Abstract
Genotype by environment (G×E) interactions for grain yield were investigated in 14 rice genotypes across eight rainfed lowland field environments in Lao PDR, in order to identify stable adapted cultivars for improved farmer livelihood and food security. G×E accounted for 20.3% of the total variance, with three vectors from ordination analysis accounting for 75.1% of the G×E-SS, in 6 genotype × 6 environment groups. PCA1 indicated water-limited yield potential, PCA2 pre-flowering stress and PCA3 post-flowering stress. Genotype groups (G1–G6) differed in adaptation to these environments. G5 (VT450-2 and TSN9) were widely adapted and high-yielding. G6 (TDK11 and TDK37) were also high-yielding, topping the rankings in three environment groups, but yielded less in Phalanxay 2012 and Phalanxay 2011, where their phenology was unstable under stress. Other genotype groups showed specific adaptations, but failed to exceed yields of G5 and G6. Hence, VT450-2 and TSN9 (G5) were the preferred genotypes for rainfed lowland in southern Lao PDR, due to their high and stable grain yields. Stability in flowering time and high yield in rainfall deficit were desirable traits for improved farmer livelihood and food security.
Classification:
1. Introduction
With the challenge to feed a projected 9 billion people in coming decades, world food production must increase by around 100% by 2050 to meet this demand (Tilman et al., Citation2011). Rice is important, as it is the second largest crop grown worldwide, and affects a significant proportion of the world’s smallholders and urban consumers (Muthayya et al., Citation2014). This group includes two thirds of the world’s poorest people, who are reliant on rice as their staple food (Timmer, Citation2014). Often, smallholder rice farmers must generate their food requirements under rainfed conditions with fragile soils and variable seasons, which may become more unpredictable with climate change (Wheeler & von Braun, Citation2013). Almost half of global rice production is generated in rainfed lowlands, where rice is grown in bunded fields with limited or no access to irrigation (McLean et al., Citation2002). This is especially the case in the Lao PDR, a small nation in South-East Asia categorized as low-income food-deficit, where a quarter of the population live in poverty in rural and remote areas (World Food Program, Citation2013). With the majority of the Lao population reliant on agriculture, food security is strongly dependent on the success of the rainfed lowland rice crop in the southern provinces of Savannakhet and Champassak, where 25% of the rice crop is produced (Eliste et al., Citation2012).
Under rainfed lowland conditions, the intent is to grow the crop in standing water, although variable climatic conditions make this difficult to control. In the Lao PDR, soils are sandy in texture, have low water-holding capacity and are low in pH and available nutrients (Linquist & Sengxua, Citation2001), making water management inherently difficult. This manifests in several different challenges: the absence of ponded water early in the season increases weed pressure, intermittent water deficit affects spikelet fertility and terminal water deficit reduces grain filling. When ponded water vanishes, soil conditions change and plants quickly encounter water deficit. With light-textured soils and variable monsoonal rainfall (Inthavong et al., Citation2011), these problems are especially important in southern Lao PDR, so cultivars are normally required with an ability to handle some rainfall deficit (Monkham et al., Citationin press; Wade, Fukai et al., Citation1999). Given that most households practice subsistence rice production as their central agricultural endeavour (Manivong et al., Citation2014), stable adapted cultivars are critical to farmer livelihood and food security.
Consequently, this paper examines the adaptation of 14 rice genotypes released in Lao PDR under rainfed lowland field conditions. Field experiments were conducted on-farm in Savannakhet and Champassak provinces in southern Lao PDR in 2011 and 2012. The objectives were to (1) assess the adaptation and field performance of 14 lowland rice genotypes in 8 province–district–year combinations, (2) consider traits needed for successful adaptation to this target population of environments and (3) identify stable adapted cultivars for improved farmer livelihood and food security.
