’ that limits the number of times a cell can divide and this phenomenon is often referred to as the ‘Hayflick limit’ [Citation8]. During each cell division, a portion of the telomere is lost due to the way DNA replication works. When telomeres become critically short, cells enter a state of replicative senescence. In this state, cells remain metabolically active but lose their ability to divide and function normally. Cellular senescence is associated with aging-related pathologies and contributes to tissue dysfunction. The cumulative impact of telomere attrition on individual cells affects tissues and organs on a systemic level. Over time, the decline in cellular function and the reduced regenerative capacity of tissues contribute to the aging phenotype [Citation9].
Another hallmark of aging is the loss of proteostasis. The term proteostasis refers to the precise regulation of protein synthesis, folding, trafficking and degradation within a cell. Maintaining proteostasis is essential because proteins are the workhorses of cellular function, responsible for executing virtually every biological process. As an organism ages, the efficiency of protein folding can diminish. This can lead to the production of misfolded or improperly folded proteins. Misfolded proteins are often dysfunctional and can aggregate, forming toxic protein clumps that interfere with normal cellular processes [Citation10]. These aggregates are a hallmark of several age-related neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease [Citation11]. Aging is also associated with a decline in the capacity of cells to degrade and clear damaged or misfolded proteins. The proteasome and lysosome, two major protein degradation systems, may become less efficient with age [Citation12]. As a result, the accumulation of damaged proteins can occur, contributing to cellular dysfunction and the aging phenotype. Moreover, chaperone function declines with age, making it more difficult for cells to fold newly synthesized proteins correctly. This can lead to the production of misfolded proteins and contribute to proteostatic imbalance [Citation13].
Arguably one of the most universal strategies for slowing down the aging process has been the reduction of the caloric intake, which leads to an increase in both average and maximum lifespan across different species ranging from worms to primates [Citation14]. And this is not a coincidence since it dramatically improves nutrient sensing, the deregulation of which is one of the hallmarks of aging. Deregulation of nutrient sensing encompasses several key aspects, one of which is insulin resistance. With age, cells may become less responsive to insulin, which leads to elevated blood sugar levels. Insulin resistance leads to type 2 diabetes and is closely linked to aging [Citation15]. Although the exact mechanisms underlying the progressive decline in insulin sensitivity with age are not yet fully understood, a study by Guo et al. [Citation16] in 2022 identified membrane-bound matrix metalloproteinase 14 (MT1-MMP/MMP14) as a significant factor regulating insulin sensitivity during aging. As individuals age, MMP14 activity increases progressively in insulin-sensitive tissues, leading to the cleavage of Insulin Receptors and subsequent suppression of insulin signaling. Additionally, Guo et al. [Citation16] demonstrated that inhibiting MT1-MMP restores Insulin Receptor expression, thereby improving insulin sensitivity in aged mice [Citation16]. Another key aspect of the deregulation of nutrient sensing is the mechanistic target of rapamycin (mTOR) pathway.mTOR is an evolutionary conserved serine/threonine kinase, with homologues found from yeast to mammals. In mammals and higher eukaryotes, mTOR is part of two multiprotein complexes—mTOR complex 1 (mTORC1) and 2 (mTORC2), which differ functionally and structurally and in their sensitivity to rapamycin and regulate essentially all aspects of anabolic metabolism. Many nutrient sensing pathways involved in longevity like insulin/insulin-like growth factor 1 (IGF-I) signaling (IIS) network converge at the mTOR pathway which makes it a central player affecting the aging process. One of its functions is to sense nutrient abundance to promote cell growth, but it can become chronically activated in aging cells. This persistent activation may contribute to the development of age-related diseases and conditions, including cancer and neurodegenerative disorders [Citation17]. AMP-activated Protein Kinase (AMPK) is another key player in the nutrient sensing pathways. AMPK is a sensor of cellular energy status. When cellular energy (in the form of ATP) is low, AMPK becomes activated and triggers processes to conserve energy and increase nutrient uptake. Recent research suggests that the effectiveness of AMPK signaling diminishes noticeably as individuals age [Citation18,Citation19].
Mitochondrial dysfunction represents yet another hallmark of aging, emphasizing the progressive decline in mitochondrial function as an organism grows older. With age, mitochondrial efficiency in generating ATP tends to diminish. This can lead to reduced cellular energy levels, impacting the functionality of tissues and organs. Cells may also become less capable of adapting to increased energy demands. Also, mitochondria are a primary source of reactive oxygen species (ROS), which are chemically reactive molecules that can damage many cellular components like DNA, proteins and lipids including the mitochondria itself. This creates a vicious cycle where dysfunctional mitochondria generate more ROS, worsening mitochondrial damage and further elevating the mitochondrial disfunction. While increased levels of mitochondrial ROS may not be the direct cause of aging according to existing evidence, they do contribute significantly to the development of age-related diseases when combined with other factors, such as epigenetic alterations or a decrease in quality control systems [Citation20]. Another aspect of mitochondrial dysfunction is disruption of the mitochondrial calcium regulation with age, potentially affecting cellular calcium signaling and leading to altered cellular functions and increased vulnerability to stress [Citation21].
