Abstract
Cultivar tests under tropical environments could be an approach to explore soybean productivity under high temperature. Twenty-nine soybean cultivars divided into five groups with temperate origin (Japanese and US) and tropical origin (Indonesian-old, Indonesian-modern and other tropical) were grown for two years in a tropical environment at Banten, Indonesia, with minimal season-to-season variation in air temperature and day-length. Temperate cultivars were earlier in flowering and shorter in duration from R1 to R5. Temperate cultivars had a seed yield of 157 g m−2 (mean temperate cultivars) compared to 249 g m−2 (tropical cultivars), which was due to having lower values of pods, seed number and TDW. In addition, the occurrence of shriveling and smaller seed size compared to plants grown in their region of origin was considerably evident in Japanese cultivars. To account for the difference of growth duration, a maturity-corrected index for yield and relevant variables was calculated to consider the amount of incident solar radiation. The yield index for all tested cultivars ranged from .49 to 1.48, and Japanese cultivars showed the lowest yield index (.67), followed by US cultivars (.87), whereas tropical cultivars had index means from 1.05 to 1.20. Although they were both of temperate origin, Japanese cultivars tended to show a lower index than US cultivars. The tendency was similar for TDW and node number. The poor performance of temperate cultivars even after correction suggests that there is a genetic variation of adaptation to a tropical environment independent of growth duration. Additionally, there was considerable performance variation within temperate cultivars.
Classification:
Introduction
Soybean production has dominated the temperate regions due to cool to moderately warm climates. The increasing demand for soybeans in economically developing regions necessitates the rapid increase of production in the tropics. Soybean production under high temperature environments has also increased in the temperate regions due to global warming. The favorable temperature for soybean range of 16–28 °C for the whole growing season (McBlain et al., Citation1987), or the ranges of 15–22 °C, 20–22 °C, and 15–22 °C as the optimum temperatures for the emergence, flowering and maturity stages, respectively (Liu et al., Citation2008), or maximum of 27 °C for the seed-filling period (Thomas et al., Citation2010). Concerns have emerged that global climate change may impact soybean production (Prasad et al., Citation2006, Citation2017).
A recent analysis of long-term data revealed that growing season temperatures had a negative impact on soybean yields and caused a 17% reduction for every 1 °C rise (Lobell & Asner, Citation2003). Moreover, studies using a temperature gradient chamber (TGC) and a model-aided analysis on farmers’ yields have demonstrated significant negative effects of increased temperature on soybean yield under a temperate climate (Tacarindua et al., Citation2012, Citation2013), and reductions in both reproductive development and biomass production were associated. However, the variability of soybean genotypes in response to high temperature has been very limited (Chebrolu et al., Citation2016; Mochizuki et al., Citation2005), which is crucially important for soybean breeding for better adaptations to high temperature.
The tropical environment with relatively higher temperatures has the potential to be employed to study plant development and agronomic performance under high temperatures. Unlike temperate regions, the mean air temperature in the tropics is relatively consistent and between 20 and 30 °C every month of the year; the differences in monthly mean temperature between cool and hot months are rarely more than 7 °C, and in some regions, the difference is only 2 °C (Monteith, Citation1977). Additionally, the day-length is approximately 12 h throughout the year at the equator (Monteith, Citation1977), while in the temperate latitudes, it ranges from 11.2 to 14.4 h at latitude 35° in Kyoto, Japan. The large seasonal change of environmental factors and associated environmental stresses in the temperate region can have considerable seasonal effects on crop performances, to which the responsiveness of plant production processes is different depending on developmental stage. Therefore, the climate in the tropics, such as that in Indonesia, may be suitable for detecting cultivar differences in soybean adaptation to a high temperature environment. The objective of this study was to evaluate the variability of growth and yield performance in soybean genotypes grown under a tropical environment.
