Abstract
Because an outgrowth of auxiliary bud in plant is regulated by light quality detected by phytochrome, branching differences in various environment or cultivars in soybean would be the results of the responses to light environment. Therefore, we analyzed differences in the number of branches of two determinate soybean cultivars, ‘Hatsusayaka’ and ‘Sachiyutaka’, between low and high planting densities in relation to light quality within the canopy. We compared changes in the ratio of red to far red (R:FR) irradiance at the ground level over time and with canopy height with those in the fraction of intercepted photosynthetically active radiation (FIPAR) and leaf area index (LAI). Regardless of time or distance from the top of the canopy, the changes in R:FR were sigmoidal, and were symmetrical with those of FIPAR and LAI. The effects of density and cultivar on FIPAR, LAI, and R:FR could be modeled with a logistic function. The number of branches was greater at low density than at high density, and in Hatsusayaka than in Sachiyutaka. However, there were no notable differences in the dynamics of R:FR between planting densities or between cultivars. Close relationships between parameters of the dynamics of the changes in R:FR and of those in FIPAR and LAI suggest that FIPAR and LAI are major factors that regulate R:FR, regardless of time or canopy depth. We found no evidence of a causal relationship between the dynamics of R:FR within the canopy over time or with canopy depth and number of branches of either cultivar.
Classification:
The quantity of solar radiation intercepted by a plant canopy directly affects dry matter production (Monteith, Citation1977), while light quality provides information on the plants’ growth environment. Changes in light quality are detected by phytochromes as changes in the ratio of red (665 nm) to far red (730 nm) irradiance (R:FR). A lowered R:FR is a warning signal for future competition, triggering plant morphogenic responses for shade avoidance, seen as enhanced elongation, accelerated flowering, increased apical dominance, and reduced tillering (Ballaré & Casal, Citation2000; Smith, Citation2000; Franklin & Whitelam, Citation2005). Shade avoidance is an essential survival strategy for plants in the natural environment, and its importance in agronomy is related to the emergence and outgrowth of auxiliary buds. For instance, R:FR in wheat is closely related to the determination of spike number through the emergence and survival of tillers (Evers et al., Citation2006; Sparkes et al., Citation2006; Toyota et al., Citation2014; Xie et al., Citation2016). In soybean, studies have shown that an increase (Kasperbauer, Citation1987) or decrease (Green-Tracewicz et al., Citation2011) in R:FR resulted in a respective increase or decrease in branching, and that greater partitioning of dry matter into branches altered R:FR within the canopy (Board, Citation2000). However, there is limited information on the relationships between the light environment in a canopy and branching in soybean.
In general, partitioning of dry matter to branches increases under low planting densities, resulting in greater branch number per plant (Weber et al., Citation1966; Carpenter & Board, Citation1997; Board Citation2000). Yield responses to different planting densities result from the emergence and growth of branches (Carpenter & Board, Citation1997; Norsworthy & Shipe, Citation2005). The plasticity of branching among soybean cultivars has an important role in yield, particularly at low planting density. For instance, considerable variation in branch development at low planting density caused differences in seed yield (Board & Kahlon, Citation2013), and branching plasticity was responsible for yield stability across planting densities in American cultivars, unlike in Japanese cultivars, in which yield declined with decreasing planting density (Agudamu et al., Citation2016). Despite the significant contribution of branch number to yield in soybean, however, information about the relationship between branching performance and the light environment within a canopy is still limited. It is, therefore, worthwhile to explore the dynamic relationship between the light quality within a canopy and branching characteristics in different cultural environments or cultivars. If a causal relationship exists, it will provide fundamentals for developing a new avenue to increase or stabilize soybean yield through cultural and genetical control of branching responses.
Here, we present empirical measurements of light interception and light quality within a soybean canopy over time and with canopy depth, and the associated morphological traits obtained by field experiments with two determinate Japanese cultivars with different branching characteristics at two planting densities. The main objective was to clarify the relationships between the changes in light quality within a soybean canopy over time and with canopy depth and the resulting changes with light interception, leaf area, leaf thickness, and leaf greenness. We also aimed at elucidating whether the light quality within the canopy is responsible for differences in branching characteristics between plant densities or cultivars.
