?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bamboo forests are expected to play an important role in mitigating climate change. However, the capacity of bamboo forests to sequester and store carbon has not been assessed on a national scale in Japan. This study estimates the changes in the carbon stock of bamboo forests (CSbamboo) in Japan from 1985 to 2005. We compiled two data sets: (1) administrative statistical data on bamboo stand area (BSA) and (2) published data on stand-level aboveground biomass for Phyllostachys pubescens Mazel ex Houz. (n = 44) and Phyllostachys bambusoides Sieb. et Zucc. (n = 13). The BSA expanded from 147 kha in 1985 to 158 kha in 2005. The average carbon density (CD) of managed and abandoned stands were 84.9 Mg C ha−1 and 115.1 Mg C ha−1 for P. pubescens and 24.1 Mg C ha−1 and 46.4 Mg C ha−1 for P. bambusoides, respectively. The CD of P. pubescens stands in Japan was higher than that in other Asian countries and regions and was comparable to the CD of other forests in Japan. The area-based method indicated that the CSbamboo increased from 10.1 ± 2.6 Tg C to 13.9 ± 1.7 Tg C during the 20-year period, representing less than 1% of the total carbon pool of the forested area. The increase in CSbamboo was primarily caused by the synergistic effect of the abandonment and range expansion of P. pubescens forests. Our results suggest that P. pubescens forests are overstocked, and the increase in the CSbamboo is undesirable.
Introduction
Global warming is one of the major consequences of anthropogenic activities associated with the overuse of fossil fuels as energy resources (Al-Ghussain Citation2019). Forests are one of the most effective carbon sinks in terrestrial ecosystems (de Wit et al. Citation2015; Palmer Citation2021) and mitigate climate change with their carbon sequestration and storage capacity (Withey et al. Citation2019; Kilpeläinen and Peltola Citation2022). Therefore, assessments of the carbon sequestration and storage of forests have become a vital issue and the focal point of recent studies (Nunery and Keeton Citation2010; Meijaard et al. Citation2014).
Bamboo is the vernacular or common term for members of a particular taxonomic group of large woody grasses (family: Poaceae, subfamily: Bambusoideae; Scurlock et al. Citation2000). There are nearly 1500 species in approximately 119 genera, native to all continents except Antarctica and Europe (Clark et al. Citation2015). The total area of bamboo forests is approximately 31.5 million ha worldwide, accounting for approximately 1% of the total global forested area (Lobovikov et al. Citation2007; Song et al. Citation2011; Zhou et al. Citation2011). Bamboo is one of the fastest-growing woody plants in the world, with a maximum growth rate of 100 cm per day during the growing season (He et al. Citation2013; Song et al. Citation2016; Yen Citation2016). Such rapid biomass accumulation implies that bamboo forests have high carbon stock production and excellent carbon sequestration potential (Zhou et al. Citation2005, Citation2011; Zhang et al. Citation2014; Nath et al. Citation2015; Wu et al. Citation2015; Lin et al. Citation2017). Bamboo is also well known to be a highly versatile material; approximately 1500 commercial applications of bamboo have been identified (Scurlock et al. Citation2000; Buckingham et al. Citation2011). It has been suggested that the use of bamboo products with relatively long life spans mitigates climate change through the long-term storage of sequestered carbon (Düking et al. Citation2011; Kuehl et al. Citation2011; Li et al. Citation2015).
In Asian countries and regions, to assess the capacity of bamboo forests to sequester and store carbon, allometric equations have been developed for estimating the biomass of various bamboos from commonly measured culm attributes such as the diameter at breast height (Yuen et al. Citation2017; Hui and Long Citation2019; Brahma et al. Citation2021). Using these equations, the biomass or carbon density (CD; carbon stock density per hectare) of bamboo stands has been quantified (Yen et al. Citation2010; Zhang et al. Citation2014; Yen Citation2015; Lin et al. Citation2017; Xu et al. Citation2018a; Abebe et al. Citation2021; Liu and Yen Citation2021) and compared with that of plantations and natural forests (Yen and Lee Citation2011; Zhou et al. Citation2011; Yen and Wang Citation2013; Wang et al. Citation2013). The CD was also scaled up to the regional or national carbon stock of bamboo forests (CSbamboo) using area-based method (Chen et al. Citation2009; Wang et al. Citation2013; Li et al. Citation2015; Xu et al. Citation2018a, Citation2018b; Devi and Singh Citation2021; Ouyang et al. Citation2022).
In Japan, Phyllostachys pubescens Mazel ex Houz. (P. pubescens) and Phyllostachys bambusoides Sieb. et Zucc. (P. bambusoides) are the major useful bamboo species (Inoue et al. Citation2013). Bamboo stands of the two species have typically been managed by thinning of old culms and harvesting bamboo shoot (Suzuki and Nakagoshi Citation2008; Inoue et al. Citation2018). Sexual reproduction of these species is episodic with the intervals ranging from several decades to more than a 100 year, and new culms are produced every year without planting (Isagi et al. Citation2016). The culms of bamboo have been traditionally used as building materials and other commodities, whereas the edible shoots are used as seasonal ingredients in Japanese cuisine (Shibata Citation2003; Utsumi et al. Citation2010; Suzuki and Nakagoshi Citation2011; Shima et al. Citation2023). There are approximately 150 kha of bamboo forests in Japan (Lobovikov et al. Citation2007), with the two species accounting for 99% of the bamboo stand area (BSA) in 1993 (Torii and Isagi Citation1997; Takano et al. Citation2017). The biomass or CD of bamboo stands has been quantified using allometric models (Isagi et al. Citation1993, Citation1997; Isagi Citation1994). However, to our knowledge, there have been no studies on the regional or national CSbamboo in Japan. Although the national carbon stocks (CS) of other forests in Japan have been assessed (Fukuda et al. Citation2003; Fang et al. Citation2005; Sasaki and Kim Citation2009; Egusa et al. Citation2020), bamboo forests have been excluded from these analyses. How much carbon is stored in bamboo forests in Japan? How is it changing? If it is changing, what factors are responsible for the change? Information regarding the CSbamboo is required to improve our understanding of carbon dynamics in bamboo forests, develop policies for mitigating future climate change, and optimize bamboo forest management strategies and practices (Li et al. Citation2015; Xu et al. Citation2018a; Wang et al. Citation2023).
