Abstract
We conducted hydroponic culture experiments to characterize root traits in a rice cultivar ‘Puluik Arang’ that has been identified in a previous study as a cultivar that is adaptable to unflooded conditions. Root morphological traits and the expression of 11 aquaporin genes in rice seedlings (cv. Puluik Arang and cv. Akitakomachi) subjected to osmotic stress by polyethylene glycol (PEG) treatments (10 and 20%) were analysed. ‘Puluik Arang’ exhibited significantly greater water uptake under 10% PEG treatment than ‘Akitakomachi’. Lateral root development was maintained in ‘Puluik Arang’ under PEG treatments. The expression of some aquaporin genes, particularly OsTIP2;1, was higher in ‘Puluik Arang’ than in ‘Akitakomachi’. Immunocytochemical analysis showed that the OsTIP2;1 protein mainly accumulated in endodermal cells. The results suggest that better lateral root development and the function of aquaporins could contribute to water uptake in ‘Puluik Arang’ under osmotic stress.
One of the biggest challenges in increasing rice production under limited water supply is improving the plant’s drought resistance. Genetic improvement and agronomic management strategies for the efficient use of available soil moisture are of utmost importance (Kamoshita, Citation2011; Serraj et al., Citation2011). Several studies related to drought resistance in rice have been conducted over the years. Various plant adaptation mechanisms to drought have been delineated in previous studies (Bouman et al., Citation2006; Fukai & Cooper, Citation1995). Biomass production in plants can be determined by water uptake multiplied by water use efficiency. Therefore, enhancing water uptake under soil moisture deficit is important for improving rice production under drought stress (Blum, Citation2009; Kobata et al., Citation1996; Nguyen et al., Citation1997).
Plant water status is regulated by the balance of water uptake through the root system and water demand by the shoot. The root system responds to various soil conditions and plays an important role in maintaining the plant’s water status by regulating water uptake under conditions of low water availability. For example, deep root growth and root thickness enable the plant to uptake water from a deep layer of soil (Kato et al., Citation2006; Uga et al., Citation2013). The plasticity of root development is also important for the adaptability to soil moisture changes (Suralta et al., Citation2008; Kano et al., Citation2011, Kano-Nakata et al., Citation2011). Several studies have investigated the molecular mechanisms of water transport in roots. The flow of water in roots occurs in a radial manner from the root surface to the central cylinder by both apoplastic and cell-to-cell pathways. Where apoplastic barriers such as Casparian bands exist, water is transported via the cell-to-cell pathway. In this pathway, water channels known as aquaporins regulate water transport (Javot & Maurel, Citation2002; Maurel et al., Citation2008). Recent studies have reported that aquaporins facilitate water transport in plant tissues in many plant species (Knipfer et al., Citation2011; Mahdieh et al., Citation2008; Murai-Hatano et al., Citation2008; Sakurai et al., Citation2008). The regulation of aquaporin gene expression, their trafficking and/or their reversible gating actively regulate the water movement in the plant and play an important role in drought resistance and/or recovery from drought (Matre et al., Citation2002; Tournaire-Roux et al., Citation2003; Lian et al., Citation2006; Guo et al., Citation2006; Hachez et al., Citation2013; Luu & Maurel, Citation2013). These studies indicate that aquaporin is a key trait that could provide a better understanding of the adaptation of different rice varieties to conditions where water availability is limited.
We previously examined the genotypic variation in biomass production under different soil moisture conditions using a rice diversity research set of germplasm developed by the National Institute of Agrobiological Science and found that an indica rice cultivar ‘Puluik Arang’ exhibited great water uptake, resulting in large biomass production under unflooded conditions (Matsunami et al., Citation2012). It was hypothesized that better development of the root system, especially lateral root development, contributed to the greater water uptake by the cultivar ‘Puluik Arang’ under conditions of low water availability. In addition, we hypothesized that the expression of root aquaporin genes could be involved in water uptake by different rice varieties under stress. Therefore, in this study, we investigated root development and expression of aquaporin genes in response to osmotic stresses in ‘Puluik Arang’, as a step towards evaluating root traits associated with better water uptake under conditions with low water availability.