Material and methods
Cultivars and agronomic practice
Field experiments were conducted from July to November 2014 and March to July 2015 in the tropical environment at Banten Assessment Institute of Agricultural Technology, Serang District, Banten Province, Indonesia (lat. 6.1°S, long. 106.2°E). Twenty-two soybean cultivars (Glycine max (L.) Merr.) were collected from the mini core-collection of world soybean by the National Institute of Agro-biological Sciences (NIAS) and the Germplasm Resources Information Network (GRIN) of the USDA, USA; four cultivars, DS24-2, DS25-1, DS34-3 and DS65-4, were provided by USDA-ARS, and three cultivars, M100-47-52-13, M150-7B-41-10, and SC-1-8, were selected from the cultivar collection of Bogor Agricultural University. All cultivars used in this experiment were of determinate type, except that DS25-1 is semi-determinate. The names of the cultivars are listed in Table , and they were selected by origin (Japan, USA, Indonesia and other countries in the tropics), maturing traits (early to mid-maturing for tropical ones and mid to late maturing for temperate ones), and representativeness of commercial cultivars. The cultivar groupings were made based on origins, making 5 groups: Japanese, US, Indonesian-old, Indonesian-modern and other tropical cultivars. The groupings were also based on region: temperate and tropical cultivars.
Table 1. Name, origin and classification of the regions of 29 soybean cultivars.
On 19 July 2014, seeds were sown and grown under field conditions with three replications using 2.4 × 2 m plots. After seedling emergence, plants were thinned to one plant per hole. Plants were arranged with 50 × 20 cm spacing. In 2015, seeds were sown on 17 March using a similar size plot with 40 × 20 cm spacing. The soil was fertilized with 5 g m−2 of N, 1.18 g m−2 of elemental P and 6.22 g m−2 of elemental K with urea, calcium superphosphate and potassium chloride, respectively. Regional recommended management programs were employed for irrigation and pest control to optimize growth conditions. Saturated soil culture were applied for irrigation following Ghulamahdi et al. (Citation2016). In short, continuous irrigation water was given through the furrows between plots, and water depth was maintained constantly to make soil layer saturated.
Phenology, agronomical traits and meteorological data
Growth stages were recorded according to Fehr et al. (Citation1971). At R8, seed yield and yield components, node number, pod number, seed number and seed size, aboveground total dry weight (TDW) and harvest index (HI) were determined by harvesting six plants per replicate. Harvested seeds were divided into three classes: full seed, shriveled seed and damaged seed. Seeds in each class were counted and weighed, and moisture content was determined by a seed moisture meter. Daily air temperatures were measured by HMP45C (Campbell Scientific, INC., Logan, UT), and incident radiation (IR) was measured by a CMP3 pyranometer (Kipp & Zonen, B.V., The Netherlands) and recorded by a CR1000 datalogger (Campbell Scientific, INC., Logan, UT). Cumulative IR from emergence to R7 (Cum IRR7) was calculated.
Maturity-corrected index
One difficulty of comparison between tropical and temperate cultivars is their different growth durations due to different sensitivities to day-length. To cope with this problem, the calculation of a maturity-corrected index was adopted from Voldeng et al. (Citation1997). The index was calculated as (actual value) (expected value)−1. Expected value for each cultivars is derived from linear regression of actual value of performances on days to maturity. Considering the resource supply of solar radiation in this study, we corrected the crop performances of yield, TDW and node using cumulative incident radiation from emergence to maturity (Cum IRR7) rather than growth duration.
Data analysis
The variability among cultivar and cultivar groups was evaluated using an analysis of variance (ANOVA) and were followed by Tukey’s test using MINITAB 16.
Results
Plant phenology
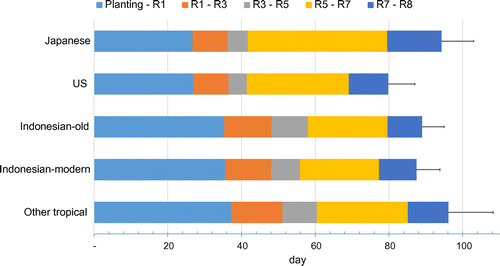
The days from seeding to R7 ranged from 64 to 95 days, and significant differences between groups were observed in the progress of the developmental stages. Japanese and US cultivar groups were the earliest in reaching R1 (26.7 and 26.9 days, respectively) compared to Indonesian-old and Indonesian-modern cultivar groups (35.3 and 35.5 days, respectively) and had shorter periods of duration from R1 to R5 with 15.0 and 14.6 days compared to the others with 20 days to 23 days, respectively. However, the seed-filling period (days from R5 to R7) for Japanese cultivars was longer compared to the other groups (Figure ). For total growth duration from emergence to R7, US cultivars were the shortest (69.1 days) and were different from the other groups, ranging from 77.3 to 85.1 days (p < .01).
