Abstract
The seeding time is an important factor affecting the process of stem yield. Nevertheless, details related to the optimum seeding time to maximize the stem yield of sweet sorghum have not been studied. This study elucidated information necessary to ascertain the optimum seeding time to obtain the maximum stem yield. We conducted an examination with three seeding times at intervals of two weeks, ranging from late May to mid-June for four years during 2009–2012, and we analyzed the relation between seeding times and stem yield based on internode characteristics of stems using two sorghum cultivars with different maturity: ‘Kazetachi’ and ‘Wray’. Differences were observed in the respective relations between the seeding times and the stem yields. For Kazetachi, the dry matter yield increased with the earlier seeding time. Thus, to expand the growing period, an earlier seeding time with good establishment is better. For Wray, a cultivar that can grow to heading in the Tohoku region, the stem dry weight was greater by seeding in early June. For cultivars such as Wray with a sufficient growing period in a given region, the optimum seeding time to obtain stable higher stem yield existed and the cultivars should be seeded when they could grow under good weather conditions at the critical time strongly influences growth.
Introduction
Sweet sorghum (Sorghum bicolor Moench), which accumulates large amounts of sugar in its stems, can be used to produce bioethanol fuel (Monk et al., Citation1984; Smith & Buxton, Citation1993). In recent years, it is also anticipated for use as a raw material for the production of biomethanol and cellulosic biofuels (Kobayashi, Citation2009). In sorghum cultivated as a biomass crop, the stem is a major organ for harvest. The dry matter yield of stems is closely related to the stem volume (Nakamura et al., Citation1997). Therefore, the stem volume can be regarded as indicating the yield capacity of the dry matter yield of stem. Increasing the stem volume is expected to increase the stem dry yield.
An important factor related to cultivation to increase the stem yield of sweet sorghum is the seeding time. In general, a later seeding time shortens the growing period and decreases the stem yield in sweet sorghum (Almodares & Mostafafi Darany, Citation2006; Teetor et al., Citation2011; Ratnavathi et al., Citation2012). Therefore, to obtain higher stem yield of sweet sorghum, an early seeding time would be better. However, in cold regions, low temperatures immediately after seeding might cause low germination rates and delay the early growth of sweet sorghum. In the Tohoku region in Japan, seeding from the end of May to the beginning of June produces a steady yield irrespective of the sweet sorghum cultivar with different heading times (Nakamura et al., Citation2010c). Nevertheless, details related to optimum seeding times to maximize the stem yield of sweet sorghum have not been studied. It is necessary to clarify what we should take into account in order to decide the optimum seeding time. In this study, we conducted examinations with three seeding times at intervals of two weeks, ranging from the end of May to mid-June in 2009–2012, and analyzed the relation between seeding times and stem yield, especially focusing on the internode characteristics of the stems.
Materials and methods
We used two sorghum cultivars with different maturity, ‘Kazetachi’ and ‘Wray’, for this study. Kazetachi is an ultra-late maturing cultivar that usually does not flower in the Tohoku region (Nakamura et al., Citation2010a). It has very strong tolerance for lodging because the stem consists of shorter and thicker internodes than other cultivars and large dry matter (Kasuga et al., Citation2014; Nakamura et al., Citation2010b) though Brix value of extracted juice from the stem is not so high. Wray (Broadhead et al., Citation1981) was used as a representative of sweet sorghum because it has a high stem yield and high sugar contents and also has smaller variations in stem characteristics among plants than other cultivars (Nakamura et al., Citation2011). It has often been used for sweet sorghum research conducted in foreign countries (Clegg et al., Citation1986; Dolciotti et al., Citation1998; Tsuchihashi & Goto, Citation2004).
The cultivars were seeded on three seeding dates from about May 20 to about June 18 for four years during 2009–2012 in an experimental field of Miyagi University, Sendai, Japan (38°13΄N, 140°48΄E) (Table ). The experimental plots in late May, early June, and mid-June were designated, respectively, as plot I, plot II, and plot III. Seeding time of plot I is regarded as the earliest period to ensure stable establishment in previous investigations in this region. The seedlings were thinned to 1 plant per hill with .85 and .15 m spacing between and within rows. Slow-release fertilizer with 12 g m−2 nitrogen (N), 10.6 g m−2 phosphate (P2O5), and 12 g m−2 potash (K2O) and quick-acting fertilizer with 4 g m−2 N, 4 g m−2 P2O5, and 4 g m−2 K2O were applied as basal fertilizer before seeding. Each plot with 17 m2 had three replications. Seven plants in the center of a row in each replication were selected to assess the growth and yield. Except for plants that received damage such as insect feeding, 16–18 plants in each plot were harvested and analyzed. The leaf number on the main stems in plots I, II, and III were recorded from about 15 June, 30 June, and 7 July, respectively, at about 7 day intervals in each year. The leaf number was defined as the number of fully expanded leaves, not including expanding leaves. The heading date of each plot was calculated by averaging the heading dates of all plants measured.