Materials and methods
1. Plant material and growth conditions
An indica cultivar ‘Puluik Arang’, which was identified as a cultivar adaptable to unflooded condition in our previous study (Matsunami et al., Citation2012), was used for hydroponics experiments. A japonica rice cultivar (O. sativa L. cv. Akitakomachi) was used as control, because this cultivar was well investigated for its aquaporin function in the previous studies (Sakurai et al., Citation2005; Sakurai-Ishikawa et al., Citation2011).
After germination in the dark for three days at 28 °C in Petri dishes, 10 seedlings were transplanted and grown in 1,200 mL of a culture solution (1.5 × 10−3 M KNO3, 1.0 × 10−3 M Ca(NO3)2, 2.5 × 10−4 M NH4H2PO4, 5.0 × 10−4 M MgSO4, 1.3 × 10−5 M Fe-EDTA, 2.3 × 10−6 M MnCl2, 1.2 × 10−5 M H3BO3, 1.9 × 10−7 M ZnSO4, 7.9 × 10−8 M CuSO4, and 7.5 × 10−9 M (NH4)6Mo7O24) in a plastic pot (95 φmm, 240 mm height). Plants were grown in a growth chamber (MLR-350H; Sanyo, Japan) under a 12-h light/12-h dark photoperiod (320 μmol s−1 m−2 photosynthetic photon flux density during the light period), with 28 °C and 70% of relative humidity. To induce osmotic stress, polyethylene glycol (PEG) 6,000 was dissolved in water at concentrations of 0 (control), 10% (w/w), and 20%, three days after transplanting; the water potentials of the PEG-treated solutions were −0.18 and −0.42 MPa, respectively. The osmotic potentials were measured using a vapour pressure osmometer (model 5520, Wescor Inc., U.S.A). PEG 6,000 has no toxic effect under well-aerated conditions, which is why we used it to mimic soil water limited conditions (Kano et al., Citation2011).
2. Measurement of water uptake, plant growth and morphological root traits
The amount of water uptake of 13-day-old plants was measured as the decrease in pot weight during a 1-h measurement period, from 3 h after lights-on, with the same sampling timing as for the expression analysis of the aquaporin genes. The measurements were taken independently three times. Evaporations were estimated from the weight change of the pot without plants.
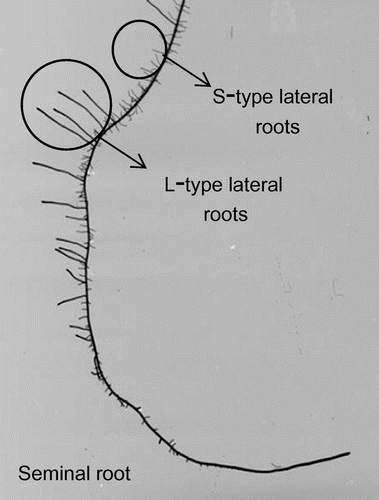
The shoot and root of the 13-day-old plants were sampled and dried at 80 °C for more than three days, and then the dry matter weight was measured. Regarding the morphological root trait measurements, root samples were fixed and stained in FAA solution and Coomassie Brilliant Blue. Images of the root systems were captured using an image scanner (GT-9800). The total root length, the root surface area and the total number of lateral roots were measured using WinRHIZO (Regent instrument Inc., Canada), an image analysis system. The number of long and thick lateral root (L-type lateral root) was counted by visual observation (Figure ), and the number of short and thin lateral root (S-type lateral root) was calculated from the number of root tip using WinRHIZO.