Figure 1. Growth duration of temperate and tropical cultivars grown in the tropics. Data are mean of growth duration of 5 – 9 cultivars within groups. Bars represent standard deviation of total growth duration of 5 – 9 cultivars within groups. Growth stages were recorded according to Fehr et al. (Citation1971). Stage indicated R1, beginning of flowering; R3, beginning of pod; R5, beginning seed-filling; R7, beginning of maturity; and R8, full maturity.
Yield and yield component
Figure shows that the yield performance of cultivars from the tropical regions performed better than those from the temperate regions. Tanbaguro and UA4805, both from temperate group were significantly lower yield (p < .05) than Tanggamus, an Indonesian-modern group. Additionally, Tanbaguro was also lower (p < .05) than four cultivars, two from Indonesian modern group and two from other tropical group (Figure ). Given the average of each group (Table ), the yield of Japanese and US cultivars was 146 and 167 g m−2, respectively, which was smaller than Indonesian-old (253 g m−2), Indonesian-modern (253 g m−2) and other tropical (242 g m−2) cultivars. The TDW for all tested cultivar groups ranged from 206 to 454 g m−2. Japanese and US cultivars were smaller (p < .01) in TDW (279 and 206 g m−2, respectively) compared to the values of Indonesian-old (374 g m−2), Indonesian-modern (411 g m−2) and other tropical cultivars (454 g m−2). The harvest index (HI) of the US and Indonesian-old cultivars (.47 and .44, respectively) was higher (p < .01) than that of Japanese and other tropical cultivars (.36 and .37, respectively). However, there was no difference in HI between cultivars from temperate to tropical regions.
Figure 2. Seed yield performance of temperate and tropical cultivars. Data are mean ± SD of 5 – 9 cultivars within groups. Different letters between cultivars indicate significant difference at the .05 probability level.
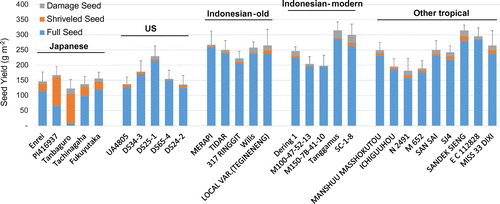
Table 2. Yield and yield component of temperate and tropical cultivars grown in the tropics.
The Japanese and US cultivars performed poorly in pod number (333 and 601 m−2, respectively) and node number (221 and 258 m−2, respectively) compared to the tropical cultivar groups (Table ). Pod number and node number of the tropical cultivar groups ranged from 1184 to 1459 and 534 m−2 to 644 m−2, respectively. On average, the pods per node of cultivars from temperate regions (1.92) were smaller than those from tropical regions (2.30). The seed sizes of Japanese cultivars (27.4 g per 100 seed) were nearly three times bigger than Indonesian cultivars (10.6 g per 100 seed) and two times bigger than US cultivars (13.6 g per 100 seed).
Variation in seed quality was observed. Japanese cultivars produced a higher number of shriveled seeds compared to US and tropical cultivars (Figure ). Unlike Japanese cultivars, the poor yield of US cultivars was not associated with low seed quality. There was a positive correlation between the ratio of shriveled seed and seed size (r = .83, data not shown).