Materials and methods
Experimental site, cultivars, and field management
Two field experiments were conducted at the Faculty of Agriculture, Kagawa University (34°16′N, 134°7′E), in 2014 and 2015, using two determinate soybean cultivars (Hatsusayaka in both years and Sachiyutaka in 2015) and two planting densities (12.8 and 18.5 plants m−2). The field had a well-drained loam soil. We established four 1.8-m × 18.0-m ridges for Hatsusayaka in 2014 and six 1.5-m × 18.0-m ridges for both cultivars in 2015, with the ridges running from north to south. Each ridge consisted of six rows in 2014 and five rows in 2015, separated by 0.3 m in both years. Seeds were sown on 18 July 2014 and 13 July 2015 at intervals of 0.09 m using a hand-powered seed drill (HS-120; Mukai Kogyo, Inc., Osaka, Japan). At the V1 [stages according to Fehr and Caviness (Citation1977)], seedlings were thinned to a spacing of 0.18 or 0.27 m, which corresponded to planting densities of 18.5 plants m−2 (hereafter, D18) and 12.8 plants m−2 (D12), respectively. The treatments were arranged in a randomized block design with six replications and plot areas of 1.8 m × 5.0 m (2014) and 1.5 m × 5.0 m (2015). The field was fertilized with compound fertilizer before sowing at rates of 20, 56, and 56 kg ha−1 of N, P2O5, and K2O, respectively. A herbicide mixture of benthiocarb, pendimethalin, and linuron (Clearturn; Kumiai Chemical Industry Co., Ltd., Tokyo, Japan) was sprayed at the manufacturer-recommended dosage after sowing to prevent weeds. Plants were sprinkled water several times by a water hose attached with a spray nozzle during the early vegetative stage, when the soil moisture seemed deficient.
Measurements
Aboveground materials from four plants per plot (for all destructive samples in 2014 and the first two destructive samples in 2015) or from three plants per plot (for the third to the sixth destructive samples in 2015) were collected at 2-week intervals: at 12, 26, 40, 54, 68, and 82 days after sowing (DAS) in 2014 and at 17, 31, 45, 59, 73, and 87 DAS in 2015. We did not sample plants in the border rows so as to avoid edge effects. We counted the numbers of branches and sub-branches (second- and third-order racemes with compound leaves) per plant. Leaf greenness of the topmost fully expanded leaf was measured with a SPAD meter (SPAD-502; Konica Minolta Inc., Tokyo, Japan). All leaf blades were then removed and spread in a single layer on a large copy stand, except for dead and yellowed parts, and images were taken with a digital camera. The leaf area per plot was measured from the digital images by means of image analysis using the LIA32 software (http://www.agr.nagoya-u.ac.jp/~shinkan/LIA32/). The dry weight of each leaf blade was determined after oven-drying at 80 °C for more than 72 h. The specific leaf area (SLA, cm2 g−1) was calculated as the total leaf area divided by the total leaf dry weight per plot.
At maturity, 20 plants from each plot were destructively sampled: on 27 October 2014 (102 DAS) and 26 October 2015 (106 DAS). The numbers of branches and sub-branches per plant were recorded.
Air temperature, daily total solar radiation, and daily precipitation were measured at a meteorological station of the Faculty of Agriculture, Kagawa University, which is located adjacent to the experimental field. The values in 2014 and 2015 were compared with the 13-year mean values from 2001 to 2013 at the station.
The fraction of photosynthetically active radiation (PAR) intercepted by the plant canopy (FIPAR) in each plot was measured weekly with a SunScan Canopy Analysis System (Delta-T Devices, Cambridge, UK). This system measures PAR above and below the canopy simultaneously, using a sunshine sensor placed at the center of the field higher than the canopy height and a 1-m probe that contains 64 PAR sensors is inserted into the canopy at the ground level, perpendicular to the row direction. The 1-m probe was connected with the sunshine sensor using a 25-m cable and was attached to a handheld computer to store the data. The mean of five measurements per plot was used to estimate LAI and FIPAR. FIPAR was calculated subtracting the ratio of PAR below the canopy to that above the canopy from one (Purcell et al., Citation2002):
R:FR at the ground level (R:FR) was measured weekly using a red/far-red sensor (SKR110; Skye Instruments, Powys, UK) and a light sensor logger (LI1500; Li-COR, Lincoln, NE, USA).
Cumulative LAI (cLAI), FIPAR, and R:FR at vertical intervals of 10 cm from the top of the canopy to the soil surface were measured on 18 September 2014 (62 DAS) and 11 September 2015 (60 DAS). The average of three (cLAI, FIPAR) or nine (R:FR) measurements per stratum was used. The greenness of about 50–60 leaves per plot chosen randomly and evenly from all heights were measured with the SPAD meter. Immediately after measurement of each leaf, the height of the leaf from the soil surface was measured.
