ABSTRACT
Sclerotia are the resting bodies of fungi. The exact biochemical properties of melanized sclerotia that allow them to remain in the soil and retain their structure are unclear. Sclerotium grains were collected from Mongolian steppe forest soils to elucidate the characteristics of melanized sclerotia in semi-arid areas. The collected sclerotia were characterized as derived from Cenococcum geophilum based on similarities in the ITS1-5.8S-ITS2 region. Using accelerator mass spectrometry 14C/12C measurement, the mean residual time of the sclerotium in the soil was estimated to be 40 years. The H2O content of the grains was 10%, and the major element compositions were 43% C, 39% O (with H), and 1.8% N. Scanning electron microscope-energy dispersive X-ray spectrometry analysis showed that C, O, and Ca were the dominant elements and Al, K, and Mg were distributed homogeneously in the grain, while Ca and P specifically coexisted in cell compartments in the form of Ca-phosphate (hydroxyapatite) with a concentric location inside the grains. Hydroxyapatite precipitation in sclerotia may suggest a form of P sequestration in slightly alkaline soils.
Introduction
One of the most common ectomycorrhizal fungi is Cenococcum geophilum Fr., which often dominates in arctic, temperate, and subtropical forests (Trappe Citation1962; LoBuglio Citation1999; Jonsson et al. Citation2000; Dickie and Reich Citation2005; Bahram et al. Citation2011), especially in abiotically stressful habitats, such as those characterized by drought (Matsuda et al. Citation2009; Obase et al. Citation2009, Citation2011) or dry seasons (Smith et al. Citation2007). Ectomycorrhizae formed by C. geophilum have ecological importance for nutrient cycling in host plants of various forest ecosystems (Courty et al. Citation2010; Fernandez et al. Citation2013). Sexual and asexual C. geophilum spore reproduction has not been observed, and mitotically derived sclerotia are the only known soil dispersal bodies in C. geophilum (Douhan et al. Citation2007). Cenococcum geophilum sclerotia are among the most common resistant propagules among ectomycorrhizal fungi in forest soils, and their survival after drought was confirmed by demonstrating their germination and ability to form ectomycorrhizae (Glassman et al. Citation2015).
Although the characteristics of sclerotia of ectomycorrhizal fungi surviving in various environments are not yet fully understood, those of C. geophilum collected from low-pH forest soils in Japan have been reported to remain in the soil for thousands of years (Watanabe et al. Citation2007a, Citation2021), and Al was found to be enriched in sclerotium grains in low-pH forest soils from Japan and Germany (Nyamsanjaa et al. Citation2021a). However, little is known about sclerotia in neutral to slightly alkaline forest soils.
Mountain soils in Mongolia are classified as tundra, meadow steppe, forest, steppe, and desert steppe soils. Mountain forest soil, including taiga cryomorphic ochro soil, taiga podzolic soil, and dark-colored derno soil, is weakly acidic (pH (H2O) 4.5 to 6.0) in the full profiles, whereas the soil pH of mountain steppe soil, including chernozem and kastanozem, is weakly acidic to neutral in the upper part of the profiles (pH (H2O) 5.8 to 6.8) and alkaline in the lower parts (Dorjgotov Citation2003). Steppe forest soil is the transition between mountain forest and mountain steppe soil. Nyamsanjaa et al. (Citation2021b) found melanized sclerotia in Mongolian steppe forest soils and studied elemental concentrations by inductively coupled plasma (ICP) analysis. They noted a higher Ca concentration compared to sclerotia from Japanese low pH forest soils, although fungal species of the sclerotia have not been identified and P concentration was not available due to the limitations of ICP analysis.
The objective of this study was to obtain knowledge on the exact biochemical properties of melanized sclerotia in slightly alkaline forest soil by microfeature observation and elemental analysis using scanning electron microscope-energy dispersive X-ray spectrometry (SEM-EDS).