Maturity-corrected index
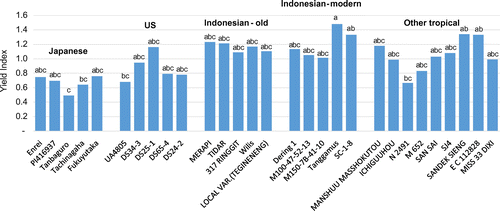
The maturity-corrected index calculated based on Cum IRR7 were observed (Table ). On average, Japanese cultivars were the smallest in yield index (.67), followed by US cultivars (.87), whereas tropical cultivars had greater yields as shown by the higher index means that ranged from 1.05 to 1.20 (Table ). Yield index for all tested cultivars ranged from .49 to 1.48 (Figure ). Tanbaguro, a Japanese cultivar, was significantly lower (p < .05) in yield index than Tanggamus and SC-1-8 (Indonesian modern group and Sandek Sieng and EC 112,828 (other tropical cultivar group). Although Japanese and US cultivars were both from temperate regions, the indices tended to be lower in the Japanese group compared to the US group according to a two-sample t-test between Japanese and US cultivars, with p = .08, and p = .005 for yield and node number, respectively, but not for TDW (p = .349) (Table ). A similar tendency was also demonstrated by the TDW index and node number index, where Japanese cultivars were markedly the lowest in the index of TDW and node number with .78 and .49, respectively, followed by the US with .89 and .83, respectively. Tropical cultivars tended to be greater in the indices of TDW and node number, which ranged from 1.02 to 1.20 and 1.15 to 1.19, respectively.
Table 3. Corrected indices of yield, TDW and node number calculated from cumulative incident solar radiation to maturity and days to maturity.
Discussion
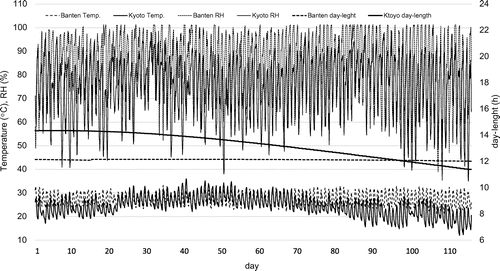
The effects of temperature and day-length and its interaction on soybean yield have been studied using controlled chamber experiments, historical data analyses and crop models (Kantolic & Slafer, Citation2001; Kumagai & Sameshima, Citation2014; Setiyono et al., Citation2007, 2010; Tacarindua et al., Citation2012, 2013; Wu et al., Citation2015). However, information on the genetic variability of responsiveness to temperature is limited, and it is not known whether cultivars adapted to temperate and tropical regions differ in adaptation to warm climates. For instance, Sanit et al. (personal communication) introduced Williams cv., an US cv., to Thailand’s breeding program early in the 1970s, but descriptions of the genetic differences in performance are not available. Field experiments regarding growth and yield responses of divergent soybean genotypes under warm climates may help to explore better adaptations to climate warming. This cannot be accomplished in mid to high latitude regions because temperatures are only high in the mid-summer and are typically proceeded and followed by moderate to low temperature seasons. This study was conducted in an environment where season-to-season variation in mean daily air temperature was at minimum an average of 27.4 °C (Figure ). For this, both early and late cultivars experienced similar temperatures through the whole growing season, while at Kyoto, late cultivars experienced lower temperatures than early cultivars due to their later growing season and declining temperatures (Figure ). An additional advantage of this study is that the photoperiod at Banten is less variable at approximately 12 h of day and night time, which is entirely short compared to the soybean cropping season in the temperate regions (Figure ).
Figure 4. Daily average air temperature, relative humidity (RH) and day-length for 2014 and 2015 at Banten (2-yr experiment) and Kyoto (common planting from June to October).
Although the above conditions made the climate range relatively narrow, considerable variations between groups in phenological stage and growth duration were observed. In temperate cultivars, the days to R1 were earlier, and the durations from R1 to R5 were shorter compared to tropical cultivars (Figure ). Short day-length, as well as warm temperatures, can hasten progress towards flowering (Gaynor et al., Citation2011; Han et al., Citation2006; Kantolic & Slafer, Citation2005, 2007; Rahman et al., in Wu et al., Citation2015; Setiyono et al., Citation2007, 2010) and increase the rate of plant development (Craufurd & Wheeler, Citation2009). The major factor influencing different growth durations could be the different responsiveness of plant development to day-length and/or temperature. In contrast, it was observed that the seed-filling period from R5 to R7 were specifically longer in Japanese cultivars.