3. Expression analyses of the rice aquaporin genes
For the investigation of the expression of aquaporin genes at mRNA levels in the roots, we sampled seminal roots and lateral roots emerged from seminal root from 13-day-old plants 3 h after lights-on, when the highest expression is observed in most plasma membrane intrinsic protein (PIP) members (Sakurai et al., Citation2005). The root samples were immediately frozen in liquid nitrogen. Approximately 100 mg (fresh weight) of the seminal roots from two or three plants was ground with a pestle. Total RNA was extracted from the ground sample using the RNeasy Plant Mini Kit (Qiagen, the Netherlands), according to the manufacturer’s instructions. First-strand cDNA was synthesized using TaKaRa RNA PCR Kit (AMV) Ver. 3.0 (Takara Bio Inc., Japan). After dilution of the synthesized cDNA (2 ng μL−1), we conducted a quantitative real-time PCR using the StepOne Real-Time PCR system (Applied Biosystems, U.S.A). The PCR was conducted using the Fast SYBR Green system (Applied Biosystems). From 33 rice aquaporin genes (Sakurai et al., Citation2005), we determined 8 PIPs and 3 tonoplast intrinsic proteins (TIPs) (OsPIP1;1, OsPIP1;2, OsPIP1;3, OsPIP2;1, OsPIP2;2, OsPIP2;3, OsPIP2;5, OsPIP2;6, OsTIP1;1, OsTIP2;1 and OsTIP2;2), which are strongly expressed in the root (Sakurai-Ishikawa et al., Citation2011). The PCR conditions were as follows: 1 cycle at 95 °C for 20 s, followed by 40 cycles at 95 °C for 3 s and at 60 °C for 30 s. Three biological replications were conducted for the determination of the expression of aquaporin genes. The sequence of primers and the calculation of the absolute copy number of expressed aquaporin mRNAs are given in Sakurai-Ishikawa et al. (Citation2011). Using sequence analysis, we confirmed the differences of sequences of expressed genes between several indica and japonica cultivars. The cultivar differences were not observed in the sequences of primer pairs used for real-time PCR in this study, indicating that these primers are suitable for comparison of cultivar differences.
4. Immunocytochemistry
Root tissue samples from 13-day-old plants of ‘Puluik Arang’ under 10% PEG treatment were excised from the lateral root initiation zone (approximately 1–2 cm from the root tip) and from the mature zone (middle position of seminal root) of the seminal root. After fixation with 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4 °C, the tissues were dehydrated through a tertiary-butyl alcohol series. Then, the tissues were embedded in paraffin blocks and sectioned into 7-μm slices using a microtome (HM355S, Thermo Fisher Scientific, Germany). After dewaxing by xylene, the sections were gradually hydrated through an ethanol series, from 100% to 30%. The sections were then treated with a blocking solution (1% bovine serum albumin in PBS-Tween20 [0.1%]) for 45 min to block intrinsic alkaline phosphatase activity. After washing in PBS, the sections were reacted with anti-aquaporin antibodies overnight. The sections were carefully washed in PBS, and then the sections were reacted with a second antibody (a 200-fold dilution of anti-rabbit goat IgG-alkaline phosphatase conjugate; Promega, U.S.A) for 4–5 h, and visualized with Western Blue (Promega) stabilized substrate for alkaline phosphatase. When a blue/purple colour was observed, the slides were rinsed with water and then dehydrated in a graded water ethanol series, ethanol–xylene and xylene. The slides were mounted with Eukitt® and observed under a microscope (BX51, Olympus, Japan).
5. Statistical analysis
Statistical analyses were performed using JMP 8 Statistical Discovery software (SAS Institute, U.S.A). The data analysis was conducted to determine the effects of the water treatment on the water uptake, shoot and root DW and root morphological traits by Tukey’s multiple means test, and significant difference between cultivars under each treatment was determined by t-test. To determine the individual and interaction effects of the treatment and cultivar for aquaporin genes expression level, a two-way analysis of variance (ANOVA) was performed.
Results and discussion
Water uptake, dry matter production and morphological root traits of the cultivars ‘Puluik Arang’ and ‘Akitakomachi’ are shown in Table . Water uptake under the 10% PEG treatment in ‘Puluik Arang’ was comparable to that under control. ‘Akitakomachi’ showed a small reduction in water uptake under the 10% PEG treatment compared to control, although the difference was not significant. Water uptake was strongly inhibited under the 20% PEG treatment regardless of the cultivar tested; the water uptake in ‘Puluik Arang’ was approximately 50% and in ‘Akitakomachi’ was approximately 30% of that under the control treatment. ‘Puluik Arang’ (172 mg plant−1 h−1) absorbed significantly larger amounts of water than ‘Akitakomachi’ (121 mg plant−1 h−1) under the 10% PEG treatment. The response of shoot dry weight to osmotic stresses was similar to that of water uptake. On the other hand, PEG treatment did not have a significant effect on root dry weight, although a small reduction was observed under the 20% PEG treatment in both cultivars.