Materials and methods
Sampling area
Soil samples containing fungal sclerotia were collected from the A horizon (0–15 cm) at a site in Terelj, Nalaikh district, Ulaanbaatar, Mongolia. In total, three soil samples (TE-1, TE-2, and TE-3), approximately 20–30 m apart, were retrieved from the Terelj site using a stainless cylinder (diameter (Ø) 8 cm × 10 cm). TE-1 and TE-2 were collected in June and August 2018 and TE-3 in June 2019. The geology of Terelj is a Mesozoic granitoid complex (Dorjgotov Citation2004). The soil was classified as Dark Kastanozem according to the Mongolian soil classification system (Dorjgotov and Shirnin Citation1976; Dorjgotov Citation2003) and Haplic Kastanozem according to the WRB/FAO-UNESCO 2014 classification system (Food and Agriculture Organization of the United Nations Citation2015). The vegetation coverage consisted of Larix sibirica Ledeb. and Pinus sibirica Du Tour forest associated with Betula fusca Pall, Aster alpinus L., Geranium pratense L., Fragaria orientalis Losinsk. Stellera chamaejasme L., Dasiphora fruticosa (L.) Rydb., and Potentilla anserina L. The annual mean temperature (1999–2017) is −0.3°C and the annual mean precipitation (2018) is 269.2 mm at Ulaanbaatar (Mongolian Statistical Information Service).
Soil analyses
Soil color was examined using a soil color meter (SPAD-503, Konica Minolta, Tokyo, Japan). Soil samples were air-dried and passed through a 2 mm sieve for pH (H2O) and C and N content analyses. Total carbon and nitrogen contents (TC and TN, respectively, g kg−1) were examined using an NC analyzer (NC-22 F, Sumika Chemical Analysis Service Ltd., Tokyo, Japan). Soil n H2O pH was measured using a pH meter (S20, Mettler Toledo, Columbus, OH, USA) in a suspension mixture of soil containing a volume of distilled water 2.5 times that of the soil (5 g). The measurement of each soil sample (TE-1, TE-2, and TE-3) was performed in triplicate, except for X-ray diffraction (XRD) analysis.
Advanced X-ray diffraction (XRD) analysis (SmartLab, Rigaku, Tokyo, Japan) was performed to identify the crystalline components of the soil samples. Approximately 80 mg of the TE-2 soil sample was powdered using an agate mortar, air-dried, and filtered through a 0.02 mm sieve for measurement. The operating conditions were as follows: Ni-filtered CuKα radiation at 45 kV and 200 mA; scan range: 10–70 °; step size: 0.02 °; scan speed: 0.7 °/min; sampling time: 0.50 s; peak angle range: 4–70 °. A one-dimensional semiconductor detector (D/tex Ultra 250) was used with a 2θ continuous scan, and the content (wt %) of the crystal phases in the samples was calculated by Rietveld analysis.
Sclerotium analyses
Sclerotia preparation and morphological analysis
Sclerotia were isolated from soil samples using tweezers in the laboratory. Approximately 150–200 grains were collected from a 100 g soil sample from TE-3 for morphological analysis. Each grain was placed into a microtube with distilled water and washed by hand for 2–3 min, and this procedure was repeated five times. The washed sclerotia were then air-dried at 25°C for 5 h. The weight and diameter of the sclerotium grains were individually measured (n = 100) using an analytical balance (AX26, Mettler Toledo, Columbus, OH, USA) and a digital microscope (VHX-1000, Keyence, Osaka, Japan), respectively. In addition, the density of sclerotia grains per unit soil was calculated by counting (grains/g soil) and weight (mg/g soil), using three soil samples (115 g, 25.7 g, and 20.3 g of soil) collected from site TE-3, in addition to the soils prepared for the above analyses.
DNA analysis
Three grains from each sampling point, TE-1 and TE-2, were used for DNA analysis. Each grain was separately added into a 1.5 mL tube containing 15 μL of PrepMan Ultra Sample Preparation Reagent (Applied Biosystems, Tokyo, Japan). The grains were then crushed using a sterilized pestle, and 15 μL of the reagent was added to the tubes. DNA was extracted according to the manufacturer’s instructions for the reagent. The obtained supernatant (approximately 30 μL) was mixed with 50 μL Tris-EDTA buffer and 100 μL PCI (phenol/chloroform/isoamylalchol = 25:24:1) and centrifuged (15,000 rpm, 15 min, 4°C) to collect the purified supernatant (approximately 80 μL). After further purification by ethanol precipitation, the pellet was air-dried, dissolved in TE buffer, and used as template DNA. Polymerase chain reaction (PCR) amplification targeting the ITS1-5.8S-ITS2 region was performed using KOD FX Neo DNA polymerase (Toyobo) with fungal universal primers ITS5-LR5 (Vilgalys and Hester Citation1990; White et al. Citation1990). The PCR mixture composition and PCR conditions were the same as those described by Takashima et al. (Citation2018), with the exception that the annealing temperature was set to 54°C. PCR products were purified using polyethylene glycol and ethanol precipitation, and a cycle sequence reaction was then performed with the BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Tokyo, Japan) in accordance with the manufacturer’s instructions using the forward primer ITS5. Cycle sequencing products were purified by ethanol precipitation, and electrophoresis was performed using an Applied Biosystems 3130xl Genetic Analyzer (Tokyo, Japan) to determine nucleotide sequences. Nucleotide similarities of the obtained sequences were compared using BlastN searches at the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/). Nucleotide sequences obtained from each sclerotium were deposited under the accession numbers OK493298–OK493300 for TE-1, and OK513037 and OK513038 for TE-2.