The purpose of this study is to estimate the change in CSbamboo in Japan from 1985 to 2005. We also compare the estimated CSbamboo with that in China and the CS of other forests (CStrees) in Japan.
Materials and methods
Changes in BSA
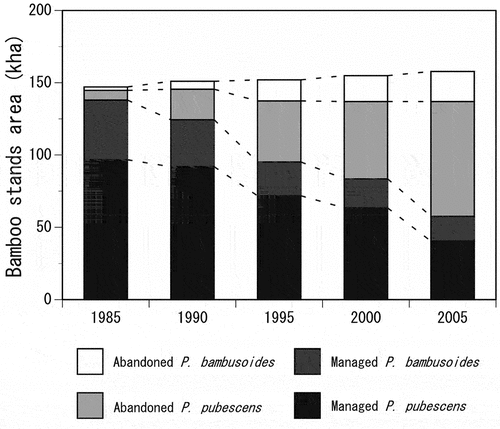
The BSA for the two decades between 1985 and 2005 was reconstructed using the procedure outlined in . Unfortunately, the BSA before and after the period from 1985 to 2005 could not be reconstructed due to unavailability of data. Since P. pubescens and P. bambusoides accounted for 99% of total BSA in Japan in 1993 (Torii and Isagi Citation1997; Takano et al. Citation2017), only these two species were considered in this study. The bamboo forests in Japan have been managed for the production of culm wood and bamboo shoots. For example, it is recommended that a stand of P. pubescens with D = 10 cm should be managed to maintain a density of 5000 culms ha−1 for bamboo shoot production and 8000 culms ha−1 for culm wood production (Uchimura Citation2009). However, the number of abandoned bamboo forests has been increasing since 1980s (Shibata Citation2003, Citation2010; Shinohara et al. Citation2014; Manabe et al. Citation2020). Therefore, the bamboo forests were classified into four types: two management conditions (managed and abandoned) × two species (P. pubescens and P. bambusoides).
Table 1. Procedures for estimating the bamboo stand area for each bamboo forest type.
We consolidated the Japan Statistical Yearbooks (published by the Statistics Bureau, Ministry of Internal Affairs and Communications, Japan). The Yearbooks shows the total BSA compiled in Forest Register Data for the establishment of National and Regional Forest Planning in Japan. The yearbooks enabled us to obtain the total BSA for 1980, 1985, 1990, 1995, 2002, and 2007. From these values, linear interpolation was used to determine the total BSA for 1984, 2000, and 2005. Note that data on the BSA for each type of bamboo forest as mentioned above were unavailable in this material.
In order to determine the BSA for each type of bamboo forest, we also consulted the working document of the Japan Forestry Agency, which was published as an appendix to the Bamboo Journal No. 1–28. This material includes managed BSA values for P. pubescens and P. bambusoides for culm wood and bamboo shoots production from 1982 to 2009. From this data, we extracted the managed BSAs for each of the two species in 1984, 1985, 1990, 1995, 2000, and 2005. The abandoned BSAs for the two species were computed by subtracting the managed BSA from the total BSA.
It was reported that the BSA ratio between P. pubescens and P. bambusoides was 3:1 in 1993 (Torii and Isagi Citation1997; Takano et al. Citation2017). Therefore, the abandoned BSA for each species in 1995 was estimated to ensure that the ratio of BSA for P. pubescens and P. bambusoides was 3:1. The abandoned BSA for each species in 1985, 1990, 2000, and 2005 were predicted as follows: Manabe et al. (Citation2020) reviewed the range expansion of bamboo forests in Japan, and showed that P. pubescens accounted for the majority of the cases of expansion. Moreover, based on their reproductive capacity, it is unlikely that P. bambusoides forests expanded their ranges (Shibata Citation2010). Hence, assuming that the decrease in managed BSA for P. bambusoides equaled the increase in abandoned BSA for the species, the abandoned BSA for P. bambusoides in 1985, 1990, 2000, and 2005 were then estimated. Note that this approach results in a negative value for the abandoned BSA of P. bambusoides prior to 1984. For this reason, the abandoned BSA for P. bambusoides in 1985 was calculated using the difference in the managed BSA for P. bambusoides between 1984 and 1985 and the assumption that the abandoned BSA for P. bambusoides in 1984 was 0 kha. Finally, the abandoned BSA for P. pubescens was predicted by subtracting the managed BSA for the two species and the abandoned BSA for P. bambusoides from the total BSA.
CD of bamboo stands
To determine the representative CD of bamboo stands in Japan, we compiled the published data on the stand-level aboveground biomass (AGB) for P. pubescens (n = 47), P. bambusoides (n = 22), and a mix of P. pubescens, P. bambusoides and Phyllostachys nigra var. henonis (n = 25) in Japan from 28 literature sources (Supplementary Appendix). Of these, data on P. pubescens (7 managed and 37 abandoned stands) and P. bambusoides (3 managed and 10 abandoned stands) pure stands, where the presence or absence of management was described in the literature, were used in the analysis, but other data are included in Supplementary Appendix.
Because of insufficient information on the belowground biomass (BGB), the AGB of each stand was extrapolated to the whole-plant biomass (WPB=AGB+BGB) as follows: According to the review by Yuen et al. (Citation2017), the stand-level root:shoot ratio (=BGB/AGB) for P. pubescens and P. bambusoides in Asia (i.e. China, Japan, Taiwan, and Korea) were 0.55 (n = 125) and 0.44 (n = 2), respectively. Others have shown that the ratio for P. pubescens stands in China was 0.37 ± 0.07 (Ouyang et al. Citation2022) and that the ratio in subtropical China ranged from 0.41 to 0.49 (Wang et al. Citation2013). Wang et al. (Citation2013) also concluded that the AGB was twice the value of the BGB, indicating that the root:shoot ratio was 0.5. In Japan, a few researchers examined both AGB and BGB for P. pubescens (Suzuki Citation1976; Isagi et al. Citation1997; Fukushima et al. Citation2015; Shimono et al. Citation2021) and P. bambusoides (Isagi Citation1994). Using these data, the ratios were 0.49 ± 0.22 for P. pubescens (n = 8) and 0.40 for P. bambusoides (n = 1). Therefore, we assumed that the root:shoot ratio were 0.5 for P. pubescens and 0.4 for P. bambusoides, and the AGB was converted into the WPB.