As a hallmark of aging, cellular senescence represents the accumulation of senescent cells within tissues and organs as an organism grows older. Over time, various stressors, including DNA damage, telomere attrition and oxidative stress, can lead to the accumulation of senescent cells in tissues. These cells remain metabolically active but are no longer capable of contributing to tissue repair and regeneration [Citation22]. Senescent cells secrete pro-inflammatory molecules as part of the senescence-associated secretory phenotype (SASP) [Citation23]. This can contribute to chronic, low-level inflammation within tissues, known as ‘inflammaging’. Inflammation is a common feature of aging and is linked to the development of age-related diseases [Citation24]. The presence of senescent cells within tissues can impair tissue homeostasis and regenerative capacity by disrupting the normal function of surrounding cells and may contribute to tissue dysfunction and age-related pathologies. While senescence can be detrimental in the context of aging, the traditional view is that it also serves as a protective mechanism against cancer by halting the proliferation of cells with damaged DNA. Although senescence is often seen as a protective mechanism against cancer, emerging evidence suggests that cells undergoing persistent senescence may, in fact, develop characteristics that promote tumorigenesis [Citation25].
Stem cell exhaustion represents yet another hallmark of aging. It is responsible for the diminishing regenerative capacity of tissues as an organism gets older. Over time, the number of functional stem cells in various tissues and organs can decline. This reduction may result from a combination of factors, including decreased stem cell self-renewal, impaired mobilization from their niches, and even programmed cell death [Citation26]. As the population of stem cells diminishes, tissues and organs become less capable of repairing damage and regenerating new cells. This leads to the gradual loss of tissue function and regenerative capacity. Stem cells themselves can become senescent, losing their regenerative potential and contributing to the overall accumulation of senescent cells within tissues [Citation27,Citation28].
Altered intercellular communication is a hallmark of the aging process, accentuating the change in the accuracy and efficiency of cellular signaling as an organism ages. Aging can disrupt the normal functioning of signaling pathways. This may lead to aberrant responses to signals or the activation of detrimental pathways, contributing to age-related diseases. Dysregulated communication between immune cells and other cell types can lead to chronic, low-level inflammation, a condition known as inflammaging as previously discussed [Citation29]. Changes in hormone production and sensitivity with age can disrupt endocrine signaling, affecting metabolism, growth, and other physiological processes. Stem cells receive signals from their microenvironment (niche) to regulate their behavior. Changes in these signaling cues can affect tissue repair and regeneration.
Paradigm shift: epigenetic age as the ultimate hallmark
Over the past decade, research in the field of aging has led to a significant paradigm shift. While the originally proposed framework of nine hallmarks of aging provided valuable insights, recent discoveries have emphasized that one of them, the epigenetic alterations, might be the ultimate hallmark that governs the aging process and overwrites the others. Epigenetic age, often abbreviated as EpiAge, represents a concept in the field of aging research. Unlike chronological age, which is simply a count of years, EpiAge is a measure of biological age based on the epigenetic modifications that accumulate in our cells and tissues over time. These modifications primarily involve DNA methylation patterns, which can be thought of as chemical marks on the DNA molecule that can influence gene activity. To calculate EpiAge, sophisticated algorithms and epigenetic clocks have been developed. These clocks examine the patterns of DNA methylation at specific sites across the genome, known as CpG sites. By comparing these patterns to a reference database of known ages, these clocks can estimate the biological age of a cell, tissue or organism with remarkable accuracy [Citation30]. Importantly, it appears that by resetting the EpiAge of cells by reprograming them to totipotent or pluripotent state, the other hallmarks of aging are also reset to a youthful state [Citation31]. This reprogramming can be done either by somatic cell nuclear transfer (SCNT), which involves transferring a somatic nucleus into an enucleated oocyte and relying on the factors found there to reprogram it to totipotency [Citation32], or through the utilization of Yamanaka factors, which lead to the induction of a pluripotent state in the treated cells. This revolutionary approach, pioneered by Shinya Yamanaka and his team in 2006 [Citation33], utilizes a set of transcription factors that can reprogram differentiated, specialized cells back into a pluripotent state. These factors include Oct4, Sox2, Klf4 and c-Myc (OSKM). Yamanaka’s groundbreaking discovery challenged the traditional dogma that once a cell becomes specialized, it is irreversibly locked into its identity. Yamanaka factors proved that cellular identity is not fixed, and with the right molecular cues, cells can regain pluripotency—the capacity to differentiate into any cell type in the body. However, the process of cellular reprograming to a pluripotent state brings with it not only a reset of the EpiAge but also a loss of cellular identity, and up until recently it was not known if the two processes can be uncoupled.