AMS 14C dating analysis
The 14C ages of the sclerotium grains were obtained using accelerator mass spectrometry (AMS) with a 3 MV tandem accelerator at the Institute of Accelerator Analysis Ltd. (Shirakawa, Japan). Sclerotium samples of approximately 3 mg (24 or 25 grains) from each sampling point, TE-1, TE-2, and TE-3 were powdered and used to measure the 14C/12C ratio. The 14C/12C sample data were normalized using 14C/12C standards. Oxalic acid provided by NIST (HOx) was used as the standard material. 14C ages were calculated according to the procedures of Stuiver and Polach (Citation1977), and calibration for calendar age was performed using the Bomb13 calibration curve (Hua et al. Citation2013; Reimer et al. Citation2020) and OxCal calibration program v4.4 (Ramsey Citation2009).
Major element CNO content, ash content, and water content analyses
First, the water content was obtained by subtracting the weight of sclerotium grains that had been dried in an oven at 105°C from those of air-dried grains. A total of 22 samples, 3, 9, and 10 samples from TE-1, TE-2, and TE-3, respectively, were used for water content analysis. The oven-dried sclerotium grains were ground to a fine powder using an agate mortar to measure the TC and TN contents using an NC analyzer. The above 9 and 10 oven-dried samples of TE-2 and TE-3 were combined into three groups to ensure sufficient weight (>1.6 mg) for NC analyzer measurement. Consequently, the total sample size was defined as 3, 3, and 3 for the major element contents of TE-1, TE-2, and TE-3, respectively. The O and H contents were obtained from the difference between the original sample weight and the total weight of the ash plus the sum of the C and N contents.
SEM-EDS analysis
SEM observation at a magnification of up to 100,000× was conducted to examine the micromorphology of the inner areas of the sclerotium samples using one grain each from TE-1 and TE-3. The samples were mounted on double-sided carbon tape and sputter-coated with osmium and platinum film prior to imaging, and the elemental composition of sclerotia was determined using a thermal field emission scanning electron microscope (JSM-7001 F, JEOL, Tokyo, Japan; accelerating voltage, 15 kV) and EDS (JED-2300 Analysis Station with the ZAF method standardless quantitative analysis program, JEOL, Tokyo, Japan).
Statistical analysis
To compare the mean weight and C, N, and water content of sclerotia in this study with those of previous studies (Watanabe et al. Citation2007b; Sakagami et al. Citation2018), t-tests were performed using IBM SPSS statistics (Version 23, Release 23.0.0.0).
Results
Soil properties
The soil color of TE-1, TE-2, and TE-3 were 7.5 YR 2/1 (black), 5 YR 2/1 (black), and 7.5 YR 3/1 (brownish black), respectively. The soil was slightly alkaline, with pH (H2O) ranging from 7.31 to 7.72. TC and TN contents of the soil samples (TE-1, TE-2, and TE-3) ranged from 10.8% to 15.0% and 0.94% to 1.05%, respectively, while the C/N ratio ranged from 11.5 to 14.2.
The mineral composition of the TE-2 soil was determined to be 42.9% quartz (SiO2), 39.6% albite NaAl(Si3O8), 16.9% sanidine K(AlSi3O8), and 0.59% rhodochrosite (MnCO3), which revealed the soil parent material as alkali feldspar granite according to the quartz–alkali feldspar–plagioclase (QAP) ternary diagram of Streckeisen (Citation1976).
Sclerotium characteristics
Sclerotium grains were spherical and black with diameters ranging between 0.55 and 1.25 mm (average ± standard deviation (SD), 0.84 ± 0.15 mm; n = 100), and grain weights ranging between 0.03 and 0.56 mg (0.16 ± 0.09 mg; n = 100). The density of the sclerotium grains were calculated as 2.26 ± 0.08 (grains/g soil) and 0.24 ± 0.06 (mg/g soil).