The obtained WPB for each stand was then multiplied by the percent carbon content (PCC) to yield the CD. Although the PCC varies slightly among bamboo sections (leaves, branches, culms, and roots; Isagi Citation1994; Yen and Lee Citation2011; Zhang et al. Citation2014) and species (Chen et al. Citation2009), we assumed the constant value of 0.5 in the PCC (Wang et al. Citation2013; Zhang et al. Citation2014; Nath et al. Citation2015; Ouyang et al. Citation2022), i.e. CD = 0.5×WPB. In short, the AGB published in the literature was converted into the CD, i.e. CD = 0.75×AGB for P. pubescens and CD = 0.70×AGB for P. bambusoides. The values of the AGB, WPB, and aboveground CD of bamboo stands cited in this study were also converted into the CD based on the same assumptions when the whole-plant CD was not shown in the literature.
The average, standard deviation (SD) and standard error (SE) of the CD for the four types of bamboo forests, i.e. managed and abandoned stands for each P. pubescens and P. bambusoides, were calculated using the compiled data. The CD was compared among the four types of bamboo forests using the Steel-Dwass test, and their averages were used as the representative CD of bamboo stands in Japan.
CS of bamboo forests
The CSbamboo in Japan was estimated from 1985 to 2005 with 5-year intervals using the area-based method (Chen et al. Citation2009; Wang et al. Citation2013; Li et al. Citation2015; Ouyang et al. Citation2022) as follows:
where i is the type of bamboo forests, i.e. i = 1: managed P. pubescens forests, i = 2: abandoned P. pubescens forests, i = 3: managed P. bambusoides forests, and i = 4: abandoned P. bambusoides forests.
In this study, we addressed the uncertainties in CD_i and CS (UCD_i and UCS, respectively) as follows: For practical assessment of the UCD_i, the half-width of the 95% confidence interval (95%CICD_i_half-width) was used (Kauffman and Donato Citation2012; Li et al. Citation2015):
The UCD_i is then expressed as:
where μCD_i is the average CD_i. In this study, the total and managed BSA were exact values with no uncertainty, while the abandoned BSA was calculated from the total and managed BSA. Hence, since it is impossible to quantify the uncertainty of the BSA, the UCS is calculated by the following equation (IPCC Citation2000):
Statistical analysis was performed using R version 4.2.2 (R Core Team Citation2023), and P < 0.05 was considered statistically significant.
Results
Changes in BSA
depicts the changes in the BSA in Japan from 1985 to 2005. The total BSA increased from 147 kha in 1985 to 158 kha in 2005 at a rate of 0.55 kha year−1 on average. Although the ratio of the managed BSA to total BSA was greater than 90% in 1985, it substantially decreased to less than 40% in 2005. In particular, the managed BSA for P. pubescens markedly decreased from 96.7 kha to 40.4 kha over the 20-year period. In contrast, the abandoned BSA for P. pubescens increased from 6.7 kha to 79.4 kha and accounted for more than half of the total BSA in 2005. The increase in the abandoned BSA between 1985 and 2005 was approximately four times greater in P. pubescens (72.7 kha) than in P. bambusoides (18.4 kha).
CD of bamboo stands
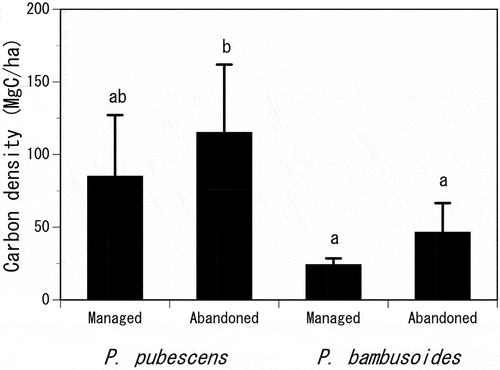
The AGB of managed and abandoned stands for P. pubescens were 113.2 ± 56.3 t ha−1 and 153.4 ± 62.3 t ha−1, respectively, whereas those for P. bambusoides were 34.4 ± 6.3 t ha−1 and 66.3 ± 28.7 t ha−1, respectively (see Supplementary Appendix). Therefore, the CD of managed and abandoned stands were estimated to be 84.9 ± 42.2 Mg C ha−1 and 115.1 ± 46.8 Mg C ha−1 for P. pubescens and 24.1 ± 4.4 Mg C ha−1 and 46.4 ± 20.1 Mg C ha−1 for P. bambusoides, respectively (average ± SD; ). For both species, the CD of abandoned stands was higher than that of managed stands, although the difference was not statistically significant (P = 0.452 for P. pubescens and P = 0.329 for P. bambusoides). The difference in the CD between managed and abandoned stands was greater for P. pubescens (30.2 Mg C ha−1) compared to P. bambusoides (22.3 Mg C ha−1).