Table 1. Seeding date.
Kazetachi and Wray were harvested, respectively, in early November and about 30 days after heading. After harvest, the length, diameter, and dry weight of each internode were recorded; the dry weights of leaves and panicle of the main stem were also recorded. The diameter was measured in the middle of the internode length. Assuming that the cross section of the internode is an ellipse, the cross-sectional area of the internode (S) was calculated using the following equation: S = abπ/4, where a stands for the diameter connecting the two mid-ribs of the alternating leaves and b denotes the diameter in the vertical direction to the diameter a. Assuming that an internode is an elliptical cylinder, the internode volume was calculated by multiplying the internode length and the internode cross-sectional area. The stem volume, the stem dry weight, and the stem length were calculated as the sum of the internode volume, the internode dry weight, and the internode length of all elongated internodes. The unit weight of the dry stem was calculated by dividing the stem dry weight by the stem volume. The node with the n-th leaf sheath was defined as the n-th node. The internode between the n-th node and the (n + 1)-th node was defined as the n-th internode (IN n). Although some tillers arose from the investigated plants during the growing season, we analyzed only the main stems because most of these tillers died and only slightly remained at harvest.
We used the method by which the plant age was indicated by the leaf number on the main stem (Goto et al., Citation1994). The age according to the leaf number (AL) is represented by AL n at which the n-th leaf blade has just expanded. The AL is represented as a natural number, but we used AL as a continuous numerical value.
We used daily mean air temperature of automated meteorological acquisition system (AMeDAS) weather data in Sendai in which the experimental field was the same region to compare the temperature environment during growing season among years.
Thickening period of internodes
In sweet sorghum, Nakamura et al. (Citation2011) revealed the relation between the leaf expansion and the internode thickening, namely the thickening pattern of internodes drawn by a sigmoidal curve. Based on this pattern, the thickening state of each internode was estimated by the measurement of leaf expansion during the growing period. Although the thickening period of IN n differs slightly depending on the internode position, the period from AL (n – 1) to AL (n + 1) was regarded as the rapid thickening period in this study because we analyzed mainly lower internodes of the main stem. The date of the rapid thickening period was estimated using the method which Fujii et al. (Citation2014a) reported.
Results
Plant growth
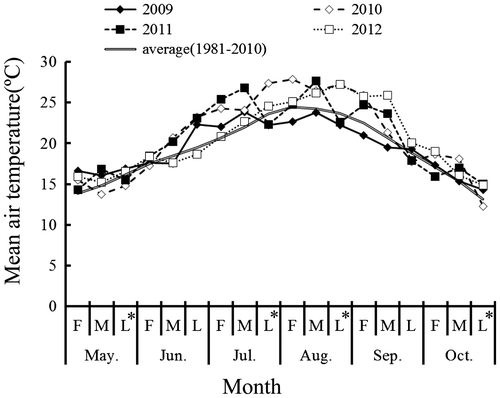
Mean air temperatures during the growing seasons of 2009–2012 are presented in Figure . During June 21–July 10, the air temperature except that in 2012 was higher than the average yearly temperature. After that period, in 2009, the air temperature was lower than the average yearly temperature from the end of July through mid-September. In 2010, it was higher than the average yearly temperature from mid-July through the beginning of September. In 2012, it was almost the same as the average yearly temperature from mid-July through the beginning of August, and was higher than the average yearly temperature from mid-August through the beginning of September.
Figure 1. Changes of air temperature during the growing season. Note. F: First ten days of the month; M: Middle ten days of the month; L: Last ten days of the month; L*: Last eleven days of the month.