In total, three and two nucleotide sequences were obtained from the TE-1 and TE-2 sclerotium grains, respectively. The DNA extracted from a grain of TE-2 was brown-pigmented, and PCR amplification failed. The sequences obtained from each site were identical to each other. BlastN searches of the longest representative sequences of each site showed high similarity to the ITS sequence of C. geophilum, with 99% similarity for TE-1 (479/482 bp, accession no. MN961132) and the group-I intron sequence of C. geophilum with 98% similarity for TE-2 (505/513 bp; accession no. JX129158). The nucleotide sequences obtained from the same sequencing primer differed between the sampling points as ITS or group-I intron regions, showing the presence/absence of the group-I intron in the 3ʹ end of the small subunit rRNA region, which is characteristic of C. geophilum (Shinohara et al. Citation1996).
The AMS 14C ages for TE-1, TE-2, and TE-3 sclerotia were determined as “Modern.” The assignment of Modern corresponds to dates after Libby’s base year of 1950 (Stuiver and Polach Citation1977). The results of calibration for post-bomb 14C data provided the calendar ages of TE-1, TE-2, and TE-3 sclerotia as 1980 calAD – 1982 calAD (82.6% probability), 1988 calAD – 1990 calAD (87.6% probability), and 1974 calAD – 1976 calAD (78.6% probability), respectively, at a 95% confidence interval ().
Table 1. The 14C dating results of sclerotia of Cenococcum geophilum in Terelj, Mongolia
The C, O (with H), and N contents in TE-1, TE-2, and TE-3 sclerotia ranged from 42.7% to 45.6%, 37.6% to 39.9%, and 1.3% to 2.1%, respectively, while the ash and water contents ranged from 4.8% to 5.2% and 7.4% to 17%, respectively. Moreover, the mean contents ± SD of C, O (with H), N, and ash of all sclerotia samples (TE-1, TE-2, and TE-3) were 43.9% ± 1.52%, 39.0% ± 1.25%, 1.85% ± 0.45%, and 5.05% ± 0.20%, respectively. The mean water content of the sclerotia was 11.7% (standard deviation, SD ± 4.87, ).
Table 2. Contents of major elements [C, N, O(H)], water, and ash in sclerotia of Cenococcum geophilum in Terelj, Mongolia. Means and standard deviations are indicated
Micromorphological features and elemental composition of sclerotium grains
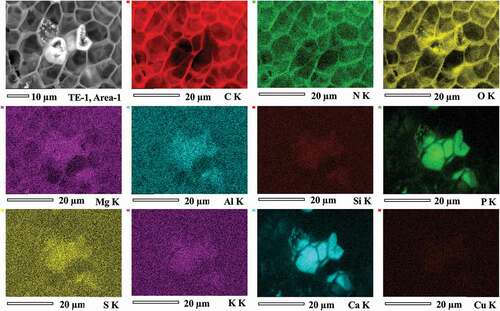
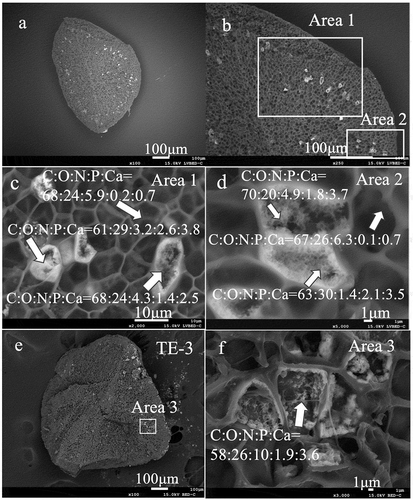
The back-scattered electron images of the opened section of the sclerotium grains of TE-1 and TE-3 are shown in . Bright areas were observed, which tended to have concentric distributions approximately 100 μm from the edge of the grain surface. The enlarged SEM images showed the acicular structures formed in the cells (, d, f). The EDS mapping of all sections revealed that sclerotium cells had strong EDS signals for C and O, and weak signals for Na, Mg, S, Si, Al, Fe, N, Cu, and Zn (, Supplemental Figures S1 and S2). The bright areas were especially rich in Ca and P (, Supplemental Figures S1 and S2). The mean contents of C, N, O, P, and Ca (atom % ±SD) in bright cells (n = 5) were 64.0 ± 4.94, 4.76 ± 3.21, 25.8 ± 4.02, 1.96 ± 0.43, and 3.42 ± 0.52, respectively (, d, f). The mean contents of C, N, O, P, and Ca (atom %) in non-bright cells (n = 2) were 68, 5.9, 24, 0.2, and 0.7 for area 1 and 67, 6.3, 26, 0.1, and 0.7 for area 2, respectively (, d). The Ca/P atom ratio of the bright cells was 1.77 (SD ± 0.22, n = 5).