In our study, the mean daily air temperature (27.4 °C) was near the maximum of the favorable temperature range of 16–28 °C for the whole growing season (McBlain et al., Citation1987), which was higher than the ranges of 15–22 °C, 20–22 °C, and 15–22 °C as the optimum temperatures for the emergence, flowering and maturity stages, respectively (Liu et al., Citation2008), and slightly higher than 27 °C, which is an optimum temperature for the seed-filling period (Thomas et al., Citation2010). In the present study, high temperatures occurred starting from emergence and continued to maturity and might advance the flowering stage and shorten the period from R1 to R5 for temperate cultivars, and in the Japanese cultivar group, high temperatures might prolong the seed-filling period. Similarly, Tacarindua et al. (Citation2013); Thomas et al. (Citation2010) reported a delay in the commencement of seed-filling under high temperature. One possible reason for a longer duration from R5 to R7 for the Japanese cultivar group could be a lower seed growth rate (Tacarindua et al., Citation2012, 2013; Thomas et al., Citation2010). This might be related to the larger seed size of Japanese cultivars, but this remains to be studied further.
Temperate cultivars show poor performances in yield compared to tropical cultivars (Figure , Table ). The low yield of temperate cultivars was attributed to pod number, node number, seed size and seed number and was associated with low TDW (Table ), thus indicating their poor adaptation to the tropics. We presumed that the early flowering and short duration from R1 to R5 was not beneficial for temperate cultivars. When grown under their environments of origin, for instance, Enrei cv., a Japanese cv., reached R1 in 30 days, took 27 days from R1 to R5 (Tacarindua et al., Citation2012), produced 28.1 nodes plant−1, 827 pods m−2, and 1626 seeds m−2 (Tacarindua et al., Citation2013). In contrast, our study showed that Enrei cv. was 5 days earlier in R1 and 14 days shorter in the duration from R1 to R5 compared to Tacarindua et al. (Citation2012) and produced 16.4 nodes plant−1, 312 pods m−2, and 563 seeds m−2, which was much lower compared to Tacarindua et al. (Citation2013). The advance of the flowering stage and the shortened growth duration of the temperate cultivars resulted in the decrease of seed yield (Tao et al., Citation2006, Citation2010; Zhang et al., Citation2016).
The difference between the temperate and tropical cultivars in growth duration caused incomparable results in yield performances. This is because different resource supplies, particularly solar radiation, can affect growth duration and differences in the amount plant organs developed (Kantolic & Slafer, Citation2001, Citation2005; Kantolic et al., Citation2013; Nico et al., Citation2015, Citation2016). The two aspects need to be separately considered if the resource supply seasonally changes. Consequently, we modified the calculation of index from Voldeng et al. (Citation1997) and corrected the crop performances of yield, TDW and node number using the value of Cum IRR7. The indices of the temperate cultivars in yield, TDW and node number were small compared to the value of the tropical cultivars even after correction. To confirm the indices based on Cum IRR7, an additional analysis based on growth duration from emergence to R7 were calculated, and both analyses were compared and had similar tendencies to our R2 calculations for yield, TDW and node number at 1.0, .99 and .99, respectively (data not shown).
Inferior yield of temperate cultivars after correction suggest that there is a genetic variation in the adaptation to warm environments independent of growth duration, and tropical cultivars are better adapted than temperate cultivars. To the best of our knowledge, genetic variability in yield response to high temperatures in soybean crop has not been reported, although cultivar differences in the response of seed development to high temperature have been recognized (Chebrolu et al., Citation2016). Since the average of HI index did not differ much between temperate and tropical cultivars group (Table ), the process of biomass production may be involved in the yield differences. The study on the comparison between temperate and tropical soybean cultivars under a tropical environment regarding biomass production and energy utilization through physiological activities becomes the subject for another report (Saryoko et al., Citation2016).
Although the average of HI of the cultivar from temperate regions were comparable to that from tropical region (Table ), Japanese cultivars had the lowest HI, indicating poor adaptation to the tropics. This result demonstrated that Japanese cultivars failed to set seed compared to other cultivars. In temperate cultivars, pod/node of US cultivars was on a par with tropical cultivar groups but not for Japanese cultivars (Table ). Other evidence of variation in temperate cultivars was in seed quality, where US cultivars produced less shriveled seed than Japanese cultivars. The performance of Indonesian cultivars could potentially be improved through enhancing pod number and HI for better yield.