Tables and respectively show the heading rate of Kazetachi and the heading date of Wray. For Kazetachi, in no plot did all plants head; however, the heading rate tended to increase with the earlier seeding time except in 2011. The heading date in Wray was 10 August (plot I in 2010) to September 6 (plot III in 2009). It tended to be earlier with the earlier seeding time in each year. Table presents the days to heading after seeding in Wray. It tended to be longer with the earlier seeding time each year.
Table 2. Heading rate of Kazetachi.
Table 3. Heading date of Wray.
Table 4. Days to heading after seeding in Wray.
In all plots, the number of expanded leaves increased linearly from the time that the sixth leaf expanded to the beginning of November in Kazetachi and to the boot stage in Wray. Table shows the total leaf number of the main stem in both cultivars at harvest. The total leaf number of the main stem in Kazetachi ranged from 29.2 (plot III in 2009) to 36.4 (plot I in 2010). It tended to increase with the earlier seeding time in each year. The total leaf number in Wray ranged from 17.9 (plot III in 2012) to 21.2 (plot I in 2012; plot III in 2010). Although the total leaf number in Wray increased with the earlier seeding time in 2009 and 2012, no common tendency was observed among years.
Table 5. Total leaf number of main stem.
Stem yield and related characteristics
Table shows the total dry weight per plant in both cultivars. For Kazetachi, the total dry weights of plot II and plot III in 2009, and of plot III in 2011, were significantly less than those of other plots in the same year. For the mean value for four years, the total dry weight tended to increase with the earlier seeding time. For Wray, the total dry weights of plot III in 2011 and plot I in 2012 were significantly less than those of other plots in the same year. Although no clear trend of the total dry weight was observed through four years in Wray, greater total dry weight was obtained stably in plot II.
Table 6. Total dry weight per plant.
Table displays the stem dry weight per plant in both cultivars. For Kazetachi, the stem dry weight was significantly less in 2009 than in other years. The stem dry weight tended to increase with the earlier seeding time through four years. For Wray in 2011, the stem dry weight in plot III was significantly less than at other seeding times. The mean value of stem dry weight for four years in plot II was greater, but no significant difference was found in the stem dry weight.
Table 7. Stem dry weight per plant.
Table portrays the stem volume per plant in both cultivars. For Kazetachi, the stem volumes of plot III in 2011 and plot I in 2012 were significantly less than for other seeding times. The stem volume tended to increase with the earlier seeding time during 2009–2011. For Wray, the stem volume of I plot in 2012 was significantly less than at other seeding times. For the mean value of four years, the stem volume of II plot was greater than those of other seeding times.
Table 8. Stem volume per plant.
Table depicts the unit weight of dry main stem in both cultivars. For Kazetachi, the unit weights of plots II and III in 2009 and that of plot III in 2012 were significantly less. Regarding the mean value of four years, the unit weight increased significantly along with the earlier seeding time. For Wray, the unit weights of plots II and III in 2009, plot III in 2010, plot III in 2011, and plots I and III in 2012 were significantly less. Regarding the mean values of four years, the unit weight of plot III was significantly lower.
Table 9. Unit weight of dry main stem.
Stem shape based on internode characteristics
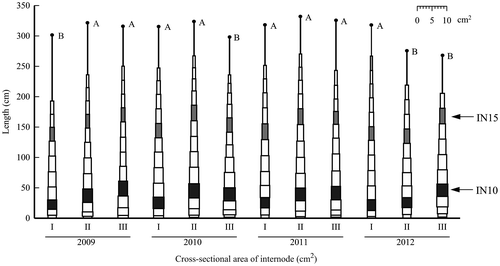
Stem shapes, which comprise internodes drawn by the cross-sectional area of the internode against the x-axis and the internode length against the y-axis, of Kazetachi and Wray are presented, respectively, in Figures and . The internode positions including the neck internode immediately below the head are not depicted in these figures. The lowest elongated internode (1 cm or more long) position was IN7 to IN9 in Kazetachi and from IN6 to IN8 in Wray. For Kazetachi, the stem length in plot I was greater than that of any other plot for every year. For Wray, the stem lengths of plot II in 2009–2011 and of plot I in 2012 were greater. In both cultivars in all plots, the internode position of the largest cross section was near the base of the stem. The cross-sectional area of the internode decreased gradually to the top. The cross-sectional areas of basal internodes of plot I were smaller than those of plots II and III in every year, irrespective of the cultivar.
Figure 2. Stem shape of Kazetachi based on internode characteristics.