Figure 1. Morphology and elemental composition (atomic ratios) of the structure of Cenococcum geophilum sclerotia determined by scanning electron microscopy energy dispersive X-ray analysis in Terelj, Mongolia. a–d, sclerotium in the Terelj-1 soil sample; e-f, sclerotium in the Terelj-3 soil sample. The arrows in c, d, and f indicate the elemental composition (atomic ratios) of the cell structures.
Discussion
In comparison to the mean weight ±SD of sclerotia in Harz, Germany: 1.34 ± 1.03 (n = 291) mg and Myoko-1A, Japan: 0.47 ± 0.59 (n = 111) mg reported by Sakagami et al. (Citation2018), the sclerotia weight in Terelj was significantly less than that in the Harz (p < 0.001, t-test) and Myoko (p < 0.001) sclerotia collected from low-pH soils.
The mean contents (± SD, n = 6) of C, O, H, N, and the mean content of water (± SD, n = 9) of sclerotium grains collected from Japanese forest soils (Myoko sclerotia) were 47.6 ± 0.58, 30.2 ± 0.57, 3.32 ± 0.06, 0.79 ± 0.01, and 11.6 ± 0.76%, respectively (Watanabe et al. Citation2007b). In comparison with these data, the mean C and water contents of Terelj sclerotia were not significantly different from those of Myoko sclerotia, whereas the content of N in Terelj sclerotia was significantly higher (p < 0.001) than those from Myoko.
and d display a mean content of P in non-bright cells of Terelj sclerotia of 0.2 in atom % (0.64 in atomic wt %) for area 1, and 0.1 in atom % (0.32 in atomic wt %) for area 2, respectively. The mean P contents inside sclerotia from acidic pH soils were detected as: from 0.7 to 3.0 (n = 3, atomic wt %) for Mt. Harz, Germany (Watanabe et al. Citation2004a), and from 0.54 to 0.83 (n = 4, atomic wt %) for Mt. Myoko, Japan (Watanabe et al. Citation2001). However, P was detected in sclerotia from both soil pH conditions (acidic and slightly alkaline), and microfeatures of mineralized phosphate were not observed in sclerotia from acidic pH forest soils (Watanabe et al. Citation2004b). As a result, characteristic of sclerotia may depending on environment including soil, and plant species.
The 14C ages of sclerotium grains in this study were obtained as the mean formation age of 24–25 sclerotia grains. The dating results could indicate the mean residual time of sclerotia grains in the soil after their formation. Consequently, the calibration results for post-bomb 14C age suggested that the C. geophilum sclerotium grains collected from Terelj soil (0–15 cm) remained in the soil for approximately 40 years. The 14C age of sclerotia collected from the low pH soil in Harz, Germany was reported to be Modern (<50 yr BP) by Sakagami et al. (Citation2018), whereas the 14C ages for the Myoko-1A and Myoko-3A sclerotia from a buried humic A horizon in Japan were reported to be 95–240 and 1020–1200 yr BP by Watanabe et al. (Citation2007a). According to a study by Sakagami et al. (Citation2018), Fourier transform infrared spectroscopy and 14C age analyses suggested that the Harz sclerotia consists of fresh cell materials. Therefore, based on the 14C data, we suggest that C. geophilum sclerotia in Terelj may contain fresh cell materials inside the sclerotia.
SEM micrographs of C. geophilum sclerotia indicated the occurrence of biomineralization by mycelial cells, with evidence provided by EDS analysis. The SEM–EDS analysis showed that Ca specifically coexisted with P in cell compartments inside sclerotia with a concentric distribution of hydroxyapatite (HAP). The Ca/P atomic ratio of the bright cells was 1.77, which suggested the presence of hydroxyapatite (Ca/P 1.67) together with their micro-morphological features, as reported by Raynaud et al. (Citation2002). The formation of hydroxyapatite in fungal mycelia with respect to the biologically induced precipitation of hydroxyapatite has been studied in vitro. Rosling et al. (Citation2007) suggested that hydroxyapatite formation within mycelial spheres is similarly induced by high local P concentrations. This could potentially represent a mechanism of extracellular P immobilization in the direct vicinity of fungal hyphae (Rosling et al. Citation2007). Recently, Li et al. (Citation2018) demonstrated that the presence of phosphate and organic acids in an alkaline environment with a lack of carbon can promote the formation of minerals such as CaCO3 and hydroxyapatite among fungal mycelia. Many microbes maintain high intracellular concentrations of polyphosphates (Beveridge Citation1989), which could release dissolved inorganic phosphate (PO43+) after they die (Gächter and Meyer Citation1993) either through spontaneous hydrolytic degradation or the enzymatic action of phosphatase enzymes such as alkaline phosphatase or phytase (Hirschler et al. Citation1990). Furthermore, this dissolved inorganic phosphate may precipitate as intra- or extracellular apatite, which is a generally insoluble form of phosphorus under neutral to alkaline conditions (Liu et al. Citation2017). The findings of the above-mentioned studies suggest that fungi are responsible for hydroxyapatite formation observed in the natural environment. To our knowledge, this was the first study to demonstrate HAP precipitation in C. geophilum sclerotia found in slightly alkaline forest soils. Moreover, the distribution of HAP precipitation inside sclerotia suggests that HAP accumulation may occur after sclerotia formation and could accumulate over 40 years based on 14C dating results.