Variation in seed quality occurred. The Japanese cultivars showed poor quality as shown by the high number of shriveled seed, but only small proportion of shrivel-ness was found in US cultivars (Figure ). In this study, the seed size of Japanese cultivars was nearly three times bigger compared to tropical cultivars and two times bigger compared to US cultivars, which exhibited a moderate occurrence of shriveled seeds (Table , Figure ). This fact also might indicate that seed quality of Japanese soybean under the tropics may result from larger seed size. If this is the case, in tropical environment production, large-seeded cultivars may be disadvantageous to that of small-seeded ones, and it is a challenge to develop a large seeded genotype that can produce high-quality seeds. In addition, the seed size of Japanese cultivars under the tropics was smaller compared to the sizes of the original description. For instance, the seed weight of Enrei cv. and Fukyutaka cv. in this study was 24.7 and 19.3 g per 100 seed (data not shown) compared to 28.9 g and 28.9 g for Enrei cv. and Fukyutaka cv., respectively (NARO, https://www.gene.affrc.go.jp/). In contrast, seed weight of Merapi cv., Wilis cv. and Tangamus cv., some well-known cultivars from Indonesia, were 8.0, 9.9 and 9.4 g per 100 seed in this study (data not shown), respectively, and were nearly similar to that described in a variety description at 8.0, 10.0 and 11.0 g, respectively (Suhartina, Citation2005). These facts were in line with Gibson and Mullen (Citation1996); Mochizuki et al. (Citation2005); Tacarindua et al. (Citation2012, 2013); Thomas et al. (Citation2010); Zhang et al. (Citation2016), who reported that soybean grown under supra-optimal temperatures result in smaller final seed size. Smaller seed size was also attributed to lower individual seed growth rate, decreased cell size (Thomas et al., Citation2010) and/or a decrease in cell number per cotyledon (Tacarindua et al., Citation2012), and increased seed shriveling (Tacarindua et al., Citation2012, Citation2013; Thomas et al., Citation2010).
In summary, the performance in seed production of soybean cultivars from temperate regions was poor under a tropical environment compared to cultivars from the tropical region. The responses in phenology and a short growth duration before the beginning of seed-filling was the primary factors of difference. The Japanese cultivars showed poor seed quality in terms of the occurrence of shriveled seed and reduced seed size. The performance of temperate cultivars in yield, TDW and node number was poor, even after a correction of data based on cumulative incident radiation that considered a shorter growth duration, suggesting that there is a genetic variation for adaptation to a tropical environment independent of growth duration. There was also variation in HI, pod setting and seed quality within temperate cultivars.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan [grant number 25257410].
Acknowledgements
We thank Dr. Ir. Muchammad Yusron, M. Phil. of AIAT Banten, IAARD, Ministry of Agriculture, for his generous support with conducting the experiments.