Partial reprogramming: a bridge to cellular rejuvenation
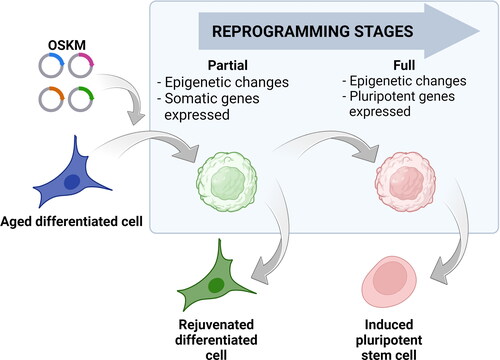
The discovery that the rejuvenation effect can indeed be uncoupled from the identity loss by a process called partial reprograming opened up exciting possibilities for interventions aimed at reversing the aging process. This process involves the controlled activation of Yamanaka factors within differentiated cells, initiating a process of dedifferentiation towards pluripotency. However, unlike full reprogramming to iPS cells, partial reprogramming is strategically halted before cells completely lose their specialized identity (). This means that the cells retain their original functions and characteristics while undergoing epigenetic rejuvenation [Citation34].
Figure 2. Cell reprogramming stages. Reprogramming of differentiated cells (blue cell) with a specific combination of transcription factors: Oct4, Sox2, Klf4 and c-Myc (OSKM) leads to the induction of pluripotency and acquiring the characteristics of pluripotent stem cell (red cell). During the reprogramming process, the cell passes through several stages including changes in DNA methylation patterns and histone modifications. This reshapes the cell’s epigenetic landscape but still leaves specific somatic genes expressed (round green cell). If the reprogramming continues, the resulting changes would lead to the activation of the pluripotent genes and, after their stabilization, the cells will become induced pluripotent cells (red cell). If the effects of Yamanaka factors are terminated before the activation of pluripotent genes, the cell can stabilize in a rejuvenated state (green cell) without losing its somatic identity. Cells in the blue box represent intermediate stages during reprogramming.
Several studies have shown that OSKM-driven age reprogramming can take place without overt dedifferentiation and loss of cellular identity (for review see [Citation31,Citation35]). An in vivo study revealed that the periodic activation of Oct4, Sox2, Klf4 and c-Myc genes, with a pattern of 3 days ‘on’ followed by 4 days ‘off’, in six-month-old wild-type mice over a period of 4 months, resulted in the rejuvenation of age-related characteristics in dentate gyrus cells. Notably, this rejuvenation occurred without an increase in mortality, as reported by Rodríguez-Matellán et al. in 2020 [Citation36]. Another investigation by Ocampo et al. [Citation37] in 2016 demonstrated that a similar cyclical expression of OSKM, with a schedule of 2 days ‘on’ and 5 days ‘off’, in a mouse model exhibiting premature aging, effectively rejuvenated four key hallmarks of aging: cellular senescence, epigenetic modifications, mitochondrial dysfunction, and DNA damage. Importantly, this rejuvenation occurred without significant histological changes. Browder et al. [Citation38] further extended these findings in 2022, illustrating that the 2 days ‘on’, 5 days ‘off’ expression of OSKM in 15-month-old mice experiencing natural aging for 7 months led to a reduction in EpiAge and rejuvenation of the skin, all while maintaining overall health.
One of the primary goals of partial reprogramming is to strike a delicate balance between rejuvenation and dedifferentiation. Too much reprogramming may lead to the loss of cell identity and function, while too little may not achieve the desired rejuvenating effect.
Literature evidence indicates that employing alternating OSKM expression for 35 cycles of 2 days ‘on’ and 5 days ‘off’ (equivalent to 35 weeks) in mice with a single copy of the OSKM transgenes does not result in a higher occurrence of teratomas. However, when subjected to only eight cycles of OSKM expression, animals possessing two copies of the transgenes exhibit escalated cell proliferation and an increased likelihood of teratoma formation in the liver, kidney and pancreas [Citation31,Citation37].
Researchers are actively working to optimize the timing and dosage combination of reprogramming factors to achieve the most effective partial reprogramming protocols. Partial reprogramming holds immense promise for addressing age-related cellular changes. By rejuvenating cells without altering their specialized roles, it offers a unique opportunity to reset the epigenetic clock, potentially reversing age-related hallmarks while maintaining tissue integrity [Citation35]. However, challenges remain in understanding the molecular intricacies of this process and ensuring its safety and efficacy for therapeutic applications.