HAP precipitation in sclerotia grains may suggest a specific form of P sequestration in slightly alkaline forest soil. Soil microbial P, Ca and organic carbon content in soil may also affect HAP accumulation in sclerotia, although this is not yet fully understood. Further studies are needed to elucidate the biochemical properties of C. geophilum sclerotia in various steppe-forest soils and their involvement in biomineralization.
Conclusions
The novel findings of this study are that sclerotia were found in slightly alkaline forest soils of the Mongolian steppe forest, which were confirmed to be produced by C. geophilum, and that hydroxyapatite accumulation was verified within sclerotia. The P sequestration in the C. geophilum sclerotium grain suggests a biochemical contribution and specific function of this structure in slightly alkaline pH forest soil ecosystems.
Geolocation information
The study was conducted in a forested landscape of Terelj areas (47°55ʹ52.0″ N, 107°25ʹ35.0″ E, 1640 m.a.s.l.).
Supplemental Material
Download Zip (20.8 MB)Acknowledgments
We thank Tomohiro Hatano, JEOL, Japan, for his technical support with the SEM-EDS analysis. We thank the anonymous reviewers for their valuable comments and advice regarding our manuscript.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The procedures of access and benefit-sharing (ABS) (Tokyo Metropolitan University) and permission to export soil and sclerotia samples (Mongolia Ministry of Environment and Tourism) were completed under the Memorandum of Understanding and Material Transfer Agreement between the National University of Mongolia and Tokyo Metropolitan University. Soil and sclerotia samples were imported with permission from the Yokohama Plant Protection Station, Ministry of Agriculture, Forestry and Fisheries, Japan.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Bahram M, Põlme S, Kõljalg U, Tedersoo L. 2011. A single European aspen (Populus tremula) tree individual may potentially harbour dozens of Cenococcum geophilum ITS genotypes and hundreds of species of ectomycorrhizal fungi. FEMS Microbiol Ecol. 75(2):313–320. doi:https://doi.org/10.1111/j.1574-6941.2010.01000.x.
- Beveridge TJ. 1989. The structure of bacteria. In: Poindexter JS, Leadbetter ER, editors. Bacteria in nature. Vol. 3: structure, physiology and genetic adaptability. New York (NY): Plenum Press; p. 1–15.
- Courty P-E, Franc A, Garbaye J. 2010. Temporal and functional pattern of secreted enzyme activities in an ectomycorrhizal community. Soil Biol Biochem. 42(11):2022–2025. doi:https://doi.org/10.1016/j.soilbio.2010.07.014.
- Dickie IA, Reich PB. 2005. Ectomycorrhizal fungal communities at forest edges. J Ecol. 93(2):244–255. doi:https://doi.org/10.1111/j.1365-2745.2005.00977.x.
- Dorjgotov D. 2003. Mongolian soil (in Mongolia with English summary). Ulaanbaatar: Institute of Geography of Mongolian Academy of Sciences: ADMON.
- Dorjgotov D. editor. 2004. Geographic atlas of Mongolia. Ulaanbaatar: Administration of Land Affairs, Geod Cartogr.
- Dorjgotov D, Shirnin G. editors. 1976. Mongolian soil-geographical area (in Mongolia). Ulaanbaatar: Academy of Sciences Publishing press.
- Douhan GW, Huryn KL, Douhan LI. 2007. Significant diversity and potential problems associated with inferring population structure within the Cenococcum geophilum species complex. Mycologia. 99(6):812–819. doi:https://doi.org/10.1080/15572536.2007.11832513.