References
- Chebrolu, K. K., Fritschi, F. B., Ye, S., Krishnan, H. B., Smith, J. R., & Gillman, J. D. (2016). Impact of heat stress during seed development on soybean seed metabolome. Metabolomics, 12, 266. doi:10.1007/s11306-015-0941-1
- Craufurd, P. Q., & Wheeler, T. R. (2009). Climate change and the flowering time of annual crops. Journal of Experimental Botany, 60, 2529–2539. doi:10.1093/jxb/erp196
- Fehr, W. R., Caviness, C. E., Burmood, D. T., & Pennington, JS. (1971). Stage of development descriptions for soybeans. Glycine max (L.) merrill Crop Science, 11, 929–931. doi:10.2135/cropsci1971.0011183X001100060051x
- Gaynor, L. G., Lawn, R. J., & James, A. T. (2011). Agronomic studies on irrigated soybean in southern New South Wales. I. Phenological adaptation of genotypes to sowing date. Crop and Pasture Science, 62, 1056–1066. doi:10.1071/CP11136
- Gibson, L. R., & Mullen, R. E. (1996). Influence of day and night temperature on soybean seed yield. Crop Science, 36, 98–104. doi:10.2135/cropsci1996.0011183X003600010018x
- Ghulamahdi, M., Chaerunisa, S. R., Lubis, I., & Taylor, P. (2016). Response of five soybean varieties under saturated soil culture and temporary flooding on tidal swamp. Procedia Environmental Sciences, 33, 87–93. doi:10.1016/j.proenv.2016.03.060
- Han, T., Wu, C., Tong, Z., Mentreddy, R. S., Tan, K., & Gai, J. (2006). Postflowering photoperiod regulates vegetative growth and reproductive development of soybean. Environmental and Experimental Botany, 55, 120–129. doi:10.1016/j.envexpbot.2004.10.006
- Kantolic, A. G., Peralta, G. E., & Slafer, G. A. (2013). Seed number responses to extended photoperiod and shading during reproductive stages in indeterminate soybean. European Journal of Agronomy, 51, 91–100. doi:10.1016/j.eja.2013.07.006
- Kantolic, A. G., & Slafer, G. A. (2001). Photoperiod sensitivity after flowering and seed number determination in indeterminate soybean cultivars. Field Crops Research, 72, 109–118. doi:10.1016/S0378-4290(01)00168-X
- Kantolic, A. G., & Slafer, G. A. (2005). Reproductive development and yield components in indeterminate soybean as affected by post-flowering photoperiod. Field Crops Research, 93, 212–222. doi:10.1016/j.fcr.2004.10.001
- Kantolic, A. G., & Slafer, G. A. (2007). Development and seed number in indeterminate soybean as affected by timing and duration of exposure to long photoperiods after flowering. Annals of Botany, 99, 925–933. doi:10.1093/aob/mcm033
- Kumagai, E., & Sameshima, R. (2014). Genotypic differences in soybean yield responses to increasing temperature in a cool climate are related to maturity group. Agricultural and Forest Meteorology, 198–199, 265–272. doi:10.1016/j.agrformet.2014.08.016
- Liu, Y., Wang, E. L., Yang, X. G., & Wang, J. (2010). Contributions of climatic and crop varietal changes to crop production in the North China Plain, since 1980s. Global Change Biology, 16, 2287–2299. doi:10.1111/j.1365-2486.2009.02077.x
- Liu, XB., Jin, J., Wang, GH., & Herbert, SJ. (2008). Soybean yield physiology and development of high-yielding practices in Northeast China. Field Crops Research, 105, 157–171. doi:10.1016/j.fcr.2007.09.003
- Lobell, D. B., & Asner, G. P. (2003). Climate and management contributions to recent trends in U.S. agricultural yields. Science, 299, 1032. doi:10.1126/science.1078475
- McBlain, B. A., Hesketh, J. D., & Bernard, R. L. (1987). Genetic effects on reproductive phenology in soybean isolines differing in maturity genes. Canadian Journal of Plant Science, 67, 105–115. doi:10.4141/cjps87-012
- Mochizuki, A., Shiraiwa, T., Nakagawa, H., & Horie, T. (2005). The effect of temperature during the reproductive period on development of reproductive organs and the occurrence of delayed stem senescence in soybean. Japanese Journal of Crop Science, 74, 339–343. doi:10.1626/jcs.74.339
- Monteith, JL. (1977). Climate. In P. T. Alvin, & T. T. Kozlowski (Eds.), Ecophysiology of Tropical Crops (pp. 1–25). New York, NY: Academic Press.