Bioelectric fields: orchestrators of embryonic development, cellular dynamics, limb regeneration and potentially aging
At its core, bioelectricity is rooted in the movement of ions, particularly sodium (Na+), potassium (K+), calcium (Ca2+) and chloride (Cl-) ions, across cell membranes. This movement generates electrical gradients and voltages, resulting in electrical potentials that can be measured and manipulated. These ionic currents are maintained by various ion channels, pumps and exchangers, which are expressed on cell membranes.
Recent research has introduced a captivating perspective on the role of bioelectric fields in development, regeneration and potentially aging. Beyond their well-established functions in excitable cells, like neurons and muscle cells, bioelectric fields have emerged as pivotal regulators in cellular processes such as proliferation, differentiation, wound healing and limb regeneration. Studies have suggested that these fields play a role in encoding and transmitting information, acting not only as a repository of memory but also as a computational layer through which collectives of non-neural cells can solve problems and have goal directed behavior, much like the collective of nerve cells in our brains [Citation39]. Although the goals of our brain differ dramatically from those of our heart and liver or from a collective of cells in the early embryo attempting to construct an organism, it seems that the underlying principles of computing the solutions to the problems that have to be solved in order to reach those goals are fundamentally the same.
As previously reviewed [Citation40,Citation41], the close association between membrane potential (Vmem) levels and events linked to cell proliferation, such as mitosis, DNA synthesis, and the overall progression of the cell cycle, has been a long-standing observation. Various cell types exhibit resting potentials spanning a broad spectrum, typically ranging from −10 mV to −90 mV, and the positioning of cells along this Vmem scale generally mirrors their proliferative potential [Citation40,Citation42]. Cells characterized by a heightened degree of polarization (a hyperpolarized Vmem), particularly somatic cells, tend to remain in a quiescent state and typically avoid undertaking mitosis [Citation40]. In contrast, cells undergoing development or those associated with cancer often display a lower degree of polarization (a depolarized Vmem) and exhibit heightened mitotic activity [Citation43]. Moreover, cells transitioning from an in vivo environment to in vitro culture tend to go through spontaneous proliferation, accompanied by Vmem depolarization. Similarly, proliferation induced by the malignant transformation of somatic cells is also marked by depolarization of Vmem [Citation43]. It is important to note that changes in membrane potential are not simply a consequence of cells passing through the cell cycle, but can actively change the direction of this transition, that is, actively changing the membrane potential can be used as a mechanism to change cell behavior [Citation40,Citation41].
The state of the membrane potential not only plays a critical role in regulating cell proliferation but can also influence their differentiation. In 2022 Sempou et al. [Citation44] showed that depolarizing the cell membranes of early Xenopus frog embryos by artificially increasing etxracellular levels of K+ ions prevents them from differentiation and delays gastrulation. They also demonstrated the same phenomenon in human embryonic stem cells in which low Vmem (depolarization) blocks differentiation and high Vmem (hyperpolarization) promotes differentiation by inhibition of mTOR [Citation44].
These findings underscore the dynamic interplay between membrane potential and various cellular states, shedding light on the role of Vmem as a crucial determinant in understanding the complex landscape of cell proliferation and differentiation.
One of the most fascinating aspects of bioelectricity is its role in regeneration. The process of limb regeneration in axolotls commences with the initiation of blastema formation, a pivotal stage in which a cluster of dedifferentiated cells assembles, with the ultimate purpose of regenerating the missing segment of the limb. Following a phase of extensive cellular proliferation and subsequent differentiation, the nascent limb becomes fully functional. However, a critical aspect to underscore is that even though the cells within the blastema undergo dedifferentiation to a state reminiscent of their embryonic origin, they retain a remarkable memory of their lineage [Citation45,Citation46]. Research has elucidated that cells originating from dedifferentiated nerve cells maintain their identity as nerve cells, while those originating from muscle cells persist as muscle cells, and so forth. This phenomenon bears a striking resemblance to the techniques employed in partial reprogramming, as discussed earlier, where cells are reprogrammed (dedifferentiated) by forced expression of Yamanaka factors to a more embryonic-like state with the purpose of rejuvenating their epigenome to a more youthful state without losing their cellular identity.