Table 1. Water uptake, dry matter production and root morphological traits of two rice cultivars in response to PEG stress.
Contrary to our expectations, morphological root traits such as root length and lateral root number were not better in ‘Puluik Arang’ than in ‘Akitakomachi’; ‘Akitakomachi’ had significantly longer root length and larger root surface area than ‘Puluik Arang’ under the control and 10% PEG treatments. Therefore, the water uptake per unit root length was significantly better in ‘Puluik Arang’ than in ‘Akitakomachi’ regardless of the treatment. We also found that the response of root development to osmotic stress was different in the two cultivars. ‘Puluik Arang’ maintained similar root length and root surface area under both PEG treatments, whereas root length and surface area were decreased by osmotic stress in ‘Akitakomachi’. Osmotic adjustment contributes to the maintenance of turgor pressure, resulting in the maintenance of root elongation under osmotic stress (Ogawa and Yamauchi, Citation2006). It has also been reported that the cell production influences root elongation under salt stress (Ogawa et al., Citation2006). Investigation of these physiological traits may help in elucidating the mechanism that maintains root development in ‘Puluik Arang’ under osmotic stress.
Lateral root development in the two cultivars was significantly different. Regardless of the treatment, the number of S-type lateral roots in ‘Akitakomachi’ (1035–1231 roots plant−1) was significantly higher than that in ‘Puluik Arang’ (781–854 roots plant−1). The PEG treatments did not affect the number of S-type lateral roots in ‘Puluik Arang’, while the number of S-type lateral roots in ‘Akitakomachi’ was reduced by osmotic stress. The S-type lateral root mostly consisted of the root length of the whole root system in this study; therefore, the maintenance of S-type lateral root development contributed to the maintenance of root length in ‘Puluik Arang’ under PEG stress. The number of L-type lateral roots was significantly higher in ‘Puluik Arang’ (20–37 roots plant−1) than in ‘Akitakomachi’ (8–15 roots plant−1). L-type lateral root development is considered a key trait for adaptation to conditions where water availability is low (Bañoc et al., Citation2000, Suralta et al., Citation2008). Toyofuku et al. (Citation2015) compared the genotypic difference in L-type lateral root formation between osmotic stress tolerant and stress sensitive genotypes and showed that under osmotic stress, L-type lateral root development was better in stress tolerant genotypes. These results indicated that L-type lateral root development is an important root trait involved in stress tolerance in rice; therefore, L-type lateral root development in ‘Puluik Arang’ might contribute towards better water uptake under osmotic stress. However, the difference in the water uptake ability of S-type lateral root and L-type lateral root still remains unknown. The progress made in the analysis of quantitative trait loci (QTL) associated with lateral root development can contribute towards elucidation of the genetic control of lateral root development (Niones et al., Citation2015). The evaluation of differences in the genetic control of development and function of S-type and L-type lateral roots will be required to clarify the role played by L-type lateral roots in conditions where water availability is low.