Changes in CS of bamboo forests
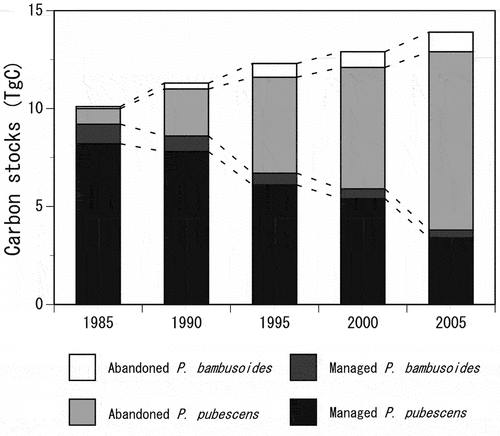
The change in CSbamboo in Japan is illustrated in . The area-based method indicated that the CSbamboo gradually increased over the 20-year period: 10.1 ± 2.6 Tg C in 1985, 11.3 ± 2.6 Tg C in 1990, 12.2 ± 2.3 Tg C in 1995, 12.9 ± 2.1 Tg C in 2000, and 13.9 ± 1.7 Tg C in 2005 (estimate ± uncertainty). The average annual increase rate of the CSbamboo between 1985 and 2005 was 0.19 Tg C year−1. The CS for P. pubescens forests accounted for approximately 90% of the CSbamboo throughout the 20-year period. The CS of the managed P. pubescens forests decreased from 8.2 Tg C to 3.4 Tg C, whereas that of the abandoned P. pubescens forests increased from 0.8 Tg C to 9.1 Tg C. In contrast, the increase in CS for P. bambusoides was considerably smaller (from 1.1 Tg C to 1.4 Tg C).
Discussion
CD of bamboo stands
When predicting the CSbamboo using an area-based method, the CD for bamboo stands is required. Therefore, this study determined the representative CD of managed and abandoned stands for each P. pubescens and P. bambusoides based on previously published data in Japan (). For the purpose of comparison, the CD for the two species in Asia is summarized in . The average CD for P. pubescens ranges from 40.7 Mg C ha−1 in Taiwan (Ouyang et al. Citation2022) to 73.8 Mg C ha−1 in Japan (Lin et al. Citation2017), whereas that for P. bambusoides is 45.7 Mg C ha−1 in Japan and South Korea (Yuen et al. Citation2017). It is apparent that the CD for both managed and abandoned P. pubescens stands is higher in Japan than in other countries and regions. However, the CD for the abandoned P. bambusoides stands is comparable to the CD for P. bambusoides stands in Japan and South Korea reported in previous study (Yuen et al. Citation2017).
Table 2. Carbon density of Phyllostachys pubescens and Phyllostachys bambusoides stands in Asian countriesa.
Why is the CD for P. pubescens stands higher in Japan? One of the possible reasons is that P. pubescens forests in Japan have been abandoned since the 1980s (Shibata Citation2003, Citation2010; Shinohara et al. Citation2014; Manabe et al. Citation2020), whereas those in other countries and districts have been intensively or extensively managed as an important forest type (e.g. Zhou et al. Citation2005, Citation2011; Chen et al. Citation2009; Li et al. Citation2015; Yen Citation2015; Liu and Yen Citation2021). When a bamboo stand is abandoned, the culm density (ρ) increases (Inoue et al. Citation2012). Since the average diameter at breast height () is negatively correlated with ρ (Yen and Lee Citation2011; Yen Citation2015; Inoue et al. Citation2018), the abandonment of bamboo stands results in a smaller
. The allometric relationship between diameter and AGB is expressed as a monotonically increasing function (Yuen et al. Citation2017; Hui and Long Citation2019; Brahma et al. Citation2021). Hence, the average AGB (
) decreases as
becomes smaller. The increase in ρ is more pronounced than the decrease in
or
due to the abandonment. In the case of P. pubescens stands in Japan (n = 415: Inoue et al. Citation2018), the variation in ρ (1,000–21,900 culms ha−1) is considerably larger than the variation in
(6.2–15.0 cm). Since CD=ρ×
, the CD is likely to increase as the bamboo stand is abandoned. Therefore, it can be expected that the higher CD in Japan is mainly produced by the difference in the management conditions of P. pubescens forests between the countries. The difference in the CD between managed and abandoned bamboo stands () could be explained by the same analogy.
The higher CD implies that the abandoned bamboo forests in Japan are possibly overstocked, resulting in self-thinning. Since the carbon loss resulting from dead bamboo via self-thinning could be compensated by newly emerging bamboo shoots every year (Inoue et al. Citation2018; NIES Citation2020), the abandonment of bamboo stands reaches a stabilized CDbamboo and ceases carbon accumulation. Conversely, the decomposition of dead bamboo produces carbon emissions (Düking et al. Citation2011). Hence, the abandoned bamboo forests could serve as carbon sources (Zhou et al. Citation2011).
This study also compares the CD for bamboo stands (CDbamboo) with the CD for other stands in Japan (CDtrees). The CDtrees values reported in previous studies range from 1.3 Mg C ha−1 in young Chamaecyparis obtusa Endl. plantations to 176.2 Mg C ha−1 in old Cryptomeria japonica D. Don plantations (Fukuda et al. Citation2003), although it varies greatly with stand type (plantations or natural forests), stand age, and species (). As Nath et al. (Citation2015) suggested, the CDbamboo is within the range of the CDtrees. This fact provides an important perspective for discussing the contribution of bamboo forests to combating climate change. It takes several decades for forests to reach the level of CDtrees, whereas bamboo forests reach the CDbamboo in only a few years. Therefore, the carbon sequestration capacity of bamboo forests would be higher than that of other forests (Chen et al. Citation2009; Yen et al. Citation2010; Yen and Lee Citation2011; Zhou et al. Citation2011).
Table 3. Carbon density of other forests in Japana.
CS of bamboo forests
It was estimated that the CSbamboo in Japan was 10.1 Tg C in 1985 and 13.9 Tg C in 2005 (). As shown in , several authors have projected the national-scale CSbamboo in China (Chen et al. Citation2009; Wang et al. Citation2013; Li et al. Citation2015; Ouyang et al. Citation2022). Even when accounting for estimation uncertainty, the CSbamboo in Japan is considerably lower than the CSbamboo in China. The area-based method determines the CSbamboo by multiplying BSA and CDbamboo. There are 6720 kha of bamboo forests in China (Li et al. Citation2015), accounting for approximately 3% of the total forest area (Chen et al. Citation2009), whereas the BSA in Japan was less than 160 kha between 1985 and 2005 (). In contrast, as discussed above, the CDbamboo is higher in Japan than in China. Therefore, the lower CSbamboo in Japan is not a result of the difference in CDbamboo but rather to the difference in BSA between Japan and China.