Note. These stem shapes are drawn by the average of length and cross-sectional area of each elongated internode, excluding the internode position including the neck internode. Black points represent the average of stem length in each plot. Uppercase letters of the same letter indicate no significant difference at the 5% level in the same year. The internode positions enclosed within a black line were elongating rapidly during August 1–20 in 2009.
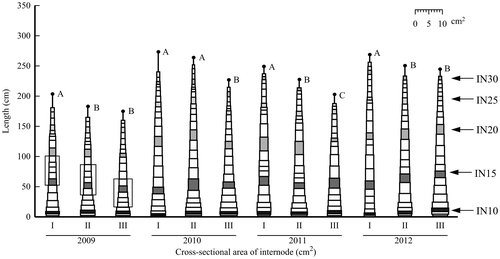
Figure 3. Stem shape of Wray based on internode characteristics.
Note. These stem shapes are drawn by the average of length and cross-sectional area of each elongated internode, excluding the internode position including the neck internode. Black points represent the average of stem length in each plot. Uppercase letters of the same letter indicate no significant difference at the 5% level in the same year.
Discussion
Regarding the relation between the seeding times and the yield through four years in Kazetachi, the total dry weight and the stem dry weight of plot I was larger than that of plot III, suggesting that the earlier seeding time can yield more dry matter (Tables and ). For Wray, the total dry weight and the stem dry weight of plot II tended to be larger, on a stable basis, through four years, suggesting that the seeding times of plot I and plot III can decrease the stem yield, depending on the year (Tables and ).
For Kazetachi cultivated in 2012, the stem volume of plot I was significantly smaller than that of plot III (Table ), but no significant difference was found in the stem dry weight among the seeding times (Table ). This result differed from that obtained in each of the other three years. In the stem shape of plot I in Kazetachi, three internodes from IN9 to IN11 at the base of stem were thinner in 2012 than in other years (Figure ). Using the relation between leaf expansion and internode thickening (Nakamura et al., Citation2011), the dates of the rapid thickening period from IN9 to IN11 were estimated, indicating that the dates were June 20–July 10 each year. Cumulative air mean temperatures through this period were 462 °Cd in 2009, 498 °Cd in 2010, 507 °Cd in 2011, and 415 °Cd in 2012, indicating that it was remarkably low in 2012. Fujii et al. (Citation2014b) reported that the stem volume is related closely to the basal internode characteristic and that the basal internode thickness influences the above internodes to the top. Therefore, in plot I in 2012 in Kazetachi, lower air temperatures during the rapid thickening period of these internodes suppressed the basal internode thickening, thereby reducing the stem volume.
For Kazetachi in 2012, in spite of the smaller stem volume in plot I, no significant difference was found in the stem dry weight among plots. This result is expected to be related to the unit weight of the dry stem. For Kazetachi in 2012, the unit weight of plot I was significantly greater than that of plot III among plots (Table ). The heading rate of plot III was lower than that of plot I (Table ), suggesting that the stem maturity of each plant would be later in plot III than in plot I at harvest. Therefore, the reason for the lack of significant difference between the stem dry weights in plot I and in plot III was considered that the stem contents were not enriched sufficiently, even though the stem volume, as stem capacity, was greater in plot III. A similar tendency was observed in Wray in 2012, but it was not as clear as that of Kazetachi (Tables and , Figure ).
For Wray, the stem volumes of plots II and III were larger than that of plot I through four years (Table ). Regarding the stem shape of each year in Wray, the three basal internodes from IN8 to IN10 were thicker with the later seeding time, except in 2011 (Figure ). The dates of the rapid thickening periods of these internodes were estimated from the numbers of leaves recorded, which revealed that the dates were from June 20 to July 10 in plot I, from June 30 to July 20 in plot II, and from July 10 to July 30 in plot III every year. The cumulative air temperatures in these periods shown in Table indicate that the temperature was lower with the earlier seeding time. Consequently, it was considered that the basal internode thickening of plot I was suppressed by low air temperatures during the rapid thickening period of these internodes.
Table 10. Cumulative air temperature during rapid thickening period of IN8-IN10 in Wray.