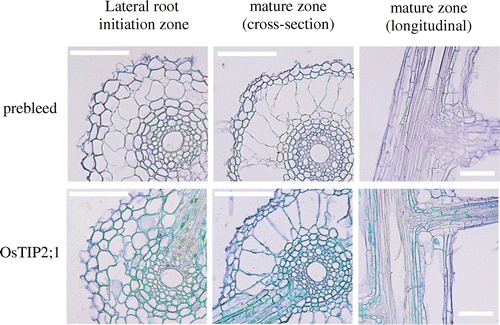
Quantitative analysis of gene expression in the roots showed that the expression levels of several aquaporin genes (e.g. OsPIP2;1, OsPIP2;2, OsPIP2;5, OsTIP2;1) were higher in ‘Puluik Arang’ than in ‘Akitakomachi’ under control and 10% PEG treatments (Figure ). In particular, the expression level of OsTIP2;1, a root-specific TIP (Sakurai et al., Citation2005; Sakurai et al., Citation2008; Sakurai-Ishikawa et al., Citation2011), was approximately seven times higher in ‘Puluik Arang’ than in ‘Akitakomachi’ under the 10% PEG treatment. Henry et al. (Citation2012) also found that the expression level of OsTIP2;1 was higher in the drought tolerant cultivar ‘Dular’ than in ‘IR 64’ during the earlier and middle part of the day under drought stress. Immunocytochemical analysis showed that the OsTIP2;1 proteins had accumulated from the central cylinder to the exodermis of the lateral root initiation zone (Figure ). In the mature zone, the accumulation of aquaporin proteins mainly occurred on the endodermis of the seminal root, and also on the endodermis of lateral roots. Casparian bands restrict water transport via an apoplastic pathway around these cells. Aquaporins that accumulate adjacent to the Casparian bands may facilitate water transport in these regions and could determine root hydraulic conductivity. Therefore, in comparison with ‘Akitakomachi’, the higher expression level of OsTIP2;1 in ‘Puluik Arang’ in response to 10% PEG treatment might be associated with better water uptake through the regulation of root water transport.
Figure 2. Comparison of the copy numbers of expressed aquaporin genes in the seminal roots and lateral roots emerged from seminal root of different cultivars under osmotic stress. Roots of 13-day-old plants were sampled three hours after lights-on. Three replicates of each cultivar for each treatment were conducted using different plant samples. Bars indicate the standard error (n = 3). Asterisk represents a significant difference between cultivars (p < 0.05, t-test). Probability from the results of ANOVA was shown to determine the individual and interaction effects of the treatment and cultivar.
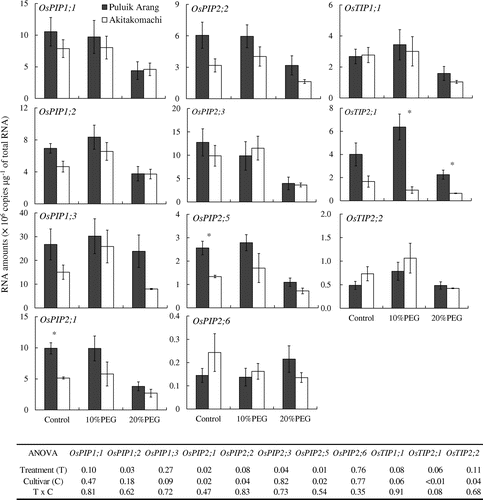
Figure 3. Immunolocalization of OsTIP2;1 in seminal root tissue of the cultivar ‘Puluik Arang’ subjected to 10% PEG treatment. Root tissue samples from 13-day-old plants were excised from the lateral root initiation zone (approximately 1–2 cm from the root tip) and from the mature zone (middle position of seminal root) of the seminal root. Bars represent 100 μm.
Previous studies have reported the effects of osmotic stress on the expression of aquaporin genes and the genotypic difference in the response of aquaporin gene expression to osmotic stresses (Guo et al., Citation2006; Lian et al., Citation2006). However, the study is limited to evaluation of the relationship between aquaporin gene expression and water uptake at the whole plant level under conditions of limited water availability. In this study, of the 11 aquaporin genes, the expression of seven aquaporin genes was significantly correlated with both the amount of water uptake and the amount of water uptake per unit root length (Table ). The results suggest that the levels of expression of aquaporin genes contribute towards the genotypic differences in water uptake at the whole plant level and water uptake per unit root length. Sakurai-Ishikawa et al. (Citation2011) found that the expression of root-specific aquaporins, such as OsPIP2;5, was closely correlated with diurnal change in transpiration. Thus, root aquaporin gene expression is regulated by both the environment around the root and the transpiration demand by the shoot. Further studies on shoot-root communication via the regulation of aquaporins are necessary to evaluate how to optimize water uptake and transport at the whole plant level under conditions of low water availability. Recent studies have revealed that aquaporins are involved not only in plant water relations but also in plant growth and development, such as tissue expansion (Maurel et al., Citation2008). Péret et al. (Citation2012) suggested that auxin-regulated aquaporin gene expression could play an important role in lateral root emergence in Arabidopsis. The investigation of the mechanisms of aquaporin function in root development in rice will contribute towards a deeper understanding of rice adaptation to various hydrological conditions.
