ABSTRACT
Bottletail squids (Cephalopoda: Sepiadariidae) spend the daytime buried in sediment; however, their burying behaviour has not yet been described in detail. In the present study, the burying pattern of a single tropical bottletail squid Sepiadarium kochii Steenstrup, 1881 is analysed for different behavioural characteristics. Burying in S. kochii consists of a rapid sequence of strong, alternating forward- and backward directed funnel jets which obscure the individual almost fully with sediment, followed by a single flinging movement of the dorsolateral arm pair to cover the remaining exposed body parts with sand. A comparison of the burying pattern of S. kochii with that of closely related bobtail squids (Cephalopoda: Sepiolidae) is drawn. Moreover, differences between these two cephalopod families in terms of the execution and duration of their burying procedure as well as its behavioural use are discussed.
Introduction
Burying in soft sediment as a means of protection is a common defensive behaviour in several animal groups inhabiting the marine environment. Defined as ‘the act of covering oneself with the substrate (or diving into it), resulting in temporary concealment’ (Hanlon and Messenger Citation2018), it has frequently been observed, amongst others, in crustaceans (Bellwood Citation2002; McGaw Citation2005) or fish (Gibson and Robb Citation1992; Lü et al. Citation2018). In cephalopods, burying behaviour has been reported for some octopus species (Hanlon and Hixon Citation1980; Guerra et al. Citation2006; Hanlon et al. Citation2008, Citation2010), the cuttlefish Sepia officinalis Linnaeus, 1758 (Mather Citation1986; Hanlon and Messenger Citation1988), and especially bobtail squids (Sepiolidae) of the subfamilies Rossiinae and Sepiolinae (Boletzky and Boletzky Citation1970; Anderson et al. Citation2002, Citation2004; Rodrigues et al. Citation2010; Drerup et al. Citation2020). Members of these sepiolid subfamilies are nocturnal and not only bury themselves as an evasive defensive behaviour but also spend the daytime buried in the sediment (Drerup et al. Citation2020).
Following Boletzky and Boletzky (Citation1970), the burying behaviour of bobtail squids can be divided into two phases. Phase 1 consists of alternating forward- and backward-directed funnel jets to displace sediment particles from below the sepiolid’s body and immerse the latter into the resulting depression. Subsequently in phase 2, bobtail squids will raise a pair of arms to sweep sediment particles from their vicinity and thereby obscure the remaining exposed body parts (Boletzky and Boletzky Citation1970; Rodrigues et al. Citation2010; Drerup et al. Citation2020). This behavioural pattern is remarkably consistent across all sepiolid species and shows little variation.
While the burying pattern of bobtail squids has been addressed in detail in several studies (Boletzky and Boletzky Citation1970; Anderson et al. Citation2002, Citation2004; Rodrigues et al. Citation2010; Drerup et al. Citation2020), the closely related but sparsely investigated family of bottletail squids (Sepiadariidae) displays a comparable burying behaviour. Bottletail squids not only share close anatomical and morphological similarities with bobtail squids, they also have a similar benthic lifestyle and spend the daytime buried in the sediment while emerging at night for hunting and mating (Reid Citation2005, Citation2016). Although their reproductive biology has been investigated to some extent (Wegener et al. Citation2013a, Citation2013b; Hooper et al. Citation2016), other behavioural aspects of this cephalopod family, such as their burying behaviour, have not been studied in detail. Here, I report the first detailed description of the burying pattern in a bottletail squid species.
Material and methods
Ethical statement
Cephalopods do not fall under the ‘Act on Humane Treatment and Management of Animals’ as national legislation in Japan (Ogden et al. Citation2017). To ensure appropriate welfare, this study followed the regulations of Directive 2010/63/EU (European Parliament and Council of the European Union Citation2010) for cephalopods (Smith et al. Citation2013).
Animal husbandry
On March 28 2019 a female tropical bottletail squid Sepiadarium kochii (mantle length 14.6 mm) was collected from Seragaki Port (Onna, Okinawa, Japan; 26°30'39.5"N 127°52'16.1"E) at dusk. The individual was kept in a 50 L aquarium (50 cm long x 25 cm wide x 40 cm deep) together with five individuals of the spotty bobtail squid Eumandya parva Sasaki, 1913 at the Marine Science station of the Okinawa Institute of Science and Technology (Onna, Okinawa, Japan; 26°30′35.6″N 127°52′12.7″E), following the husbandry conditions described in Drerup et al. (Citation2020). The tank was connected to a flow-through system supplied with natural sea water and its bottom was covered with a 3 cm layer of subtidal sediment. Lighting was maintained by using fluorescent light tubes and followed a 10:14 LD cycle from 08:00–18:00. S. kochii and the five E. parva were fed with local mysid shrimps (Neomysis japonica Nakazawa, 1910).