The stem length of Wray was shorter in plot III than in plot II through four years (Figure ). In sweet sorghum, the later seeding time reportedly shortened the growing period from germination to heading, indicating the shorter stem length (Almodares & Mostafafi Darany, Citation2006). The shorter stem length in plot III contributed to the short period from seeding to heading (Table ). This result suggests that the stem volume in plot III might be smaller in spite of the larger basal internodes, depending on the year (Table ). Furthermore, the unit weight of dry matter in plot III was lower than that in plot II (Table ). Results obtained for plot II show that a greater stem volume and dry matter yield would be obtained; in plot I and plot III, the stem volume and the dry matter yield would be less, depending on the year.
For Kazetachi in 2009, the stem dry weight tended to be less; the stem volume was significantly less than in other years, irrespective of the seeding time (Tables and ). In addition, the stem length was much less than in other years (Figure ). The total leaf number of the main stem was significantly less than in other years (Table ). These results suggest that the smaller total leaf number, corresponding to the fewer internodes, is one reason for the shorter stems in 2009. The total leaf number of the main stem is determined when the panicle initiated. Nakamura et al. (Citation1996) reported that about 13 unexpanded leaves existed at the panicle initiation stage in a late maturing variety. Based on this result, the times of panicle initiation stages in plots I, II, and III were estimated, respectively, as the times when L18, L17, and L15 fully expanded in Kazetachi in 2009 (Table ). In addition, the date when those leaves fully expanded was estimated as about 10 August every seeding time by the recorded leaf number. The cumulative mean air temperature during August 1–10 in each year were estimated as 225 °Cd in 2009, 279 °Cd in 2010, 249 °Cd in 2011, and 251 °Cd in 2012. Cumulative hours of sunlight during this period were 14.2 h in 2009, 75.4 h in 2010, 42.8 h in 2011, and 70.7 h in 2012, reflecting an extremely short time in 2009. These results suggest that lower air temperature and short hours of sunlight in this period promote the panicle initiation of Kazetachi and decrease the leaf initiation rate. To evaluate the effect of lower temperature and insufficient sunlight on the internode length in 2009, the rapidly elongating positions of internodes during August 1–20 were estimated. The internode positions were IN15 to IN19 in plot I, IN14 to IN18 in plot II, and IN12 to IN16 in plot III, which are enclosed in a box with a line in Figure . The lengths of these internodes were less than those of internodes in the same internode positions in other years. This result suggests that the final length of internodes, which was elongated rapidly under low temperature, would be shorter. The shorter length of internodes was regarded as one reason that the stem length was less in 2009 in addition to the fewer leaves in Kazetachi.
In conclusion, differences between the cultivars were observed in the relation between the seeding times and the stem yield by analysis based on the internode characteristics. For Kazetachi, the dry matter yield increased with the earlier seeding time (Table ). This result is consistent with those of previous reports (Almodares & Mostafafi Darany, Citation2006; Ratnavathi et al., Citation2012; Teetor et al., Citation2011). For higher temperatures in the latter half of the growing stage in a year similar to 2012, the stem volume increased with the later seeding time (Table ). However, in 2012, the unit weight of dry main stem was lower in the later seeding time than in the earlier seeding time. Consequently, no significant difference was found in the stem dry weight (Table ). For growing sorghum cultivar Kazetachi, to expand the growing period, the earlier seeding time with good establishment is better, for example, around May 20 in Sendai in Tohoku, Japan.
For Wray, one cultivar that can grow to heading in the Tohoku region, the stem dry weight of II plot tended to be greater through four years (Table ). This suggests that early June is the better seeding time for Wray in Sendai, not as early as possible. This result differed from the results described in previous reports (Almodares & Mostafafi Darany, Citation2006; Ratnavathi et al., Citation2012; Teetor et al., Citation2011). For cultivars with a sufficient growing period in a given region, some optimum seeding time exists to obtain the maximum stem yield substantially. Seeding must be done when the cultivars can grow under good weather conditions at the critical time during which the growth can be positively influenced.
Abbreviations
AL n, n-th age in leaf number.
IN n, the n-th internode number.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Almodares, A., & Mostafafi Darany, S. M. (2006). Effects of planting date and time of nitrogen application on yield and sugar content of sweet sorghum. Journal of Environmental Biology, 27, 601–605.