Table 2. Correlation coefficients between the expression levels of 11 aquaporin genes versus the amount of water uptake and the amount of water uptake per unit root length.
In conclusion, the results of this study highlight genotypic differences in root development and aquaporin gene expression under osmotic stress in hydroponic culture. Moreover, we also found that the maintenance of lateral root development in ‘Puluik Arang’ under PEG stress was better than that in ‘Akitakomachi’. The L-type lateral root development was significantly better in ‘Puluik Arang’ than in ‘Akitakomachi’. We also found that expression levels of some aquaporin genes, especially OsTIP2;1, were higher in ‘Puluik Arang’ than in ‘Akitakomachi’ under 10% PEG treatment. Data indicate that better maintenance of lateral root development and the function of aquaporin could contribute to water uptake in ‘Puluik Arang’ under osmotic stress. In addition to better morphological development of the root system under conditions of low water availability, the improvement in water uptake per unit root length may be a way of enhancing the extraction of water retained by the soil. Further studies to evaluate the functions of L-type lateral roots and/or aquaporins are required in order to explore the possibilities of enhancing water uptake in rice grown under conditions where water availability is low.
Notes on contributors
Maya Matsunami is a JSPS (the Japanese Society for the Promotion of Science) research fellow and currently works at NARO (National Agriculture and Food Research Organization) Tohoku Agricultural Research Centre, Morioka, Japan. Her research interests are mainly on water and nutrient uptake of rice plant under stressed conditions. Her most recent article was `Genotypic variation in biomass production at the early vegetative stage among rice cultivars subjected to deficient soil moisture regimes and its association with water uptake capacity', Plant Production Science, 15(2012): 82-91.
Kyoko Toyofuku received her PhD from the Faculty of Bioagricultural Sciences at Nagoya University, Japan. Her research covers the areas of sugar transport and signalling, stress responses and gene regulations in rice and leafy vegetables. She works now in the Department of Biological Production at the Akita Prefectural University as a postdoctoral research fellow. Her current interest is the expression mechanisms of plant root plasticity on the osmotic stress tolerance focused on nutrient uptake and transport by lateral root.
Junko Ishikawa-Sakurai is working as a researcher at NARO Institute of Crop Science, Tsukuba, Japan. Her research interests are mainly on mechanism of plant water relation and nutrient distribution in different organs. She is the author and co-author of many articles about rice aquaporin including `Identification of 33 rice aquaporin genes and analysis of their expression and function', Plant Cell Physiology, 46 (2005):1568-1577.
Atsushi Ogawa is an associate professor, Crop Science Laboratory, Department of Biological Production, Akita Prefectural University, Japan. His research interests are the relationship between the change of root morphology and the uptake of water and nutrients under water deficit conditions on crops. His most recent work includes `Genotypic variation in osmotic stress tolerance among rice cultivars and its association with L-type lateral toots development' Plant Production Science., 18 (2015):246-253, written with Maya Matsunami, and Kyoko Toyofuku.
Toshinori Matsunami, agronomist, is now working as a researcher at NARO Tohoku Agricultural Research Center, Morioka, Japan. His research interest relates to rice and soybean crop rotation system. His most recent work includes `Growth characteristics related to high productivity and good quality of sparsely planted rice' Japanese Journal of Crop Science, 85 (2016): 67-76.
Makie Kokubun is Emeritus Professor at Tohoku University, Sendai, Japan. His research interests are physiological and agronomical aspects on crop productivity. He is author of many books and articles, including `Edible Crops' Yokendo, Tokyo, pp.513, 2010, and `Genetic and cultural improvement of soybean for waterlogged conditions in Asia' Field Crops Research, 152 (2013) : 3-7.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank Prof. M. Maeshima (Nagoya University, Japan) for kindly providing anti-OsTIP2;1 antibodies.