Changes in FIPAR, LAI, and R:FR over DAS and in the vertical distribution of FIPAR, cLAI, and R:FR with canopy depth were fitted to a logistic function (Figure ) (Sparkes et al.,. Citation2006; Xie et al., Citation2016):(1)
Figure 1. Schematic curve of a logistic function [Equation (Equation1(1) )] fitted to the increase in FIPAR or LAI or the reduction of R:FR over time (DAS) or distance (cm) from the top of the canopy. Aupper, upper asymptote; Alow, lower asymptote; Onset and End, beginning and completion of increase (or decrease). Duration (or distance) of increase (or decrease) is DAS or distance (cm) from Onset to End (t1 − t0).
![Figure 1. Schematic curve of a logistic function [Equation (Equation1(1) )] fitted to the increase in FIPAR or LAI or the reduction of R:FR over time (DAS) or distance (cm) from the top of the canopy. Aupper, upper asymptote; Alow, lower asymptote; Onset and End, beginning and completion of increase (or decrease). Duration (or distance) of increase (or decrease) is DAS or distance (cm) from Onset to End (t1 − t0).](/cms/asset/ed09dea9-7087-49f1-9b5e-8b44408f4fe4/tpps_a_1294464_f0001_b.gif)
where Y is FIPAR, cLAI, LAI, or R:FR; x is DAS for the changes in FIPAR, LAI, and R:FR over time (DAS) or distance (cm) from the top of the canopy; A is the lower asymptote and A + C is the upper asymptote and B is the doubled the relative rate of increase in FIPAR, cLAI, or LAI or of decrease in R:FR at time M (DAS) or distance M (cm) from the top of the canopy when the rate of increase or of reduction is at a maximum and when FIPAR, cLAI, LAI, or R:FR reaches A + 0.5C. The beginning of the increase (or decrease) of Y is Onset (t0: at A + 0.1C, t0 = M − 2.1972/B), and the completion is End (t1: at A + 0.9C, t1 = M + 2.1972/B). The duration (or distance) of the increase (or decrease) is the DAS or distance (cm) from Onset to End (t1 − t0) (Xie et al., Citation2016).
The responses of R:FR to the increase of LAI were fitted to an exponential function (Figure ) (Edwards et al., Citation2005):(2)
Figure 2. Schematic curve of an exponential function [Equation (Equation2(2) )] fitted to the relationships between R:FR and TransPAR (– · –) and LAI ( – ).
![Figure 2. Schematic curve of an exponential function [Equation (Equation2(2) )] fitted to the relationships between R:FR and TransPAR (– · –) and LAI ( – ).](/cms/asset/1cbb2b32-d015-4060-8540-3fbb01d7e47d/tpps_a_1294464_f0002_b.gif)
where Y is R:FR, x is LAI, β0 + α is the asymptote, and β1 (negative) represents the responsiveness of R:FR to the changes of LAI. Because Equation (Equation2(2) ) did not fit the response of R:FR to the increase in FIPAR, we used the fraction of PAR transmitted by a canopy (TransPAR; PAR below / above the canopy) instead. In this case, x in Equation (Equation2
(2) ) is TransPAR and β1 (positive) represents the responsiveness of R:FR to the changes in TransPAR.
Statistical analysis
Data for 2015 were analyzed by means of a two-way ANOVA using a model for a randomized block design with six replications to evaluate the effects of plant density, cultivar, and their interaction on the number of branches and sub-branches per plant. The effect of plant density on the number of branches and sub-branches per plant in 2014 was evaluated by t-test. Regression analysis was carried out to quantify the relationships between variables estimated by Equation (Equation1(1) ). Statistical analysis, including non-linear regression analysis to estimate the values of A, C, B, and M for Equation (Equation1
(1) ) and of α, β0, and β1 for Equation (Equation2
(2) ), was performed in the JMP statistical software (SAS Institute Japan, Tokyo, Japan).
Results
Meteorological conditions
Table shows the mean temperature, daily total solar radiation, and precipitation during the experiments in 2014 and 2015 and the 13-year means (2001–2013). In both years, there were many cloudy and rainy days throughout the experimental period. Almost all mean temperatures in both years were significantly lower than the 13-year mean. The mean radiation in August and October 2014 and from July to September in 2015 was significantly lower than the 13-year mean, while that in October 2015 was significantly higher. The precipitation in August and October 2014 and July 2015 was significantly higher than the 13-year mean, and that in July 2014 and October 2015 was significantly lower.