Table 4. National-scale carbon stock of bamboo forests in China.
In addition, we compare the CSbamboo with the CS of other forests in Japan (CStrees). summarizes the CStrees estimates in Japan from previous studies (Fukuda et al. Citation2003; Fang et al. Citation2005; Sasaki and Kim Citation2009; Egusa et al. Citation2020). Despite the larger variation in the CStrees estimate and uncertainty in the CSbamboo, the CSbamboo is significantly smaller than the CStrees. The CSbamboo predicted in this study accounts for less than 1% of the total national CStrees excluding bamboo forests estimated by Egusa et al. (Citation2020). Since CDbamboo is within the range of CDtrees, the lower portion of CSbamboo to CStrees could also be attributed to the difference in area between bamboo and other forests. The lower CSbamboo implies that bamboo forests in Japan play a lesser role as carbon sinks compared to other forests in Japan as well as bamboo forests in China. However, as discussed above, the higher carbon sequestration capacity of bamboo forests should be emphasized.
Table 5. Carbon stock of other forests in Japan.
The increase in CSbamboo suggests that bamboo forests in Japan act as carbon sinks. What is the determinant of the increase in the CSbamboo in Japan? Since CSbamboo=BSA×CDbamboo, the increases in both BSA and CDbamboo are attributed to the increase in the CSbamboo. Let us now consider the reason for the increase in CSbamboo by dissecting each increase in BSA and CDbamboo. In China, the BSA increases with the afforestation of bamboo (Chen et al. Citation2009; Li et al. Citation2015). However, culm wood and bamboo shoots production have been shown to decline in Japan (Shibata Citation2003, Citation2010; Shinohara et al. Citation2014; Manabe et al. Citation2020); thus, an increase in BSA due to afforestation over a 20-year period is unlikely. In fact, the managed BSA for both species examined in this study monotonically decreased during the 20 years (). In contrast, many studies have shown increases in BSA through the asexual reproduction since the 1980s; the range expansion of bamboo forests to adjacent stands or farms by shooting new culms from their rhizome systems (Torii and Isagi Citation1997; Shibata Citation2003, Citation2010; Suzuki and Nakagoshi Citation2008; Shinohara et al. Citation2014; Suzuki Citation2015; Manabe et al. Citation2020). Since the range expansion of P. bambusoides forests is unlikely (Shibata Citation2010; Manabe et al. Citation2020), the increase in the total BSA could be caused by the range expansion of P. pubescens forests. Of course, the expanded bamboo forests are accounted for as abandoned BSA.
In addition, the CDbamboo of the abandoned stands was greater than that of the managed stands for the two species examined in this study, although the differences were not statistically significant (). The difference in the CDbamboo between managed and abandoned stands was also greater for P. pubescens compared to P. bambusoides. This implies that the impact of the abandonment of bamboo forests on the increase in CSbamboo could be greater for P. pubescens. For these reasons, the increase in the CSbamboo is mainly caused by the synergistic effect of the increase in BSA owing to range expansion and the increase in the CDbamboo resulting from the abandonment of P. pubescens forests. This is consistent with the rapid increase in CS for the abandoned P. pubescens forests (). Therefore, it must be stated that the increase in CSbamboo in Japan is undesirable.
Estimates of CSbamboo in this study include various uncertainties. For example, the CDbamboo is assumed to be constant for each bamboo forests type. However, studies have shown that the CDbamboo varies with the anthropogenic (Zhou et al. Citation2011; Yen Citation2015; Mao et al. Citation2016, Citation2017) as well as the environmental factors (Wang et al. Citation2013; Li et al. Citation2015, Xu et al. Citation2018b; Liu and Yen Citation2021; Ouyang et al. Citation2022). Moreover, while the shoot:root ratio is assumed to be a constant at 0.5 for P. pubescens and 0.4 for P. bambusoides in this study, it also varies within and among sites, and is typically chaotic (Yuen et al. Citation2017; Kobayashi et al. Citation2023; Wang et al. Citation2023). In addition, the PCC, which is assumed to be a constant at 0.5, differs slightly between leaves, branches, culms and roots (Isagi Citation1994; Yen et al. Citation2010; Zhang et al. Citation2014). Among them, only the uncertainty in CDbamboo was taken into account when predicting CSbamboo. Therefore, it should be noted that the CSbamboo is an approximation and that the actual uncertainty in CSbamboo could be even greater. Moreover, the soil organic carbon of bamboo forests and the bamboo products are also important carbon sinks (e.g. Chen et al. Citation2009; Zhou et al. Citation2011; Li et al. Citation2015; Nath et al. Citation2015; Mao et al. Citation2016; Abebe et al. Citation2021; Orrego et al. Citation2022). In the future, it will be necessary to assess CSbamboo more accurately and precisely in Japan by considering these factors.
Conclusions
In this study, we predicted the change in the CSbamboo in Japan from 1985 to 2005. The results showed that the BSA increased from 147 kha to 158 kha. The CD of P. pubescens stands was higher in Japan compared to other Asian countries because of the abandonment. The CSbamboo in Japan increased from 10.2 ± 2.6 Tg C to 13.9 ± 1.7 Tg C during the 20-year period, which accounted only for less than 1% of the total CS of the forested area. The increase in the CSbamboo was mainly produced by the synergistic effect of the abandonment and range expansion of P. pubescens forests. These findings suggest that the abandoned P. pubescens forests are probably overstocked, and the increase in CSbamboo in Japan is undesirable. It is necessary to manage the bamboo forests and utilize the harvested bamboo to maintain their carbon sequestration capacity properly.
Supplemental Material
Download MS Word (40 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/13416979.2023.2229984
Additional information
Funding
References
- Abebe S, Minale AS, Teketay D, Jayaraman D, Long TT. 2021. Biomass, carbon stock and sequestration potential of Oxytenanthera abyssinica forests in lower beles River Basin, Northwestern Ethiopia. Carbon Balance Manag. 16(1):29. doi: 10.1186/s13021-021-00192-5.