Funding
This research was financially supported in part by a Grant-in-Aid for the JSPS (the Japanese Society for the Promotion of Science) fellowship awarded to M. M.
References
- Bañoc, D. M., Yamauchi, A., Kamoshita, A., Wade L. J., & Pardales, J. R. Jr. (2000). Genotypic variations in response of lateral root development to fluctuating soil moisture in rice. Plant Production Science, 3, 335–343.
- Blum, A. (2009). Effective use of water (EUW) and not water use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research, 112, 119–123.10.1016/j.fcr.2009.03.009
- Bouman, B. A. M., Humphreys, E., Tuong, T. P., & Barker, R. (2006). Rice and water. Advances in Agronomy, 92, 187–237.
- Fukai, S., & Cooper, M. (1995). Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crop Research, 40, 67–86.10.1016/0378-4290(94)00096-U
- Guo, L., Wang, Z. Y., Lin, H., Cui, W. E., Chen, J., Liu, M., ... Gu, H. (2006). Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Research, 16, 277–286.10.1038/sj.cr.7310035
- Hachez, C., Besserer, A., Chevalier, A. S., & Chaumont, F. (2013). Insights into plant plasma membrane aquaporin trafficking. Trends in Plant Science, 18, 344–352.10.1016/j.tplants.2012.12.003
- Henry, A., et al. (2012). Root attributes affecting water uptake of rice (Oryza sativa) under drought. Journal of Experimental Botany, 63, 4751–4763.10.1093/jxb/ers150
- Javot, H., & Maurel, C. (2002). The role of aquaporins in root water uptake. Annals of Botany, 90, 301–313.10.1093/aob/mcf199
- Kamoshita, A. (2011). Current status of research on improvement of drought resistance in rice (Oryza sativa L.). Japan Journal of Crop Science 80: 1-12.*
- Kano, M., Inukai, Y., Kitano, H., & Yamauchi, A. (2011). Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant Soil, 342, 117–128.10.1007/s11104-010-0675-9
- Kano-Nakata, M., Inukai, Y., Wade, L. J., Siopongco, J. D. L. C., & Yamauchi, A. (2011). Root development, water uptake, and shoot dry matter production under water deficit conditions in two CSSLs of rice: Functional roles of root plasticity. Plant Production Science, 14, 307–317.10.1626/pps.14.307
- Kato, Y., Abe, J., Kamoshita, A., & Yamagishi, J. (2006). Genotypic variation in root growth angle in rice (Oryza sativa L.) and its association with deep root development in upland fields with different water regimes. Plant Soil, 287, 117–129.10.1007/s11104-006-9008-4
- Knipfer, T., Besse, M., Verdeil, J. L., & Fricke, W. (2011). Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. Journal of Experimental Botany, 62, 4115–4126.10.1093/jxb/err075
- Kobata, T., Okuno T., & Yamamoto T. (1996). Contributions of capacity for soil water extraction and water use efficiency to maintenance of dry matter production in rice subjected to drought. Japan Journal of Crop Science. 64: 652-662.*
- Lian, H., Yu, X., Lane, D., Sun, W. N., Tang, Z. C., & Su, W. A. (2006). Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Research, 16, 651–660.10.1038/sj.cr.7310068
- Luu, D.T. & Maurel, C. (2013). Aquaporin trafficking in plant cells: An emerging membrane-protein model. Traffic, 14, 629–635.10.1111/tra.2013.14.issue-6
- Mahdieh, M., Mostajeran, A., Horie, T., & Katsuhara, M. (2008). Drought stress alters water relations and expression PIP-type aquaporin genes in Nicotiana tabacum plants. Plant and Cell Physiology, 49, 801–813.10.1093/pcp/pcn054
- Matsunami, M., Matsunami, T., Ogawa, A., Toyofuku, K., Kodama, I., & Kokubun, M. (2012). Genotypic variation in biomass production at the early vegetative stage among rice cultivars subjected to deficient soil moisture regimes and its association with water uptake capacity. Plant Production Science, 15, 82–91.10.1626/pps.15.82