Experimental setup
Filming of the burying behaviour of S. kochii was delayed until five days after capture in an attempt to ensure the animals’ acclimatisation to captivity. The experimental setup used in this study is identical to that in Drerup et al. (Citation2020). The burying procedure was recorded in a 0.8 L aquarium (10 cm long x 6.5 cm wide x 14.4 cm deep) using a video camera (PXW-FS5 with SEL-35F14Z lens, Sony Corporation, Tokyo, Japan; recording quality: 1080p/60 fps) under the stimulus of a light panel (RX-12 TD, FalconEyes Ltd., Hong Kong). The bottom of the aquarium was covered with a 3 cm layer of subtidal sediment taken from Seragaki Bay (26°30′19.9″N 127°52′56.1″E). To avoid visual disturbances, a barrier was placed around the aquarium. After S. kochi was introduced into the experimental setup, the burying behaviour was filmed three times, with 20 min between each recording.
Results
After settling on the sediment, S. kochii rested moderately motionless on the sediment for 125, 136 and 321 s (). Shortly before the start of each burying procedure, the observed individual exhibited a couple of gentle body contractions. In all three observations, the actual burying pattern was then initiated by the ejection of a forward-directed funnel jet, immediately followed by a backward-directed funnel jet. Subsequently, either two or three further sets of funnel jets were conducted, resulting in a total number of either 6 or 8 funnel jets. This sequence took between 4–6 s and no pauses in between the funnel jet sets were observed (). During the last set of funnel jets, S. kochii changed its body patterning rapidly from its common orange colouration to a pale-whitish colouration with small, either black or brown chromatophore spots, which resembled the overall colouration of the sediment.
Figure 1. Burying behaviour in Sepiadarium kochii. A – B, Settling position on the sediment, A, lateral view, B, anterior view. C – H, Lateral view: Alternating forward- and backward directed funnel jets to immerse the body into the sediment, with C – D, showing the first set of funnel jets, E – F, the second set of funnel jets and G – H, the third set of funnel jets. I – J, Ventral view: Flinging movement of the dorsolateral arm pair (marked with red circles) to obscure the remaining exposed body parts with sediment, with I, stretching the arm pair above the sediment, and J, explosive movement to fling sediment over the head and dorsal mantle side. K, Lateral view: positioning of funnel on the dextrolateral side and subsequent blowout to displace overlying sediment particles. L – M, Fully buried individual after raising its body slightly upwards to uncover the eyes, L, lateral view, M, anterior view, the black arrow depicts the funnel. Scale bars = 10 mm.
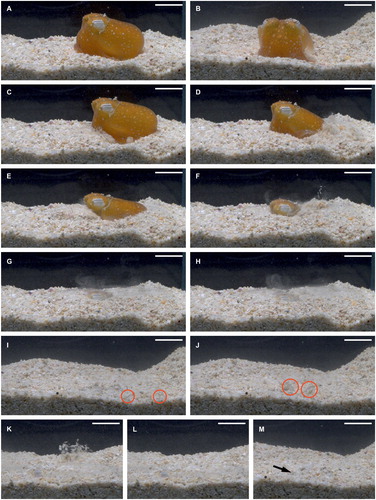
Table 1. Observations of different characteristics of the burying behaviour in Sepiadarium kochii
While on one occasion no further burial activity was observed, in the remaining two observations the individual proceeded with its burying behaviour either 3 or 14 sec after the last funnel jet. S. kochii flung its dorsolateral arm pair from an anterolateral position to disperse more sediment grains over its body and especially over its head. In both observations, only one flinging arm movement was performed which took approximately 3 s (). The whole burying pattern took between 4 s (first observation lacking any arm movements) and 23 s (third observation including arm movements and 14 s resting period), resulting in an average duration of 12.7 s ().
In all three observations, the investigated individual of S. kochii, despite being fully obscured by sediment, raised its body slightly to uncover its eyes. Afterwards, it placed its funnel on either lateral side, accompanied with an initial, forceful blowout to displace overlying sediment. Thereafter, S. kochii remained motionless in the sediment and no further behavioural actions were observed.