Table 1. Monthly mean temperatures, daily total solar radiation, and precipitation during the experiments in 2014 and 2015, and the 13-year means (2001–2013) at the study site.
Plant development
Hatsusayaka began flowering (R1) on 20 August 2014 (33 DAS), and Sachiyutaka and Hatsusayaka began flowering on 17 and 18 August 2015, respectively (35 and 36 DAS). The plants began to set seed (R5) on 18 September 2014 (62 DAS) and 9 September 2015 (58 DAS) and reached the full seed stage (R6) on 22 September 2014 (66 DAS) and 24 September 2015 (71 DAS) in both cultivars and at both densities.
Branches emerged later in 2014 than in 2015; there were no branches at 33 DAS in 2014, whereas the average number across cultivars and densities was 3.0 at 31 DAS in 2015 (Figure ). In 2014, the number of branches tended to be higher in D12 than in D18 throughout growth. In 2015, the number of branches was higher in Hatsusayaka and in D12 than in Sachiyutaka and D18 throughout growth. At maturity, the number of branches was significantly greater and the number of sub-branches tended to be greater in D12 than in D18 (Table ). In 2015, the effects of cultivar and density on the numbers of branches and sub-branches were significant, but with no significant cultivar × density interaction (Table ). The average numbers of branches and sub-branches were 21 and 16% higher in Hatsusayaka than in Sachiyutaka, and 30 and 16% higher in D12 than in D18.
Figure 3. Changes in the number of branches per plant of Hatsusayaka (H) or Sachiyutaka (S) at normal (D12) and high (D18) densities in (a) 2014 and (b) 2015. Values are mean ± S.E. (n = 6).
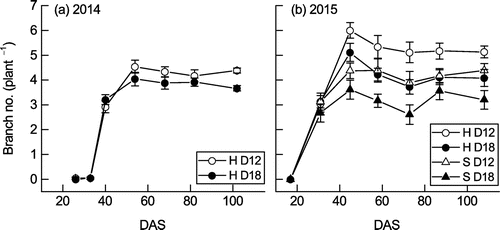
Table 2. Numbers of branches and sub-branches per plant at maturity.
Table 3. Onset, End, duration, and asymptote of sigmoidal curves of LAI, FIPAR, and R:FR over time at normal (D12) and high (D18) plant densities in 2014 and 2015.
Changes in R:FR, FIPAR, and LAI over time
FIPAR and LAI increased as a sigmoidal function of time, and R:FR decreased symmetrically to both (Figure ). There were no particular differences in the timing of Onset and End in R:FR between planting densities or cultivars, except for an earlier Onset and a later End in Sachiyutaka in D18, which lengthened the duration of the R:FR decrease (Table ). The asymptote of R:FR was lower in D18 than in D12, and in Hatsusayaka than in Sachiyutaka. LAI in 2014 was higher in D18 than in D12 throughout the growth period, and that in 2015 was higher in D18 than in D12 and in Hatsusayaka than in Sachiyutaka after around R1. Although the End of FIPAR was earlier and FIPAR at the End was higher in D18 than in D12 in both years and in both cultivars, there were no differences between cultivars.
Figure 4. Changes in (a–c) R:FR, (d–f) fraction of intercepted PAR (FIPAR), and (g–i) LAI over time in the canopy of Hatsusayaka (H) and Sachiyutaka (S) plants grown at normal (D12) and high (D18) densities in 2014 and 2015. Values are mean ± S.E. (n = 6). All variables were fitted to a logistic function [Equation (Equation1(1) )]. The Onset and End (x and y), duration, asymptote, and root mean square error of the fitted lines are shown in Table .
![Figure 4. Changes in (a–c) R:FR, (d–f) fraction of intercepted PAR (FIPAR), and (g–i) LAI over time in the canopy of Hatsusayaka (H) and Sachiyutaka (S) plants grown at normal (D12) and high (D18) densities in 2014 and 2015. Values are mean ± S.E. (n = 6). All variables were fitted to a logistic function [Equation (Equation1(1) )]. The Onset and End (x and y), duration, asymptote, and root mean square error of the fitted lines are shown in Table 3.](/cms/asset/b26a670d-9ce6-4361-8dcb-f989322c067f/tpps_a_1294464_f0004_b.gif)
Table 4. Pearson’s correlation coefficients (r) for the relationships between Onset, End, asymptote, and duration (or distance) of R:FR and those of FIPAR and LAI with changes over time or canopy depth.