Another very useful model for studying regeneration is the larval stage of the amphibian Xenopus laevis. Many of the bioelectric signals that govern the process of regeneration and blastema formation have been extensively researched in this model using approaches such as fluorescent reporter dyes [Citation47] and ion-selective vibrating probes [Citation48]. Using these methods, it has been shown that the process of regeneration requires several specific bioelectric events to occur in a precise order and within certain timeframes. When the tail is amputated, the normal regeneration bud becomes depolarized. During the 24-h post-amputation window, the regeneration bud has to be repolarized for the regeneration process to proceed. Normally, this is achieved through the flux of H+ ions driven endogenously by the action of the V-ATPase pump, which is specifically upregulated in existing wound cells by 6 h post-amputation. If this rapid repolarization fails to occur, regeneration does not occur [Citation49,Citation50]. Notably, induction of H+ flux is sufficient to rescue the regeneration process in otherwise non-regenerative conditions, even when using a non-native H+ pump, such as the yeast PMA H+ pump. These results show that as long as the cell membrane is repolarized during the specific time window, regeneration can be rescued despite the means used to achieve this [Citation49,Citation50].
These observations expand the resemblances between the events during the process of partial reprogramming and those occurring in the process of regeneration, as they are both time-sensitive and require at least the following two steps. Firstly, the cells have to partially dedifferentiate, which is probably not only coupled but induced by their depolarization. And secondly, the cells must be pushed back towards their differentiated state, which seems to be achieved by their rapid repolarization occurring in a specific time window.
As pointed above, bioelectric fields are not only a consequence of regenerative processes but can actively instruct and guide tissue repair and growth. As an example, in the process of limb regeneration observed in some amphibian species, gradients of bioelectric fields are established along the regenerating limb. These bioelectric gradients effectively serve to ‘map’ the locations of various tissues and cell types. This spatial information plays a crucial role in guiding the accurate development of the new limb, encompassing the positioning of bones, muscles, and nerves. Moreover, altering the bioelectric fields through exposure of the limb stub to an ionophore cocktail designed to selectively modify the bioelectric state can initiate the regenerative growth of an entire limb in adult Xenopus frogs that are normally non-regenerative [Citation51]. Furthermore, scientific studies have demonstrated that manipulation of the bioelectric fields can lead to remarkable outcomes. Disruption of the Xenopus laevis homolog of Kir2.1, a channel linked to Anderson-Tawil Syndrome in humans, results in craniofacial defects. Optogenetic activation of a non-specific cation channel or a hydrogen pump, causing similar Vmem changes as Kir2.1 disruption, reproduces these defects. Conversely, disruption of a sodium-hydrogen exchanger, anticipated to be electroneutral and have no impact on Vmem, does not disturb craniofacial development [Citation52,Citation53].
In 2012, Pai et al. [Citation54] showcased the potential for organ-level reprogramming through manipulation of endogenous bioelectric patterns via forced expression of specific ion channels. The induced change in the targeted membrane potential (Vmem) by the expression of these ion channels in the frog embryo resulted in the development of ectopic structures, such as complete eyes, even in regions typically incapable of forming eyes, such as the gut [Citation54]. Notably, the induction of ectopic eyes was not restricted to a single ion channel construct; various constructs leading to the same change in Vmem gave rise to the same ectopic eye formation [Citation54]. Those findings indicate that developmental significance lies in the variations of Vmem across cells, rather than specific ion channels or ions [Citation52,Citation53].
While the precise mechanism by which alterations in membrane potential are converted into epigenetic changes remains not entirely comprehended, existing data strongly suggests that fluctuations in cytosolic calcium concentration, triggered by voltage or ligand gated Ca2+ channels, significantly contribute to this phenomenon. As a second messenger calcium modulates a plethora of cell processes often via binding to calmodulin, which regulates calmodulin kinases (CaMKs). As an illustration, a rise in cytoplasmic calcium triggers the activation of the serine/threonine phosphatase, calcineurin, through the calcium–calmodulin pathway. Calcineurin, in turn, dephosphorylates NFAT family members, enabling their translocation into the nucleus. Once in the nucleus, they collaborate with activator protein 1 (AP-1) to modulate the transcription of a diverse set of genes in a cell-type-dependent manner [Citation55]. Additional examples illustrate how shifts in the cytosolic concentration of Ca2+ can modify the epigenetic state of cells by influencing various classes of histone acetyltransferases (HATs) and deacetylases (HDACs) leading to specific histone modifications [Citation56,Citation57].
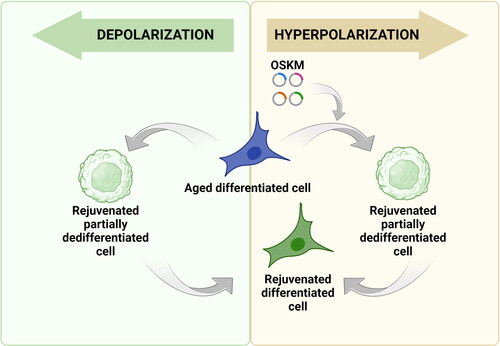
Partial reprogramming techniques have shown great promise in rejuvenating aging cells. However, concerns regarding the loss of cell identity during this process necessitate the development of innovative approaches to enhance the efficiency of reprogramming while safeguarding cellular identity. One potential avenue involves exploring the fact that artificially depolarizing the cell membrane leads to dedifferentiation and potentially reprogramming of the epigenome to a more youthful state, much like in the process of partial reprogramming using Yamanaka factors ().