- Al-Ghussain L. 2019. Global warming: review on driving forces and mitigation. Environ Prog Sustain. 38(1):13–21. doi: 10.1002/ep.13041.
- Brahma BB, Nath AJ, Deb C, Sileshi GW, Sahoo UK, Das AK. 2021. A critical review of forest biomass estimation equations in India. Trees, For People. 5:100098. doi:10.1016/j.tfp.2021.100098.
- Buckingham K, Jepson P, Wu L, Rao IVR, Jiang S, Liese W, Lou Y, Fu M. 2011. The potential of bamboo is constrained by outmoded policy frames. AMBIO. 40(5):544–548. doi: 10.1007/s13280-011-0138-4.
- Chen X, Zhang X, Zhang Y, Booth T, He X. 2009. Changes of carbon stocks in bamboo stands in China during 100 years. For Ecol Manag. 258(7):1489–1496. doi: 10.1016/j.foreco.2009.06.051.
- Clark LG, Londoño X, Ruiz-Sanchez E. 2015. Bamboo taxonomy and habitat. In: Liese W Köhl M, editors. The plant and its uses. Bamboo: Springer, Cham; p. 1–30. doi: 10.1007/978-3-319-14133-6_1.
- Devi AS, Singh KS. 2021. Carbon storage and sequestration potential in aboveground biomass of bamboos in North East India. Sci Rep. 11(1):837. doi: 10.1038/s41598-020-80887-w.
- de Wit HA, Austnes K, Hylen G, Dalsgaard L, de Wit HA. 2015. A carbon balance of Norway: terrestrial and aquatic carbon fluxes. Biogeochemistry. 123(1–2):147–173. doi: 10.1007/s10533-014-0060-5.
- Düking R, Gielis J, Liese W. 2011. Carbon flux and carbon stock in a bamboo stand and their relevance for mitigating climate change. J Am Bamboo Soc. 24:1–7.
- Egusa T, Kumagai T, Shiraishi N. 2020. Carbon stock in Japanese forests has been greatly underestimated. Sci Rep. 10(1):7895. doi: 10.1038/s41598-020-64851-2.
- Fang J, Oikawa T, Kato T, Mo W, Wang Z. 2005. Biomass carbon accumulation by Japan’s forests from 1947 to 1995. Global Biogeochem Cy. 19:1–10. doi:10.1029/2004GB002253.
- Fukuda M, Iehara T, Matsumoto M. 2003. Carbon stock estimates for sugi and hinoki forests in Japan. For Ecol Manag. 184(1–3):1–16. doi: 10.1016/S0378-1127(03)00146-4.
- Fukushima K, Usui N, Ogawa R, Tokuchi N. 2015. Impacts of moso bamboo (Phyllostachys pubescens) invasion on dry matter and carbon and nitrogen stocks in a broad-leaved secondary forest located in Kyoto, western Japan. Plant Spec Biol. 30(2):81–95. doi: 10.1111/1442-1984.12066.
- He CY, Cui K, Zhang JG, Duan AG, Zeng YF. 2013. Next-generation sequencing-based mRNA and microRNA expression profiling analysis revealed pathways involved in the rapid growth of developing culms in moso bamboo. BMC Plant Biol. 13(1):119. doi: 10.1186/1471-2229-13-119.
- Hui B, Long T. 2019. A manual for bamboo forest biomass and carbon assessment. Beijing: International Bamboo and Rattan Organization.
- Inoue A, Sakamoto S, Suga H, Kitazato H, Sakuta K. 2013. Construction of one-way volume table for the three major useful bamboos in Japan. J For Res. 18(4):323–334. doi: 10.1007/s10310-012-0366-x.
- Inoue A, Sato M, Shima H. 2018. Maximum size-density relationship in bamboo forests: case study of Phyllostachys pubescens forests in Japan. For Ecol Manag. 425:138–144. doi:10.1016/j.foreco.2018.05.044.
- Inoue A, Tateishi H, Sakuta K, Yamamoto K, Mizoue N, Kitahara F. 2012. Relationships of light environment to stand attributes in a stand of bamboo, Phyllostachys Pubescens. Ecol Eng. 38(1):135–139. doi: 10.1016/j.ecoleng.2011.09.007.
- IPCC. 2000. Good practice guidance and uncertainty management in national greenhouse gas inventories. Geneva: Intergovernmental Panel on Climate Change.
- Isagi Y. 1994. Carbon stock and cycling in a bamboo Phyllostachys bambusoides stand. Ecol Res. 9(1):47–55. doi: 10.1007/BF02347241.
- Isagi Y, Kawahara T, Kamo K. 1993. Biomass and net production in a bamboo Phyllostachys bambusoides stand. Ecol Res. 8(2):123–133. doi: 10.1007/BF02348524.
- Isagi Y, Kawahara T, Kamo K, Ito H. 1997. Net production and carbon cycling in a bamboo Phyllostachys pubescens stand. Plant Ecol. 130(1):41–52. doi: 10.1023/A:1009711814070.
- Isagi Y, Oda T, Fukushima K, Lian C, Yokogawa M, Kaneko S. 2016. Predominance of a single clone of the most widely distributed bamboo species Phyllostachys edulis in East Asia. J Plant Res. 129(1):21–27. doi: 10.1007/s10265-015-0766-z.
- Kauffman JB, Donato DC 2012. Protocols for the measurement, monitoring and reporting of structure, biomass and carbon stock in mangrove forests. Center for International Forestry Research, Bogor.
- Kilpeläinen A, Peltola H. 2022. Carbon sequestration and storage in European forests. In: Hetemäki L, Kangas J Peltola H, editors. Forest bioeconomy and climate change. Cham: Springer International Publishing; p. 113–128. doi: 10.1007/978-3-030-99206-4_6.
- Kobayashi K, Ohashi M, Fujihara M, Kitayama K, Onoda Y. 2023. Rhizomes play significant roles in biomass accumulation, production and carbon turnover in a stand of the tall bamboo Phyllostachys edulis. J For Res. 28(1):42–50. doi: 10.1080/13416979.2022.2090669.