The timings (x) of Onset and End and the asymptote of R:FR were significantly correlated with those of FIPAR and LAI, except for x of End in FIPAR (p = 0.504) (Table ).
Distribution of R:FR, FIPAR, and cLAI with canopy depth
The distribution of R:FR was sigmoidal (Figure (a–c), and was symmetrically opposite to those of FIPAR and cLAI (Figure (d–i)). In 2014, R:FR was higher in D12 than in D18 at most heights above the ground. In 2015, the decrease in R:FR at around 70 cm was faster in Hatsusayaka than in Sachiyutaka at both densities. The height of the Onset of R:FR decrease was lower and the End was higher in Hatsusayaka (Table ). The distribution of FIPAR was sigmoidal, with little difference between densities and cultivars (Figure (d–f)). FIPAR of Hatsusayaka in 2015 began to increase at a higher position than that of Sachiyutaka; however, the difference in FIPAR between cultivars disappeared below about 40 cm in both densities because maximum FIPAR had been reached. cLAI was greater in 2015 (at 60 DAS; Figure (h, i)) than in 2014 (at 62 DAS; Figure (g)). The increase in cLAI from the top to the bottom of the canopy began at a higher position in D12 than in D18 in 2014, but cLAI at the bottom of the canopy was the same in both densities. In 2015, the increase in cLAI from the top of the canopy began at a higher position in Hatsusayaka, and cLAI was higher in Hatsusayaka than in Sachiyutaka from 70 cm to the bottom of the canopy.
Figure 5. Distribution of (a–c) R:FR, (d–f) fraction of intercepted PAR (FIPAR), and (g–i) cumulative LAI (cLAI) from the soil surface to the top of the canopy at 62 DAS in 2014 and at 60 DAS in 2015. Values are mean ± S.E. (n = 6). All variables were fitted to a logistic function [Equation (Equation1(1) )]. The Onset and End (x and y), distance, asymptote, and root mean square error of the fitted lines are shown in Table .
![Figure 5. Distribution of (a–c) R:FR, (d–f) fraction of intercepted PAR (FIPAR), and (g–i) cumulative LAI (cLAI) from the soil surface to the top of the canopy at 62 DAS in 2014 and at 60 DAS in 2015. Values are mean ± S.E. (n = 6). All variables were fitted to a logistic function [Equation (Equation1(1) )]. The Onset and End (x and y), distance, asymptote, and root mean square error of the fitted lines are shown in Table 4.](/cms/asset/10805fee-150c-47ff-ac2a-45ed32ee49ff/tpps_a_1294464_f0005_b.gif)
Table 5. Onset, End, distance, and asymptote of sigmoidal curves of LAI, FIPAR, and R:FR with canopy depth at normal (D12) and high (D18) plant densities in 2014 and 2015.
Although the correlations were not significant, there were positive trends between the timing (x) of Onset in R:FR and FIPAR (p = 0.074) and LAI (p = 0.090) (Table ). R:FR at the End was significantly correlated with FIPAR and LAI at the End. The asymptote of R:FR was significantly correlated with those of FIPAR and LAI.
Relationship between R:FR and LAI or FIPAR
R:FR decayed exponentially as LAI increased with time and increased exponentially as TransPAR increased with time, and the relationships fitted Equation (Equation2(2) ) well (Figure ). R:FR responded similarly with canopy depth (Figure ).
Figure 6. Responses of R:FR to changes in (a, b) LAI and (c, d) TransPAR of the canopies of Hatsusayaka (○●) and Sachiyutaka (□■) plants grown at normal (D12, ○□) and (b) high (D18, ●■) densities over time in 2014 and 2015. Values are mean ± S.E. (n = 6). All variables were fitted to an exponential function [Equation (Equation2(2) )].
![Figure 6. Responses of R:FR to changes in (a, b) LAI and (c, d) TransPAR of the canopies of Hatsusayaka (○●) and Sachiyutaka (□■) plants grown at normal (D12, ○□) and (b) high (D18, ●■) densities over time in 2014 and 2015. Values are mean ± S.E. (n = 6). All variables were fitted to an exponential function [Equation (Equation2(2) )].](/cms/asset/8f344f1d-1530-48bd-babb-ba10b53a97ca/tpps_a_1294464_f0006_b.gif)
Figure 7. Responses of R:FR to changes in (a–c) LAI and (d–f) TransPAR with canopy depth of Hatsusayaka and Sachiyutaka plants grown at normal (D12) and high (D18) densities at (a, d) 62 DAS in 2014 and (b–f) 60 DAS in 2015. Values are mean ± S.E. (n = 6). All variables were fitted to an exponential function [Equation (Equation2(2) )].