- Fernandez CW, McCormack ML, Hill JM, Pritchard SG, Koide RT. 2013. On the persistence of Cenococcum geophilum ectomycorrhizas and its implications for forest carbon and nutrient cycles. Soil Biol Biochem. 65:141–143. doi:https://doi.org/10.1016/j.soilbio.2013.05.022.
- Food and Agriculture Organization of the United Nations. 2015. World reference base for soil resources 2014. World soil resources report 106. [accessed 2018 Jan 11]. http://www.fao.org/3/i3794en/I3794en.pdf.
- Gächter R, Meyer JS. 1993. The role of microorganisms in mobilization and fixation of phosphorus in sediments. Hydrobiologia. 253(1–3):103–121. doi:https://doi.org/10.1007/BF00050731.
- Glassman SI, Peay KG, Talbot JM, Smith DP, Chung JA, Taylor JW, Vilgalys R, Bruns TD. 2015. A continental view of pine-associated ectomycorrhizal fungal spore banks: a quiescent functional guild with a strong biogeographic pattern. New Phytol. 205(4):1619–1631. doi:https://doi.org/10.1111/nph.13240.
- Hirschler A, Lucas J, Hubert J-C. 1990. Bacterial involvement in apatite genesis. FEMS Microbiol Ecol. 73(3):211–220. doi:https://doi.org/10.1111/j.1574-6968.1990.tb03943.x.
- Hua Q, Barbetti M, Rakowski AZ. 2013. Atmospheric radiocarbon for the period 1950-2010. Radiocarbon. 55(4):2059–2072. doi:https://doi.org/10.2458/azu_js_rc.v55i2.16177.
- Jonsson L, Anders D, Tor-Erik B. 2000. Spatiotemporal distribution of an ectomycorrhizal community in an oligotrophic Swedish Picea abies forest subjected to experimental nitrogen addition: above- and below-ground views. Forest Ecol Manag. 132(2–3):143–156. doi:https://doi.org/10.1016/S0378-1127(99)00220-0.
- Li T, Hu Y, Zhang B. 2018. Biomineralization induced by Colletotrichum acutatum: a potential strategy for cultural relic bioprotection. Front Microbiol. 9:1884. doi:https://doi.org/10.3389/fmicb.2018.01884.
- Liu Y, Cheng Z, Branco B, Marra J. 2017. Speciation and mobility of phosphate in the eutrophic ponds at prospect park, Brooklyn, New York, USA. J Geosci Environ Prot. 5:26–36.
- LoBuglio KF. 1999. Cenococcum. In: Cairney JWG, John WG, Chambers SM, editors. Ectomycorrhizal fungi key genera in profile. Berlin: Springer; p. 287–309.
- Matsuda Y, Hayakawa N, Ito S. 2009. Local and microscale distributions of Cenococcum geophilum in soils of coastal pine forests. Fungal Ecol. 2(1):31–35. doi:https://doi.org/10.1016/j.funeco.2008.10.002.
- Mongolian Statistical Information Service. 1999-2017. Nature and Environment/Table/Multiyear average of climate, by station, by year/Average Air temperature, by station, by month, year. [accessed 2021 Jun 26]. https://www.1212.mn/.
- Nyamsanjaa K, Oyuntsetseg B, Watanabe M. 2021b. Melanized sclerotia grains from Mongolian steppe forest soils. In: Watanabe M, editor. Sclerotia grains in soil- A new perspective from Pedosclerotiology. Singapore: Springer Nature; p. 193–208.
- Nyamsanjaa K, Watanabe M, Sakagami N, Oyuntsetseg B. 2021a. Metal accumulation in sclerotium grains collected from low pH forest soils. J Environ Sci Health Part A. 56(3):303–309. doi:https://doi.org/10.1080/10934529.2021.1872316.
- Obase K, Cha JY, Lee JK, Lee SY, Lee JH, Chun KW. 2009. Ectomycorrhizal fungal communities associated with Pinus thunbergii in the eastern coastal pine forests of Korea. Mycorrhiza. 20(1):39–49. doi:https://doi.org/10.1007/s00572-009-0262-1.
- Obase K, Lee JK, Lee SY, Chun KW. 2011. Diversity and community structure of ectomycorrhizal fungi in Pinus thunbergii coastal forests in the eastern region of Korea. Mycoscience. 52(6):383–391. doi:https://doi.org/10.1007/S10267-011-0123-6.
- Ramsey BC. 2009. Bayesian analysis of radiocarbon dates. Radiocarbon. 51(1):337–360. doi:https://doi.org/10.1017/S0033822200033865.