Discussion
The burying behaviour of S. kochii is similar to that reported for bobtail squids, such as E. parva (Drerup et al. Citation2020) or Sepiola atlantica d'Orbigny, 1842 (Rodrigues et al. Citation2010), and can likewise be divided into two phases (following Boletzky and Boletzky Citation1970). Interestingly, in bobtail squids both phases of the burying behaviour, consisting of alternating funnel jets followed by repeating arm sweeping movements, are needed for a successul burying procedure. In contrast, the observed individual of S. kochii was able to almost completely obscure itself with sediment by performing a series of funnel jets, and only used its arms in two of the three observations to continue its burying behaviour. Moreover, the way the observed bottletail squid used its arms within the second phase of its burying behaviour differs from the observations reported for bobtail squids, such as E. parva or S. atlantica. These species perform their arm movements in a sweeping fashion to gather sediment from around the animal (Rodrigues et al. Citation2010; Drerup et al. Citation2020), whereas S. kochii, in contrast, flings sediment from an anterolateral position to cover its exposed body parts. Despite these minor differences, the general two-phased burying pattern observed in both bobtail squids (Sepiolidae) as well as bottletail squids (Sepiadariidae) supports the monophyletic relationship of these two cephalopod families within the clade Sepiolida, as suggested by Anderson and Lindgren (Citation2021).
As the burying behaviour of S. kochii discussed in the present study was recorded using the same experimental setup and sediment as for the bobtail squid E. parva in Drerup et al. (Citation2020), a direct comparison of their burying characteristics is possible. Identical to S. kochii, on average six or eight funnel jets were ejected by E. parva within phase 1. However, the average duration of phase 1 for S. kochii was 5.0 s, which is significantly shorter compared to the 9.2 s reported for E. parva (Drerup et al. Citation2020), indicating a higher frequency of ejecting funnel jets in S. kochii. After the completion of phase 1, E. parva performed on average 11 arm sweeps within phase 2, which took on average 12.2 s (Drerup et al. Citation2020). This clearly indicates the importance of the second phase for bobtail squids to complete their burying process, compared to S. kochii which performed only one arm flinging movement in two of the three observations.
The behavioural ecology of bottletail squids has not been investigated in detail yet and their preferred anti-predator responses are generally unknown. In a direct comparison, the funnel jets of S. kochii seem to be more powerful and can displace more sediment compared to those of E. parva. Whether the higher frequency of ejecting funnel jets along with its more forceful execution in S. kochii is solely linked to the size difference compared to E. parva, or rather derives from the ecological need to complete its burying behaviour as quickly as possible, needs to be addressed in future studies. However, as S. kochii can obscure most of, or even all of its body with sediment only by ejecting funnel jets within a couple of seconds, it can be hypothesised that burying is the primary escape technique in this species. In contrast, E. parva has shown a series of other primary escape responses such as inking and jetting away (Drerup et al. Citation2020), so this bobtail squid species might bury in the sediment to rest or protect itself from the environment rather than to avoid imminent predation.
While the observations of the burying behaviour of S. kochii in the present study are based on only one individual of this species, they follow observations taken from footage of wild bottletail squids (pers. obs.), mainly Sepioloidea lineolata Quoy & Gaimard, 1832 and Sepiadarium austrinum Berry, 1921, obtained through the Cephalopod Citizen Science Project (https://www.cephalopodcitizenscience.com/) and social media channels. Similar to S. kochii, these bottletail squids performed a rapid series of funnel jets, occasionally followed by an arm flinging movement. However, future studies comprising observations on several individuals of a botttletail squid species should be carried out to confirm the present observations and highlight potential individual differences within a species.
Acknowledgements
The author would like to thank Michael Kuba, Tamar Gutnick, Teresa Iglesias, Keishu Asada, Takahiro Nishibayashi, Zdenek Lajbner, Ryuta Nakajima and Jonathan Miller of the OIST Marine Science Station for their help and support. CD was supported by the Okinawa Institute of Science and Technology (OIST) Research Internship Program.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Anderson, F.E. & Lindgren, A.R. (2021) Phylogenomic analyses recover a clade of large-bodied decapodiform cephalopods. Molecular Phylogenetics and Evolution 156, 107038.
- Anderson, R.C., Mather, J. & Steele, C. (2002) The burying behavior of the sepiolid squid Euprymna scolopes Berry, 1913 (Cephalopoda, Sepiolidae). Western Society of Malacologists - Annual Report 33, 1–7.
- Anderson, R.C., Mather, J. & Steele, C. (2004) Burying and associated behaviors of Rossia pacifica (Cephalopoda: Sepiolidae). Vie et Milieu 54, 13–20.