![Figure 7. Responses of R:FR to changes in (a–c) LAI and (d–f) TransPAR with canopy depth of Hatsusayaka and Sachiyutaka plants grown at normal (D12) and high (D18) densities at (a, d) 62 DAS in 2014 and (b–f) 60 DAS in 2015. Values are mean ± S.E. (n = 6). All variables were fitted to an exponential function [Equation (Equation2(2) )].](/cms/asset/f91df9c5-c0ac-4c1b-aa69-15d6dc06f294/tpps_a_1294464_f0007_b.gif)
Leaf greenness and SLA
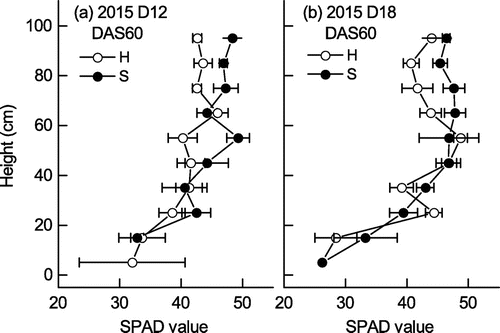
The SPAD values were higher in Sachiyutaka than in Hatsusayaka at most heights above ground, particularly above 70 cm, at 60 DAS in 2015, with means of 44.0 (D12) and 44.1 (D18) in Sachiyutaka versus 40.2 (D12) and 42.0 (D18) in Hatsusayaka (Figure ). They gradually decreased from the top to the bottom of the canopy at both densities in both cultivars.
Figure 8. Distribution of SPAD values with canopy depth at 60 DAS in 2015 at (a) normal (D12) and (b) high (D18) plant densities. Values are mean ± S.E. (n = 6).
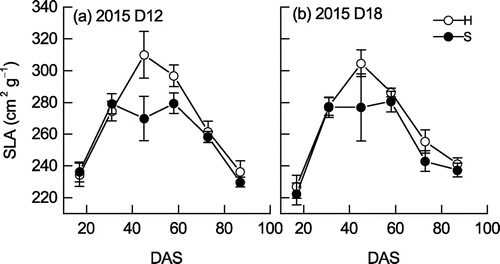
SLA was significantly larger in D12 than in D18 in Sachiyutaka at 73 DAS (Figure ). SLA was larger in Hatsusayaka than in Sachiyutaka throughout the growth period, and was significantly larger at 45 and 58 DAS in D12.
Discussion
Cloudy and rainy days in summer and fall were in common in both years. In 2014, western Japan had record high precipitation, low temperatures, and a low number of sunshine hours due to a stationary rain front and typhoons. These extremes during the early growth stage in 2014 delayed soybean growth. In 2015, in contrast, very high yield and dry matter accumulation were obtained in spite of low temperatures and low radiation (Maitree and Toyota, Citationin press). The lower number of branches and smaller LAI in 2014 than in 2015 is attributable to this climate difference between the two years. Nevertheless, plant growth was normal in both years, and no appreciable damage was observed.
A previous report showed that R:FR in the soybean canopy decreased nearly linearly over time (Board, Citation2000), whereas our more frequent measurements showed that it decreased sigmoidally, while FIPAR and LAI increased sigmoidally (Figure ). Similarly, R:FR decreased sigmoidally from the top of the canopy down, symmetrically opposite to FIPAR and cLAI (Figure ). To our knowledge, this is the first study to show the distribution of R:FR with canopy depth in soybean. The patterns of change in all variables were similar both over time and with canopy depth, and were modeled by the same logistic function at both plant densities in both cultivars.
The close relationships of R:FR with LAI and TransPAR were well explained by an exponential function [Equation (Equation2(2) )] (Figures and ). The positive relationships between R:FR and TransPAR are similar to a previous report of close, positive linear relationships between R:FR and photosynthetic photon flux density transmittance in maize, soybean, and wheat canopies (Sattin et al., Citation1994).