- Matre, P., Morillon, R., Barrieu, F., North, B. G., Nobel, P. S., & Chispeels, M. J. (2002). Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology, 130, 2101–2110.10.1104/pp.009019
- Maurel, C., Verdoucq, L., Luu, D. T., & Santoni, V. (2008). Plant aquaporins: Membrane channels with multiple integrated functions. Annual Review of Plant Biology, 59, 595–624.10.1146/annurev.arplant.59.032607.092734
- Murai-Hatano, M., Kuwagata, T., Sakurai, J., Nonami, H., Ahamed, A., Nagasuga, K., ... Okada, M. (2008). Effect of low root temperature on hydraulic conductivity of rice plants and the possible role of aquaporins. Plant and Cell Physiology, 49, 1294–1305.10.1093/pcp/pcn104
- Nguyen, H.T., Babu, R. C., & Blum, A. (1997). Breeding for drought resistance in rice: Physiology and molecular genetics considerations. Crop Science, 37, 1426–1434.10.2135/cropsci1997.0011183X003700050002x
- Niones, J.M., Inukai, Y., Suralta, R. R., & Yamauchi, A. (2015). QTL associated with lateral root plasticity in response to soil moisture fluctuation stress in rice. Plant Soil., 391, 63–75.10.1007/s11104-015-2404-x
- Ogawa, A., Kitamichi, K., Toyofuku, K., & Kawashima, C. (2006). Quantitative analysis of cell division and cell death in seminal root of rye under salt stress. Plant Production Science, 9, 56–64.10.1626/pps.9.56
- Ogawa, A., & Yamauchi, A. (2006). Root osmotic adjustment under osmotic stress in maize seedlings 1. Transient change of growth and water relations in roots in response to osmotic stress. Plant Production Science, 9, 27–38.10.1626/pps.9.27
- Péret, B., Li, G., Zhao, J., Band, L. R., Voß, U., Postaire, O., ... Bennett, M.J. (2012). Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biology, 14,991–998.
- Sakurai, J., Ishikawa, F., Yamaguchi, T., Uemura, M., & Maeshima, M. (2005). Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant and Cell Physiology, 46, 1568–1577.10.1093/pcp/pci172
- Sakurai, J., Ahamed, A., Murai, M., Maeshima, M., & Uemura, M. (2008). Tissue and cell-specific localization of rice aquaporins and their water transport activities. Plant and Cell Physiology, 49, 30–39.10.1093/pcp/pcm162
- Sakurai-Ishikawa, J., Murai-Hatano, M., Hayashi, H., Ahamed, A., Fukushi, K., Matsumoto, T., & Kitagawa, Y. (2011). Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant, Cell & Environment, 34, 1150–1163.10.1111/j.1365-3040.2011.02313.x
- Serraj, R., McNally, K. L., Slamet-Loedin, I., Kohli, A., Haefele, S. M., Atlin, G., & Kumar, A. (2011). Drought resistance improvement in rice: an integrated genetic and resource management strategy. Plant Production Science, 14, 47–55.
- Suralta, R.R., Inukai, Y. & Yamauchi, A. (2008). Genotypic variations in responses of lateral root development to transient moisture stress in rice cultivars. Plant Production Science, 11, 324–335.10.1626/pps.11.324
- Tournaire-Roux, C., Sutka, M., Javot, H.., Gout, E., Gerbeau, P., Luu, D. T., ... Maurel, C. (2003). Cytosolic pH regulates root water transport during anoxic stress thorough gating of aquaporins. Nature, 425, 393–397.10.1038/nature01853
- Toyofuku, K., Matsunami, M., & Ogawa, A. (2015). Genotypic variation in osmotic stress tolerance among rice cultivars and its association with L-type lateral root development. Plant Production Science, 18, 246–253.10.1626/pps.18.246
- Uga, Y., Sugimoto, K., Ogawa, S., Rane, J., Ishitani, M., Hara, N., ... Yano, M. (2013). Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics, 45, 1097–1102.10.1038/ng.2725