- Kuehl Y, Henley G, Lou Y. 2011. The climate change challenge and bamboo: mitigation and adaptation. Beijing: International Bamboo and Rattan Organization.
- Lin MY, Hsieh IF, Lin PH, Laplace S, Ohashi M, Chen TH, Kume T. 2017. Moso bamboo (Phyllostachys pubescens) forests as a significant carbon sink? A case study based on 4-year measurements in central Taiwan. Ecol Res. 32(6):845–857. doi: 10.1007/s11284-017-1497-5.
- Liu YH, Yen TM. 2021. Assessing aboveground carbon storage capacity in bamboo plantations with various species related to its affecting factors across Taiwan. For Ecol Manag. 481:118745. doi:10.1016/j.foreco.2020.118745.
- Li P, Zhou G, Du H, Lu D, Mo L, Xu X, Shi Y, Zhou Y. 2015. Current and potential carbon stocks in moso bamboo forests in China. J Environ Manage. 156:89–96. doi:10.1016/j.jenvman.2015.03.030.
- Lobovikov M, Paudel S, Piazza M, Ren H, Wu J. 2007. World bamboo resources: a thematic study prepared in the framework of the global forest resources assessment 2005. Rome: Food and Agriculture Organization of the United Nations.
- Manabe T, Shibata S, Hasegawa H, Ito K. 2020. Trends and issues of landscape ecological studies on range expansion of bamboo forests in Japan: perspective for sustainable use of bamboo forests. Landscape Ecol Manag. 25:119–135. (in Japanese with English summary).
- Mao F, Li P, Zhou G, Du H, Xu X, Shi Y, Mo L, Zhou Y, Tu G. 2016. Development of the BIOME-BGC model for the simulation of managed moso bamboo forest ecosystems. J Environ Manage. 172:29–39. doi:10.1016/j.jenvman.2015.12.013.
- Mao F, Zhou G, Li P, Du H, Xu X, Shi Y, Mo L, Zhou Y, Tu G. 2017. Optimizing selective cutting strategies for maximum carbon stocks and yield of moso bamboo forest using BIOME-BGC model. J Environ Manage. 191:126–135. doi:10.1016/j.jenvman.2017.01.016.
- Meijaard E, Wunder S, Guariguata MR, Sheil D. 2014. What scope for certifying forest ecosystem services? Ecosyst Serv. 7:160–166. doi:10.1016/j.ecoser.2013.12.008.
- Nath AJ, Lal R, Das AK. 2015. Managing woody bamboos for carbon farming and carbon trading. Global Ecol Conserv. 3:654–663. doi:10.1016/j.gecco.2015.03.002.
- NIES. 2020. Land use, land use change and forestry, in: greenhouse gas inventory office of Japan and ministry of the environment, Japan. National Greenhouse Gas Inventory Report of Japan 2020, Center for Global Environmental Research, National Institute for Environmental Studies, Japan, Tsukuba.
- Nunery JS, Keeton WS. 2010. Forest carbon storage in the northeastern United States: net effects of harvesting frequency, post-harvest retention, and wood products. For Ecol Manag. 259(8):1363–1375. doi: 10.1016/j.foreco.2009.12.029.
- Orrego M, Ugawa S, Inoue A, Laplace S, Kume T, Koga S, Hishi T, Enoki T. 2022. Climate, soil, and plant controls on early-stage litter decomposition in Moso bamboo stands at a regional scale. Front For Glob Change. 5:921028. doi:10.3389/ffgc.2022.921028.
- Ouyang M, Yang C, Tain D, Pan J, Chen G, Su H, Yan Z, Ji C, Tang Z, Fang J. 2022. A field-based estimation of moso bamboo forest biomass in China. For Ecol Manag. 505:119885. doi:10.1016/j.foreco.2021.119885.
- Palmer L. 2021. How trees and forests reduce risks from climate change. Nat Clim Chang. 11(5):374–377. doi: 10.1038/s41558-021-01041-6.
- R Core Team. 2023. R: a language and environment for statistical computing. [accessed 2023 April 6]. http://www.R-project.org.
- Sasaki N, Kim S. 2009. Biomass carbon sinks in Japanese forests: 1966–2012. Forestry. 82(1):105–115. doi: 10.1093/forestry/cpn049.
- Scurlock JMO, Dayton DC, Hames B. 2000. Bamboo: an overlooked biomass resources? Biomass Bioenerg. 19(4):229–244. doi: 10.1016/S0961-9534(00)00038-6.
- Shibata S. 2003. Phyllostachys pubescens and Japanese. J Jpn Soc Reveget Tech. 28(3):406–411. (in Japanese). doi:10.7211/jjsrt.28.406.
- Shibata S. 2010. Bamboo forest management for new effective utilization of bamboo resources. Shinrin Kagaku. 58:15–19. (in Japanese).
- Shima H, Inoue A, Sato M. 2023. Bamboo: A mechanically optimum design in nature. In: Palombini F Nogueira F, editors. Bamboo Science and Technology. Singapore: Springer Nature Singapore; p. 1–29. doi: 10.1007/978-981-99-0015-2_1.
- Shimono K, Katayama A, Abe H, Enoki T. 2021. Carbon and nitrogen storage of dead woody debris in an abandoned moso bamboo forest. Bull Kyushu Univ For. 102:9–14. (in Japanese with English summary).
- Shinohara Y, Kume T, Ichihashi R, Komatsu H, Otsuki K. 2014. Moso bamboo forests in Japan: what are the effects of their area expansion on ecosystem services?. J Jpn For Soc. 96(6):351–361. (in Japanese with English summary). doi:10.4005/jjfs.96.351.
- Song X, Peng C, Zhou G, Gu H, Li Q, Zhang C. 2016. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of moso bamboo (Phyllostachys heterocycla). Sci Rep. 6(1):25908. doi: 10.1038/srep25908.
- Song X, Zhou G, Jiang H, Yu S, Fu J, Li W, Wang W, Ma Z, Peng C. 2011. Carbon sequestration by Chinese bamboo forests and their ecological benefits: assessment of potential, problems, and future challenges. Environ Rev. 19(NA):418–428. doi: 10.1139/a11-015.