The numbers of branches and sub-branches were greater at the low planting density (D12) than at the high planting density (D18) (Figure ; Table ), as previously reported (Carpenter & Board, Citation1997; Norsworthy & Shipe, Citation2005; Agudamu et al., Citation2016). The numbers were significantly higher in Hatsusayaka than in Sachiyutaka throughout growth (Figure ) and at maturity (Table ), also as previously reported (Saruta et al., Citation2012). Kasperbauer (Citation1987) and Green-Tracewicz et al. (Citation2011) showed that an increase in R:FR led to greater branch development in soybean, and a decrease led to lesser branch development. Board (Citation2000) showed that greater dry matter partitioning into branches altered R:FR within the canopy. However, there is no report of a causal relationship between R:FR and the number of branches of a cultivar.
There were no differences in the timing of Onset and End in R:FR between planting densities or between cultivars over time, but the asymptote of R:FR was lower in D18 than in D12, and was lower in Hatsusayaka than in Sachiyutaka. R:FR was higher in D12 than in D18 at most height above ground, and R:FR decreased more sharply at around 70 cm in Hatsusayaka than in Sachiyutaka. The height of the Onset in R:FR was lower, but the End was higher, in Hatsusayaka than in Sachiyutaka. These different responses of R:FR to planting density and by cultivar may be fundamentally regulated by FIPAR and LAI. Because the significant correlation between the timings of changes in R:FR over time and those in FIPAR and LAI suggest that FIPAR and LAI are major factors that regulate the changes in R:FR. The changes in R:FR with canopy depth showed a similar trend, suggesting that these changes are regulated by FIPAR and cLAI.
Hatsusayaka produced more branches than Sachiyutaka at both low and high planting densities (Figure ). R:FR in D18 decreased more sharply in Hatsusayaka than in Sachiyutaka (Figure (c)), and R:FR at the End of the decrease and the asymptote were lower in Hatsusayaka (Table ). On the other hand, Hatsusayaka had lower SPAD values (Figure ) and higher SLA (Figure ). These results indicate that Hatsusayaka had a lower chlorophyll content and thinner leaves, associated with a lower R:FR, than Sachiyutaka (Figures and ). This association is consistent with results in Rumex obtusifolius (McLaren & Smith, Citation1978). Although we did not count the leaves, the larger LAI at a higher position in the canopy in Hatsusayaka might be the consequence of a larger number of leaves, because the larger number of branches in Hatsusayaka could have more nodes, and consequently more leaves. The lower chlorophyll content and thinner leaves associated with lower R:FR in Hatsusayaka suggest that although the chlorophyll content per unit leaf area in Hatsusayaka was low, its greater leaf area compensated for the absorption of PAR, resulting in a lower R:FR.
Conclusions
Soybean plants growing at low density (D12) had more branches than those growing at high density (D18), and Hatsusayaka had more branches than Sachiyutaka. The asymptote of R:FR estimated by the logistic function was lower in D18 than in D12, and was lower in Hatsusayaka than in Sachiyutaka. R:FR was higher in D12 than in D18 at most height above ground, and R:FR decreased more in Hatsusayaka than in Sachiyutaka. However, no notable difference was observed in the dynamics of R:FR between planting densities or between cultivars. The significant correlations between the timings of Onset and End of R:FR over time and those in FIPAR and LAI confirmed that FIPAR and LAI are major factors that regulate R:FR at the bottom of the canopy. Similarly, FIPAR and cLAI tended to regulate the change in R:FR with canopy depth. We found no evidence for a causal relationship between the dynamics of R:FR within the canopy over time or with canopy depth and number of branches of either cultivar.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported in part by JSPS KAKEN [grant number 24650022] to MT.
| Abbreviations | ||
| FIPAR | = | fraction of intercepted photosynthetically active radiation |
| DAS | = | days after sowing |
| LAI | = | leaf area index |
| PAR | = | photosynthetically active radiation |
Acknowledgment
The authors thank the Western Region Agricultural Research Center, National Agriculture and Food Research Organization, for providing the Hatsusayaka seeds.