- NARO. (2016). Database plant search – evaluation data queries. [Data set]. Retrieved August 1, 2016, from https://www.gene.affrc.go.jp/
- Nico, M., Miralles, D. J., & Kantolic, A. G. (2015). Post-flowering photoperiod and radiation interaction in soybean yield determination: Direct and indirect photoperiodic effects. Field Crop Research, 176, 45–55. doi:10.1016/j.fcr.2015.02.018
- Nico, M., Mantese, A. I., Miralles, D. J., & Kantolic, A. G. (2016). Soybean fruit development and set at the node level under combined photoperiod and radiation conditions. Journal of Experimental Botany, 67, 365–377. doi:10.1093/jxb/erv475
- Prasad, P. V. V., Boote, K. J., & Allen, L. H., Jr. (2006). Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain-sorghum [Sorghum bicolor (L.) Moench] are more severe at elevated carbon dioxide due to higher tissue temperatures. Agriculture and Forest Meteorology, 139, 237–251. doi:10.1016/j.agrformet.2006.07.003
- Prasad, P. V. V., Thomas, J. M. G., & Narayanan, S. (2017). Global warming effect. In B. Thomas, B. G. Murray, & D. J. Murphy (Eds.), Encyclopedia of Applied Plant Science (2nd ed.). New York, NY: Academic Press. pp. 289–299. 10.1016/B978-0-12-394807-6.00013-7
- Saryoko, A., Homma, K., Lubis, I., & Shiraiwa T. (2016, March 28–29). Performance of temperate and tropical soybean cultivars under tropical environment. 2. Physiological activity and biomass production in crop canopy [Abstract]. The 241st Meeting of the Crop Science Society of Japan, Mito, Japan.
- Setiyono, T. D., Weiss, A., Specht, J., Bastidas, A. M., Cassman, K. G., & Dobermann, A. (2007). Understanding and modeling the effect of temperature and day length on soybean phenology under high-yield conditions. Field Crop Research, 100, 257–271. doi:10.1016/j.fcr.2006.07.011
- Setiyono, T. D., Cassman, K. G., Specht, J. E., Dobermann, A., Weiss, A., Yang, H., & … De Bruin, J. L., (2010). Simulation of soybean growth and yield in near-optimal growth conditions. Field Crops Research, 119, 161–174. doi:10.1016/j.fcr.2010.07.007
- Suhartina. (2005). Deskripsi varietas unggul kacang-kacangan dan umbi-umbian [ Variety description for legumes and tubers crop]. Malang: Indonesian Legumes and Tuber Crops Research Institute **.
- Tacarindua, C. R. P., Shiraiwa, T., Homma, K., Kumagai, E., & Sameshima, R. (2012). The response of soybean seed growth characteristics to increased temperature under near-field conditions in a temperature gradient chamber. Field Crops Research, 131, 26–31. doi:10.1016/j.fcr.2012.02.006
- Tacarindua, C. R. P., Shiraiwa, T., Homma, K., Kumagai, E., & Sameshima, R. (2013). The effects of increased temperature on crop growth and yield of soybean grown in a temperature gradient chamber. Field Crops Research, 154, 74–81. doi:10.1016/j.fcr.2013.07.021
- Tao, FL., Yokozawa, M., Xu, YL., Hayashi, Y., & Zhang, Z. (2006). Climate change and trends in phenology and yield of field crops in China, 1981–2000. Agriculture and Forest Meteorology, 138, 89–92. doi:10.1016/j.agrformet.2006.03.014
- Thomas, J. M. G., Boote, K. J., Pan, D., & Allen, L. H., Jr. (2010). Elevated temperature delays onset of the reproductive growth and reduces seed growth rate of soybean. Journal of AgroCrop Science, 1, 19–32. doi:10.1016/S0167-8809(00)00224-3
- Wu, T. T., Li, J. Y., Wu, C. X., Shi, S. U. N., Mao, T. T., Jiang, B. J., & … Han, T. F. (2015). Analysis of the independent- and interactive-photo-thermal effects on soybean flowering. Journal of Integrative Agriculture, 14, 622–632. doi:10.1016/S2095-3119(14)60856-X
- Voldeng, H. D., Cober, E. R., Hume, D. J., Gillard, C., & Morrison, M. J. (1997). Fifty-eight years of genetic improvement of short-season soybean cultivars in Canada. Crop Science, 37, 428–431. doi:10.2135/cropsci1997.0011183X003700020020x
- Zhang, L., Zhu, L., Yu, M., & Zhong, M. (2016). Warming decreases photosynthates and yield of soybean [Glycine max (L.) Merrill] in the North China Plain. The Crop Journal, 4, 139–146. doi:10.1016/j.cj.2015.12.003