Figure 3. Potential applications of membrane potential change in cell reprogramming. Yellow box: Since hyperpolarization is associated with the differentiated cell state, there is a possibility that the experimental increase in membrane potential can be used to slow down and even stop the process of cellular reprogramming carried out with Yamanaka factors. Such ‘stretching’ of the process creates an opportunity to stabilize cells before the activation of pluripotency genes, but after the already occurring epigenetic changes ensuring cellular rejuvenation. Green box: Conversely, forced membrane depolarization and following repolarization of aged differentiated cells may be an alternative to the transfection-induced approach for partial reprogramming. Because lowering the membrane potential by manipulating ion concentration or activity of ion channels are able to delay or block or potentially revert differentiation, there is a possibility that this approach could prove to be an effective way of cell rejuvenation.
As discussed above, there is a high resemblance between the processes of partial reprogramming and the process of regeneration, which brings to mind the idea that artificially depolarizing the cells will partially dedifferentiate them (potentially rejuvenating their epigenome). The subsequent artificial repolarization within the appropriate time window will bring them back to their differentiated state, allowing them to perform their original function, but hopefully for much longer. If this holds true, partial reprogramming through artificial depolarization of the cell membrane followed by artificial repolarization may prove to be a superior approach. At least judging from what is observed in the process of blastemal formation, where the cells retain memory of their identity during the process.
Alternative potential application of modulating the Vmem to enhance rejuvenation by partial reprogramming using Yamanaka factors is to use artificial hyperpolarization of the cell membrane as a safeguard against cell identity loss during the process. As discussed above, low Vmem (depolarized) prevents embryonic stem cells from differentiation, and high Vmem (hyperpolarized) promotes cell differentiation. It is only logical to assume that the opposite is also true, and hyperpolarizing the cells will prevent them from dedifferentiating. If this is the case, artificially hyperpolarizing the cell membrane can be used to enhance partial reprogramming by Yamanaka factors in two ways. Firstly, it will add an additional level of safety by preventing unwarranted dedifferentiation of the cells, which is one of the main hurdles of this emerging technology. Secondly, it will potentially expand the window for partial reprogramming, improving the efficiency of epigenetic rejuvenation.
In the context of aging and rejuvenation, the intriguing question arises: Could bioelectric fields hold the secret to how cells can regain youthfulness? If the manipulation of bioelectric fields can instruct cells to dedifferentiate and form a blastemal during limb regeneration, could manipulating bioelectric fields potentially become a superior alternative to partial reprogramming using Yamanaka factors? Manipulating bioelectric fields, either by altering ion channels or applying external electric or electromagnetic fields, presents an exciting avenue for influencing cellular behavior and potentially resetting the epigenetic clock and reversing the aging process, as discussed in earlier sections. While the precise mechanisms by which bioelectricity influences aging and rejuvenation are still being elucidated, it is becoming increasingly clear that this dynamic force in biology has a pivotal role in shaping the destiny of cells and tissues.
Some concerns may arise about the effectiveness of cell rejuvenation by manipulation of bioelectric fields considering the limitations imposed by the temporal and partial nature of the process of membrane depolarization or hyperpolarization. However, it is possible that temporal and partial modulation of the cell’s membrane potential may prove to be sufficient for achieving the desired effect on rejuvenation at the cellular level. This consideration is based on the cyclical and time-dependent nature of methods such as partial reprogramming. Exploring innovative approaches to cellular rejuvenation is essential for advancing our understanding of the complex field of aging biology and is a necessary initial step toward whole-body rejuvenation.
Conclusions
The emerging understanding of the relationship between aging, rejuvenation, and bioelectric fields presents an exciting new frontier in the quest to enhance human health and longevity. This review emphasizes the significant impact of membrane potential on key biological processes, including cell proliferation, differentiation, limb regeneration and possibly aging. We explore the concept of modulating cellular membrane potential (Vmem) as a potential approach for cellular rejuvenation, which may offer a complementary or superior strategy compared to traditional partial reprogramming methods using Yamanaka factors. The insights gained from the study of bioelectric fields and their role in cellular dynamics suggest potential therapeutic interventions targeting age-related changes at a cellular level. In particular, the parallels between the processes of partial reprogramming and regeneration provide valuable lessons on how cells might regain their youthfulness while retaining their functional identity. This new approach may open alternative avenues for slowing down or even reversing the aging process and treating age-related diseases.