- Broadhead, D. M., Freeman, K. C., & Zummo, N. (1981). Registration of Wray sweet sorghum. Crop Science, 21, 987.10.2135/cropsci1981.0011183X002100060048x
- Clegg, M. D., Gorz, H. J., Maranville, J. W., & Haskins, F. A. (1986). Evaluation of agronomic and energy traits of Wray sweet sorghum and the N39×Wray hybrid. Energy in Agriculture, 5, 49–54.10.1016/0167-5826(86)90005-7
- Dolciotti, I., Mambelli, S., Grandi, S., & Venturi, G. (1998). Comparison of two Sorghum genotypes for sugar and fiber production. Industrial Crops and Products, 7, 265–272.10.1016/S0926-6690(97)00057-5
- Fujii, A., Nakamura, S., & Goto, Y. (2014a). Relation between stem growth processes and internode length patterns in sorghum cultivar ‘kazetachi’. Plant Production Science, 17, 185–193.10.1626/pps.17.185
- Fujii, A., Nakamura S., & Goto Y. (2014b). Analysis of stem volume based on pattern of internode characteristics in sweet sorghum. Japanese Journal of Crop Science, 83, 112–117.10.1626/jcs.83.112
- Goto, Y., Nakamura, S., Sakai, K., & Hoshikawa, K. 1994. Analysis of elongation and thickening of internodes in sweet sorghum (sorghum bicolor moench). Japanese Journal of Crop Science, 63, 473–479.10.1626/jcs.63.473
- Kasuga, S. 2014. Evaluation of the characteristics of commercial varieties of sorghum and sudangrass for dry matter production. Bull. Shinshu University, AFC 12, 41–45.
- Kobayashi, M. 2009. Outline of biomass gasification methanol synthesis and vision of perennial grass for biomass feedstock. Japanese Journal Grassland Science, 55, 284–287.
- Monk, R. L., Miller, F. R., & McBee, G. G. (1984). Sorghum improvement for energy production. Biomass, 6, 145–153.10.1016/0144-4565(84)90017-9
- Nakamura, S., Nakajima, N., Nitta, Y., & Goto, Y. (2011). Analysis of successive internode growth in sweet sorghum using leaf number as a plant age indicator. Plant Production Science, 14, 299–306.10.1626/pps.14.299
- Nakamura, S., Saito, M., & Goto, Y. 1996. Unexpanded leaf number just before panicle differentiation stage on sweet sorghum. Japanese Journal of Crop Science, 65 ( Extra issue 2), 67–68.
- Nakamura, S., Saito, M., & Goto, Y. 1997. Effect of fertilizer application method on characteristics of internode of sweet sorghum. Japanese Journal of Crop Science, 66 ( Extra issue 1), 68–69.
- Nakamura, S., Saito, M., Sato, A., Tagaya, T., Aoki, K., Nitta, Y., & Goto, Y. 2010a. Effect of different seeding times on the growth of sorghum variety ‘Kazetachi’. Japanese Journal of Crop Science, 79, 226–227.
- Nakamura, S., Saito, M., Sato, A., Tagaya, T., Aoki, K., Nitta, Y., & Goto, Y. 2010b. Effect of different seeding times on the internode characteristics of sorghum variety ‘Kazetachi’. Japanese Journal of Crop Science, 79, 228–229.
- Nakamura, S., Sato, A., Tagaya, T., Aoki, K., Nitta, Y., & Goto, Y. 2010c. Characteristics of stem of sweet sorghum Cultivar ‘Wray’. Tohoku Journal of Crop Science, 53, 29–32.
- Ratnavathi, C. V., Kumar, S. R., Kumar, B. S. V., Krishna, D. G., & Patil, J. V. (2012). Effect of time of planting on cane yield and quality characters in sweet sorghum. Journal of Sustainable Bioenergy Systems, 2, 1–9.10.4236/jsbs.2012.21001
- Smith, G. A., & Buxton, D. R. (1993). Temperate zone sweet sorghum ethanol production potential. Bioresource Technology, 43, 71–75.10.1016/0960-8524(93)90086-Q
- Teetor, V. H., Duclos, D. V., Wittenberg, E. T., Young, K. M., Chawhuaymak, J., Riley, M. R., & Ray, D. T. (2011). Effects of planting date on sugar and ethanol yield of sweet sorghum grown in Arizona. Industrial Crops and Products, 34, 1293–1300.10.1016/j.indcrop.2010.09.010
- Tsuchihashi, N., & Goto, Y. (2004). Cultivation of sweet sorghum (sorghum bicolor (L.) moench) and determination of its harvest time to make use as the raw material for fermentation, practiced during rainy season in dry land of Indonesia. Plant Production Science, 7, 442–448.10.1626/pps.7.442