References
- Agudamu, Yoshihira, T., & Shiraiwa, T. (2016). Branch development responses to planting density and yield stability in soybean cultivars. Plant Production Science, 19, 331–339.10.1080/1343943X.2016.1157443
- Ballaré, C. L., & Casal, J. J. (2000). Light signals perceived by crop and weed plants. Field Crops Research, 67, 149–160.10.1016/S0378-4290(00)00090-3
- Board, J. (2000). Light interception efficiency and light quality affect yield compensation of soybean at low plant populations. Crop Science, 40, 1285–1294.10.2135/cropsci2000.4051285x
- Board, J. E., & Kahlon, C. S. (2013). Morphological responses to low plant population differ between soybean genotypes. Crop Science, 53, 1109–1119.10.2135/cropsci2012.04.0255
- Carpenter, A. C., & Board, J. E. (1997). Branch yield components controlling soybean yield stability across plant populations. Crop Science, 37, 885–891.10.2135/cropsci1997.0011183X003700030031x
- Edwards, J. T., Purcell, L. C., & Karcher, D. E. (2005). Soybean yield and biomass responses to increasing plant population among diverse maturity groups: II. Light interception and utilization. Crop Science, 45, 1778–1785.
- Evers, J. B., Vos, J., Andrieu, B., & Struik, P. C. (2006). Cessation of tillering in spring wheat in relation to light interception and red: Far-red ratio. Annals of Botany, 97, 649–658.10.1093/aob/mcl020
- Fehr, W. R., & Caviness, C. E. (1977). Stages of soybean development. In Cooperative extension service, agriculture and home economics experiment station (pp. 1–12). Ames, Iowa: Iowa State University of Science and Technology.
- Franklin, K. A., & Whitelam, G. C. (2005). Phytochromes and shade-avoidance responses in plants. Annals of Botany, 96, 169–175.10.1093/aob/mci165
- Green-Tracewicz, E., Page, E. R., & Swanton, C. J. (2011). Shade avoidance in soybean reduces branching and increases plant-to-plant variability in biomass and yield per plant. Weed Science, 59, 43–49.
- Kasperbauer, M. J. (1987). Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiology, 85, 350–354.10.1104/pp.85.2.350
- Maitree, L., & Toyota, M. (in press). A high seed yield and associated attributes of dry matter production achieved by recent Japanese soybean cultivars. Plant Production Science. doi: 10.1080/1343943X.2017.1294463
- McLaren, J. S., & Smith, H. (1978). Phytochrome control of the growth and development of Rumex obtusifolius under simulated canopy light environments. Plant, Cell & Environment, 1, 61–67.10.1111/pce.1978.1.issue-1
- Monteith, J. L. (1977). Climate and the efficiency of crop production in Britain. Philosophical Transactions of the Royal Society B: Biological Sciences, 281, 277–294.10.1098/rstb.1977.0140
- Norsworthy, J. K., & Shipe, E. R. (2005). Effect of row spacing and soybean genotype on mainstem and branch yield. Agronomy Journal, 97, 919–923.10.2134/agronj2004.0271
- Purcell, L. C., Ball, R. A., Reaper, J. D., & Vories, E. D. (2002). Radiation use efficiency and biomass production in soybean at different plant population densities. Crop Science, 42, 172–177.10.2135/cropsci2002.0172
- Saruta, M., Takada, Y., Matsunaga, R., Hajika, M., Takahashi, M., & Komatsu, K. (2012). A new soybean cultivar ‘Hatsusayaka’, with tolerance of delayed leaf senescence and suitability for tofu processing. Bulletin of NARO Western Region Agricultural Research Center, 11, 81–99*.
- Sattin, M., Zuin, M. C., & Sartorato, I. (1994). Light quality beneath field-grown maize, soybean and wheat canopies – red: Far red variations. Physiologia Plantarum, 91, 322–328.10.1111/ppl.1994.91.issue-2
- Smith, H. (2000). Phytochromes and light signal perception by plants-an emerging synthesis. Nature, 407, 585–591.10.1038/35036500
- Sparkes, D. L., Holme, S. J., & Gaju, O. (2006). Does light quality initiate tiller death in wheat? European Journal of Agronomy, 24, 212–217.10.1016/j.eja.2005.08.003
- Toyota, M., Tatewaki, N., Morokuma, M., & Kusutani, A. (2014). Tillering responses to high red/ far-red ratio of four Japanese wheat cultivars. Plant Production Science, 17, 124–130.10.1626/pps.17.124
- Weber, C. R., Shibles, R. M., & Byth, D. E. (1966). Effect of plant population and row spacing on soybean development and production. Agronomy Journal, 58, 99–102.10.2134/agronj1966.00021962005800010034x
- Xie, Q., Mayes, S., & Sparkes, D. L. (2016). Optimizing tiller production and survival for grain yield improvement in a bread wheat × spelt mapping population. Annals of Botany, 117, 51–66.10.1093/aob/mcv147