Authors’ contributions
BA and RP – conceptualization; BA and IV – original draft preparation; GN, AM and RP – substantial critical review and editing; BA and IV – visualization; GN and BA – funding acquisition.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Lemoine M. Defining aging. Biol Philos. 2020;35(5):1. doi:10.1007/s10539-020-09765-z.
- López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–12. doi:10.1016/j.cell.2013.05.039.
- Papamichos-Chronakis M, Peterson CL. Chromatin and the genome integrity network. Nat Rev Genet. 2013;14(1):62–75. doi:10.1038/nrg3345.
- Mladenov M, Lubomirov L, Grisk O. Oxidative stress, reductive stress and antioxidants in vascular pathogenesis and aging. Antioxidants. 2023;12(5):1126. doi:10.3390/antiox12051126.
- Aguilera A, García-Muse T. Causes of genome instability. Annu Rev Genet. 2013;47(1):1–32. doi:10.1146/annurev-genet-111212-133232.
- Nian L, Xiaohua L, Rongcheng L, et al. Types of DNA damage and research progress. Nucleosides Nucleotides Nucleic Acids. 2023:1–21. doi:10.1080/15257770.2023.2277194.
- Lu W, Zhang Y, Liu D, et al. Telomeres-structure, function, and regulation. Exp Cell Res. 2013;319(2):133–141. doi:10.1016/j.yexcr.2012.09.005.
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–76. doi:10.1038/35036093.
- Rossiello F, Jurk D. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135–147. doi:10.1038/s41556-022-00842-x.
- Hetz C, Zhang K. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21(8):421–438. doi:10.1038/s41580-020-0250-z.
- Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84(1):435–464. doi:10.1146/annurev-biochem-060614-033955.
- Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019;20(7):421–435. doi:10.1038/s41580-019-0101-y.
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi:10.1038/nature10317.
- Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23(1):56–73. doi:10.1038/s41580-021-00411-4.
- Guo X, Asthana P, Gurung S, et al. Regulation of age-associated insulin resistance by MT1-MMP-mediated cleavage of insulin receptor. Nat Commun. 2022;13(1):3749. doi:10.1038/s41467-022-31563-2.
- Guo X, Asthana P, Gurung S, et al. Regulation of age-associated insulin resistance by MT1-MMP-mediated cleavage of insulin receptor. Nat Commun. 2022;13(1):3749. doi:10.1038/s41467-022-31563-2.
- Papadopoli D, Boulay K, Kazak L. mTOR as a central regulator of lifespan and aging. F1000Res. 2019;8:F1000 Faculty Rev-998. 2019;8. doi:10.12688/f1000research.17196.1.
- Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11(2):230–241. doi:10.1016/j.arr.2011.12.005.
- Ge Y, Zhou M, Chen C, et al. Role of AMPK mediated pathways in autophagy and aging. Biochimie. 2022;195:100–113. doi:10.1016/j.biochi.2021.11.008.
- Guo Y, Guan T, Shafiq K, et al. Mitochondrial dysfunction in aging. Ageing Res Rev. 2023;88:101955. doi:10.1016/j.arr.2023.101955.
- Müller M, Ahumada-Castro U, Sanhueza M, et al. Mitochondria and calcium regulation as basis of neurodegeneration associated with aging. Front Neurosci. 2018;12:470. doi:10.3389/fnins.2018.00470.
- Di Micco R, Krizhanovsky V, Baker D, et al. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95. doi:10.1038/s41580-020-00314-w.
- Correia-Melo C, Passos JF. Demystifying the role of mitochondria in senescence. Mol Cell Oncol. 2016;3(4):e1162896. doi:10.1080/23723556.2016.1162896.
- Park MH, Kim DH, Lee EK, et al. Age-related inflammation and insulin resistance: a review of their intricate interdependency. Arch Pharm Res. 2014;37(12):1507–1514. doi:10.1007/s12272-014-0474-6.
- Schmitt CA, Wang B, Demaria M. Senescence and cancer—role and therapeutic opportunities. Nat Rev Clin Oncol. 2022;19(10):619–636. doi:10.1038/s41571-022-00668-4.
- Liu B, Qu J, Zhang W, et al. A stem cell aging framework, from mechanisms to interventions. Cell Rep. 2022;41(3):111451. doi:10.1016/j.celrep.2022.111451.
- Waś H, Czarnecka J. [Stem cells and senescence]. Postepy Biochem. 2014;60(2):161–176.
- Picerno A, Stasi A, Franzin R, et al. Why stem/progenitor cells lose their regenerative potential. World J Stem Cells. 2021;13(11):1714–1732. doi:10.4252/wjsc.v13.i11.1714.
- Fafián-Labora JA, O’Loghlen A. Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol. 2020;30(8):628–639. doi:10.1016/j.tcb.2020.05.003.