- Bellwood, O. (2002) The occurrence, mechanics and significance of burying behaviour in crabs (Crustacea: Brachyura). Journal of Natural History 36, 1223–1238.
- Boletzky, S.V. & Boletzky, M.V.V. (1970) Das Eingraben in Sand bei Sepiola und Sepietta (Mollusca, Cephalopoda). Revue Suisse de Zoologie 77, 536–548.
- Drerup, C., Sykes, A.V. & Cooke, G.M. (2020) Behavioural aspects of the spotty bobtail squid Euprymna parva (Cephalopoda: Sepiolidae). Journal of Experimental Marine Biology and Ecology 530–531, 151442.
- European Parliament & Council of the European Union (2010) Directive 2010 ⁄ 63 ⁄ EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union L276, 33–79.
- Gibson, R. & Robb, L. (1992) The relationship between body size, sediment grain size and the burying ability of juvenile plaice, Pleuronectes platessa L. Journal of Fish Biology 40, 771–778.
- Guerra, A., Rocha, F., González, Á.F. & González, J.L. (2006) First observation of sand-covering by the lesser octopus Eledone cirrhosa. Iberus 24, 27–31.
- Hanlon, R.T. & Hixon, R.F. (1980) Body patterning and field observations of Octopus burryi Voss, 1950. Bulletin of Marine Science 30, 749–755.
- Hanlon, R.T. & Messenger, J.B. (1988) Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Philosophical Transactions of the Royal Society of London B: Biological Sciences 320, 437–487.
- Hanlon, R.T. & Messenger, J.B. (2018) Cephalopod Behaviour. Cambridge University Press, Cambridge.
- Hanlon, R.T., Conroy, L.-A. & Forsythe, J.W. (2008) Mimicry and foraging behaviour of two tropical sand-flat octopus species off North Sulawesi, Indonesia. Biological Journal of the Linnean Society 93, 23–38.
- Hanlon, R.T., Watson, A.C. & Barbosa, A. (2010) A “mimic octopus” in the Atlantic: flatfish mimicry and camouflage by Macrotritopus defilippi. The Biological Bulletin 218, 15–24.
- Hooper, A.K., Wegener, B.J. & Wong, B.B. (2016) When should male squid prudently invest sperm? Animal Behaviour 112, 163–167.
- Lü, H., Chapelsky, A., Fu, M., Xi, D., Zhang, Z. & Zhang, X. (2018) Effect of sand grain size on substrate preference and burial behaviour in cultured Japanese flounder juvenile, Paralichthys olivaceus. Aquaculture Research 49, 1664–1671.
- Mather, J.A. (1986) Sand digging in Sepia officinalis: assessment of a cephalopod mollusc's “fixed” behavior pattern. Journal of Comparative Psychology 100, 315–320.
- McGaw, I.J. (2005) Burying behaviour of two sympatric crab species: Cancer magister and Cancer productus. Scientia Marina 69, 375–381.
- Ogden, B.E., Pang, W., Agui, T. & Lee, B.H. (2017) Laboratory animal laws, regulations, guidelines and standards in China mainland, Japan, and Korea. ILAR Journal 57, 301–311.
- Reid, A. (2005) Family Sepiadariidae. In: Jereb, P. & Roper, C.F. (Eds), Cephalopods of the World. An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date. Volume 1. Chambered Nautiluses and Sepioids (Nautilidae, Sepiidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO, Rome, pp. 204–207.
- Reid, A. (2016) Cephalopods of Australia and sub-Antarctic Territories. CSIRO Publishing, Clayton, Australia.
- Rodrigues, M., Garci, M.E., Troncoso, J.S. & Guerra, A. (2010) Burying behaviour in the bobtail squid Sepiola atlantica (Cephalopoda: Sepiolidae). Italian Journal of Zoology 77, 247–251.
- Smith, J.A., Andrews, P.L.R., Hawkins, P., Louhimies, S., Ponte, G. & Dickel, L. (2013) Cephalopod research and EU Directive 2010/63/EU: requirements, impacts and ethical review. Journal of Experimental Marine Biology and Ecology 447, 31–45.
- Wegener, B.J., Stuart-Fox, D., Norman, M.D. & Wong, B.B. (2013a) Spermatophore consumption in a cephalopod. Biology Letters 9, 20130192.
- Wegener, B.J., Stuart-Fox, D.M., Norman, M.D. & Wong, B.B. (2013b) Strategic male mate choice minimizes ejaculate consumption. Behavioral Ecology 24, 668–671.