- Suzuki K. 1976. Productivity of moso-chiku (Phyllostachys edulis) stands. Trans J Jpn For Soc. 87:223–224. (in Japanese).
- Suzuki S. 2015. Chronological location analyses of giant bamboo (Phyllostachys pubescens) groves and their invasive expansion in a satoyama landscape area, western Japan. Plant Spec Biol. 30(1):63–71. doi: 10.1111/1442-1984.12067.
- Suzuki S, Nakagoshi N. 2008. Expansion of bamboo forests caused by reduced bamboo-shoot harvest under different natural and artificial conditions. Ecol Res. 23(4):641–647. doi: 10.1007/s11284-007-0422-8.
- Suzuki S, Nakagoshi N. 2011. Sustainable management of Satoyama bamboo landscapes in Japan. In: Hong S, Wu J, Kim J Nakagoshi N, editors. Landscape Ecology in Asian Cultures. Tokyo: Springer; p. 211–220. doi: 10.1007/978-4-431-87799-8_15.
- Takano KT, Hibino K, Numata A, Oguro M, Aiba M, Shiogama H, Takayabu I, Nakashizuka T. 2017. Detecting latitudinal and altitudinal expansion of invasive bamboo Phyllostachys edulis and Phyllostachys bambusoides (Poaceae) in Japan to project potential habitats under 1.5°C-4.0°C global warming. Ecol Evol. 7(23):9848–9859. doi: 10.1002/ece3.3471.
- Torii A, Isagi Y. 1997. Range expansion of bamboo species in southern areas of Kyoto Prefecture, Japan. Jpn J Ecol. 47:31–41. (in Japanese with English summary).
- Uchimura E. 2009. Utilization of bamboo resource into modern days. Soshinsya, Tokyo. (in Japanese).
- Utsumi Y, Murata I, Shiiba Y, Miyajima Y, Inoue S. 2010. The traditional name and usage of plants in Okawachi area, Shiiba Village III. Lianas and Bamboos. Bull Kyushu Univ For. 91:15–18. (in Japanese).
- Wang S, Kobayashi K, Takanashi S, Liu CP, Li DR, Chen SW, Cheng YT, Moriguchi K, Dannoura M. 2023. Estimating divergent forest carbon stocks and sinks via a knife set approach. J Environ Manage. 330:117114. doi:10.1016/j.jenvman.2022.117114.
- Wang B, Wei WJ, Liu CJ, You WZ, Niu X, Man RZ. 2013. Biomass and carbon stock in moso bamboo forests in subtropical China: characteristics and implications. J Trop For Sci. 25:137–148.
- Withey P, Johnston C, Guo J. 2019. Quantifying the global warming potential of carbon dioxide emissions from bioenergy with carbon capture and storage. Renew Sust Energ Rev. 115:109408. doi:10.1016/j.rser.2019.109408.
- Wu W, Liu Q, Zhu Z, Shen Y. 2015. Managing bamboo for carbon sequestration, bamboo stem and bamboo shoots. Small-scale For. 14(2):233–243. doi: 10.1007/s11842-014-9284-4.
- Xu M, Ji H, Zhuang S, Nath A. 2018a. Carbon stock of moso bamboo (Phyllostachys pubescens) forests along a latitude gradient in the subtropical region of China. PLoS One. 13(2):e0193024. doi: 10.1371/journal.pone.0193024.
- Xu L, Shi Y, Zhou G, Xu X, Liu E, Zhou Y, Zhang F, Li C, Fang H, Chen L. 2018b. Structural development and carbon dynamics of moso bamboo forests in Zhejiang Province, China. For Ecol Manag. 409:479–488. doi:10.1016/j.foreco.2017.11.057.
- Yen TM. 2015. Comparing aboveground structure and aboveground carbon storage of an age series of moso bamboo forests subjected to different management strategies. J For Res. 20(1):1–8. doi: 10.1007/s10310-014-0455-0.
- Yen TM. 2016. Culm height development, biomass accumulation and carbon storage in an initial growth stage for a fast-growing moso bamboo (Phyllostachys pubescens). Bot Stud. 57(1):10. doi: 10.1186/s40529-016-0126-x.
- Yen TM, Ji YJ, Lee JS. 2010. Estimating biomass production and carbon storage for a fast-growing makino bamboo (Phyllostachys makinoi) plant based on the diameter distribution model. For Ecol Manag. 260(3):339–344. doi: 10.1016/j.foreco.2010.04.021.
- Yen TM, Lee JS. 2011. Comparing aboveground carbon sequestration between moso bamboo (Phyllostachys heterocycla) and China fir (Cunninghamia lanceolata) forests based on the allometric model. For Ecol Manag. 261(6):995–1002. doi: 10.1016/j.foreco.2010.12.015.
- Yen TM, Wang CT. 2013. Assessing carbon storage and carbon sequestration for natural forests, man-made forests, and bamboo forests in Taiwan. Int J Sust Dev World Ecol. 20(5):455–460. doi: 10.1080/13504509.2013.811445.
- Yuen JQ, Fung T, Ziegler AD. 2017. Carbon stocks in bamboo ecosystems worldwide: estimates and uncertainties. For Ecol Manag. 393:113–138. doi:10.1016/j.foreco.2017.01.017.
- Zhang H, Zhuang S, Sun B, Ji H, Li C, Zhou S. 2014. Estimation of biomass and carbon storage of moso bamboo (Phyllostachys pubescens Mazel ex Houz.) in southern China using a diameter–age bivariate distribution model. Forestry. 87(5):674–682. doi: 10.1093/forestry/cpu028.
- Zhou BZ, Fu MY, Xie JZ, Yang XS, Li ZC. 2005. Ecological functions of bamboo forest: research and application. J For Res. 16(2):143–147. doi: 10.1007/BF02857909.
- Zhou G, Meng C, Jiang P, Xu Q. 2011. Review of carbon fixation in bamboo forests in China. Bot Rev. 77(3):262–270. doi: 10.1007/s12229-011-9082-z.