- Raynaud S, Champion E, Bernache-Assollant D, Thomas P. 2002. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials. 23(4):1065–1072. doi:https://doi.org/10.1016/S0142-9612(01)00218-6.
- Reimer PJ, Austin WEN, Bard E, Bayliss A, Blackwell PG, Ramsey CB, Butzin M, Cheng H, Edwards RL, Friedrich M, et al. 2020. The IntCal20 northern hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon. 62(4):725–757. doi:https://doi.org/10.1017/RDC.2020.41
- Rosling A, Suttle KB, Johansson E, Van Hees PAW, Banfield JF. 2007. Phosphorous availability influences the dissolution of apatite by soil fungi. Geobiology. 5(3):265–280. doi:https://doi.org/10.1111/j.1472-4669.2007.00107.x.
- Sakagami N, Guo Y, Watanabe M. 2018. Physicochemical characteristics of Cenococcum sclerotia formed in different types of forest soil. Soil Microorg. 72(1):50–55.
- Shinohara ML, LoBuglio KF, Rogers SO. 1996. Group-I intron family in the nuclear ribosomal RNA small subunit genes ofCenococcum geophilum isolates. Curr Genet. 29(4):377–387. doi:https://doi.org/10.1007/BF02208619.
- Smith ME, Douhan GW, Rizzo DM. 2007. Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytol. 174(4):847–863. doi:https://doi.org/10.1111/j.1469-8137.2007.02040.x.
- Streckeisen A. 1976. To each plutonic rock its proper name. Earth Sci Rev. 12(1):1–33. doi:https://doi.org/10.1016/0012-8252(76)90052-0.
- Stuiver M, Polach HA. 1977. Discussion: reporting of 14C data. Radiocarbon. 19(3):355–363. doi:https://doi.org/10.1017/S0033822200003672.
- Takashima Y, Seto K, Degawa Y, Guo Y, Nishizawa T, Ohta H, Narisawa K. 2018. Prevalence and intra-family phylogenetic divergence of burkholderiaceae-related endobacteria associated with species of Mortierella. Microbes Environ. 33(4):417–427. doi:https://doi.org/10.1264/jsme2.ME18081.
- Trappe JM 1962. Cenococcum graniforme - its distribution, ecology, mycorrhiza formation, and inherent variation [dissertation]. Seattle (WA): University of Washington.
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 172(8):4238–4246. doi:https://doi.org/10.1128/jb.172.8.4238-4246.1990.
- Watanabe M, Fujitake N, Ohta H, Yokoyama T. 2001. Aluminum concentrations in sclerotia from a buried humic horizon of volcanic ash soils in Mt. Myoko, Central Japan. Soil Sci Plant Nutr. 47(2):411–418. doi:https://doi.org/10.1080/00380768.2001.10408404.
- Watanabe M, Genseki A, Sakagami N, Inoue Y, Ohta H, Fujitake N. 2004b. Aluminum oxyhydroxide polymorphs and some micromorphological characteristics in sclerotium grains. Soil Sci Plant Nutr. 50(8):1205–1210. doi:https://doi.org/10.1080/00380768.2004.10408595.
- Watanabe M, Inoue Y, Sakagami N, Bolormaa O, Kawasaki K, Hiradate S, Fujitake N, Ohta H. 2007b. Characterization of major and trace elements in sclerotium grains. Eur J Soil Sci. 58(3):786–793. doi:https://doi.org/10.1111/j.1365-2389.2006.00868.x.
- Watanabe M, Ohishi S, Pott A, Hardenbicker U, Aoki K, Sakagami N, Ohta H, Fujitake N. 2004a. Soil chemical properties and distribution of sclerotium grains in forest soils, Harz Mts., Germany. Soil Sci Plant Nutr. 50(6):863–870. doi:https://doi.org/10.1080/00380768.2004.10408547.
- Watanabe M, Sakagami N, Tonosaki K. 2021. Dating of sclerotia grains in Andosol profiles. In: Watanabe M, editor. Sclerotia grains in soil- A new perspective from Pedosclerotiology. Singapore: Springer Nature; p. 119–137.
- Watanabe M, Sato H, Matsuzaki H, Kobayashi T, Sakagami N, Maejima Y, Ohta H, Fujitake N, Hiradate S. 2007a. 14C ages and δ13C of sclerotium grains found in forest soils. Soil Sci Plant Nutr. 53(2):125–131. doi:https://doi.org/10.1111/j.1747-0765.2007.00121.x.
- White TJ, Bruns TD, Lee SB, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide Meth Appl. 18:315–322.