- Kabacik S, Lowe D, Fransen L, et al. The relationship between epigenetic age and the hallmarks of aging in human cells. Nat Aging. 2022;2(6):484–493. doi:10.1038/s43587-022-00220-0.
- Burgstaller JP, Brem G. Aging of cloned animals: a mini-review. Gerontology. 2017;63(5):417–425. doi:10.1159/000452444.
- Wang X, Qu J, Li J, et al. Epigenetic reprogramming during somatic cell nuclear transfer: recent progress and future directions. Front Genet. 2020;11:205. doi:10.3389/fgene.2020.00205.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi:10.1016/j.cell.2006.07.024.
- Simpson DJ, Olova NN, Chandra T. Cellular reprogramming and epigenetic rejuvenation. Clin Epigenetics. 2021;13(1):170. doi:10.1186/s13148-021-01158-7.
- Singh PB, Zhakupova A. Age reprogramming: cell rejuvenation by partial reprogramming. Development. 2022;149(22): dev200755. doi:10.1242/dev.200755.
- Rodríguez-Matellán A, Alcazar N, Hernández F, et al. In vivo reprogramming ameliorates aging features in dentate gyrus cells and improves memory in mice. Stem Cell Rep. 2020;15(5):1056–1066. doi:10.1016/j.stemcr.2020.09.010.
- Ocampo A, Reddy P, Martinez-Redondo P, et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167(7):1719–1733.e12. doi:10.1016/j.cell.2016.11.052.
- Browder KC, Reddy P, Yamamoto M, et al. In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nat Aging. 2022;2(3):243–253. doi:10.1038/s43587-022-00183-2.
- Levin M, Martyniuk CJ. The bioelectric code: an ancient computational medium for dynamic control of growth and form. Biosystems. 2018;164:76–93. doi:10.1016/j.biosystems.2017.08.009.
- Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep. 2009;5(3):231–246. doi:10.1007/s12015-009-9080-2.
- Abdul Kadir L, Stacey M, Barrett-Jolley R. Emerging roles of the membrane potential: action beyond the action potential. Front Physiol. 2018;9:1661. doi:10.3389/fphys.2018.01661.
- Binggeli R, Weinstein RC. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986;123(4):377–401. doi:10.1016/s0022-5193(86)80209-0.
- Cone CD.Jr. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30(1):151–181. doi:10.1016/0022-5193(71)90042-7.
- Sempou E, Kostiuk V, Zhu J, et al. Membrane potential drives the exit from pluripotency and cell fate commitment via calcium and mTOR. Nat Commun. 2022;13(1):6681. doi:10.1038/s41467-022-34363-w.
- Laursen L. Salamander cells remember their origins in limb regeneration. Nature. 2009; doi:10.1038/news.2009.614.
- Flowers GP, Sanor LD, Crews CM. Lineage tracing of genome-edited alleles reveals high fidelity axolotl limb regeneration. Elife. 2017;6:e25726..
- Ozkucur N, Epperlein HH, Funk RH. Ion imaging during axolotl tail regeneration in vivo. Dev Dyn. 2010;239(7):2048–2057. doi:10.1002/dvdy.22323.
- Reid B, Song B, Zhao M. Electric currents in Xenopus tadpole tail regeneration. Dev Biol. 2009;335(1):198–207. doi:10.1016/j.ydbio.2009.08.028.
- Adams DS, Masi A, Levin M. H + pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134(7):1323–1335. doi:10.1242/dev.02812.
- McLaughlin KA, Levin M. Bioelectric signaling in regeneration: mechanisms of ionic controls of growth and form. Dev Biol. 2018;433(2):177–189. doi:10.1016/j.ydbio.2017.08.032.
- Tseng A, Levin M. Cracking the bioelectric code: probing endogenous ionic controls of pattern formation. Commun Integr Biol. 2013;6(1):e22595. doi:10.4161/cib.22595.
- Adams DS, Uzel SGM, Akagi J, et al. Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J Physiol. 2016;594(12):3245–3270. doi:10.1113/JP271930.
- George LF. Developmental bioelectricity: Investigating the role of ion channels in development. University of Colorado Denver, Anschutz Medical Campus ProQuest Dissertations Publishing. 2021; p. 28774803.
- Pai VP, Aw S, Shomrat T, et al. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139(2):313–323. doi:10.1242/dev.073759.
- Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21(2):91–103. doi:10.1016/j.tcb.2010.09.011.
- Di Giorgio E, Brancolini C. Regulation of class IIa HDAC activities: it is not only matter of subcellular localization. Epigenomics. 2016;8(2):251–269. doi:10.2217/epi.15.106.
- Backs J, Backs T, Bezprozvannaya S, et al. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008;28(10):3437–3445. doi:10.1128/MCB.01611-07.