2. Materials and methods
All 14 genotypes were evaluated across 24 experiments in Lao PDR, comprising 2 years, 2 provinces, 2 districts per province and 3 farms per district. The 3 farms per district were used as replicates, so the 14 genotypes were evaluated in 8 province–district–year combinations (environments E). In 2011, the experiments were conducted in Phalanxay and Phin districts of Savannakhet Province, and Phonthong and Moulapamouk districts of Champassak Province. In 2012, the experiments were repeated, but with Soukhouma replacing Phonthong in Champassak. Within each farm, each of the 14 genotypes was sown with a plot size of 5.0 m × 10.0 m, with .20 m row spacing and .20 m between hills. Plots for each experiment were randomized in the farmer’s field, and each plot was established by transplanting from adjacent seedbeds and harvested by hand, as indicated in Table , which was consistent with standard farmer practice. Flowering time, harvest time and plant height were recorded, with grain yield obtained from 2-m sections of 10 central rows (4 m−2). For simplicity, the eight environments were referred to by their environment code, e.g. Phalanxay 2011 is Pha1 (Table ). The 14 genotypes were all photoperiod non-sensitive, improved glutinous indica varieties released in Lao PDR from crosses originally made in Laos, Thailand, Vietnam or IRRI (Inthapanya et al., Citation2006), and reputed to offer a range of adaptations to wet or dry seasons, upper or lower terraces, diseases and pests, and in eating quality, and were popular in one or more districts (Table ). There was no single check, as recommendations varied between districts, and the intent was to explore adaptation. Genotypes were identified by their genotype code, e.g. Tha Dok Kham 37 is TDK37 (Table ).
Table 1. The eight environments used to discriminate lowland rice genotypes.
Table 2. Genotypes evaluated in lowland rice experiments in 8 environments in Lao PDR in 2011 and 2012. Adaptation, flowering date, time to flowering (d), grain fill (d), crop duration (d), plant height (cm) and grain yield (t ha−1) are shown (For grain yield, l.s.d. = .22; p = .05).
The soil at Phalanxay had a pH of 4.8, organic C .56 g kg−1, total N .08%, available P 4.10 mg kg−1 and exchangeable K 10.97 cmol kg−1. In Phin, pH was 4.5, with organic C .91 g kg−1, total N .07%, available P 2.46 mg kg−1 and exchangeable K 10.97 cmol kg−1. At Moulapamouk, total N was .07%, but the soil pH was 5.5, organic C 1.15 g kg−1, available P 1.14 mg kg−1 and exchangeable K 13.0 cmol kg−1. In Phonthong the soil had a pH of 4.9, organic C .32 g kg−1, total N .05%, available P 1.58 mg kg−1 and exchangeable K 5.91 cmol kg−1. In Soukhouma, pH was 5.1, with organic C .97 g kg−1, total N .05%, available P 1.37 mg kg−1 and exchangeable K 6.92 cmol kg−1. Each site received 30, 30 and 30 kg ha−1 of N, P and K, respectively, at transplanting.
Long-term weather data showed temperatures ranged from 15 to 35 °C, with the lowest minimums in December–January after the wet season, and the highest maximums in March–May towards the end of the dry season (Table ). Temperatures followed similar patterns at the sites, but Champassak in the south was warmer, with higher evaporative demand. Mean annual rainfall was higher in Champassak (2,044 mm) than Savannakhet (1,452 mm), but in all cases, there was a pronounced dry season from November to March, with an average of only 70 mm of rain being received during those 5 months. In 2011, the rains were later than average, while in the 2012 wet season, all sites had a dry finish from October onwards (Table ). Soukhouma 2012 encountered generally favourable conditions, so was used as the reference environment in the Results and Discussion. Phalanxay 2011 encountered pre-flowering stress, including gall midge. Phin 2012 encountered stress during heading and grain filling, so stress levels intensified post-flowering in Phin 2012.
Table 3. Long-term mean monthly maximum and minimum temperature (○C) and pan evaporation (mm), and monthly rainfall (mm) in 2011 and 2012 relative to the long-term mean monthly rainfall (mm), for Savannakhet and Champassak in Lao PDR.
Pattern analysis was used to examine the grain yield of 14 genotypes under 8 environments (province–district–year combinations), with the 3 farms per district used as replicates for each environment. Yield data were extracted from appropriate single-environment RCBD analyses. The effects of environment, genotype and the G×E interaction were considered fixed, with replicate random and nested within environments. G×E interactions were analysed using the pattern analysis tool in CropStat (DeLacy et al., Citation1996). This method involved the joint application of cluster analysis and ordination to a transformed G×E matrix. Since the objective was to understand genotype adaptation for breeding and evaluation, the G×E matrix was transformed by environment standardization (Cooper, Citation1999). The transformed data were clustered using an agglomerative hierarchical algorithm based on minimizing incremental sum of squares (Ward, Citation1963). Scores for both genotypes and environments from the two-component interaction principal components model were computed for PCA1, PCA2 and PCA3, and plotted as biplots, with environment points at the end of vectors labelled as in Table , and genotype points as symbols labelled as in Table .
Using data for Soukhouma 2012 as a favourable reference environment, 3 change parameters were calculated for each genotype group in each environment group, based on data for time to flowering (days), duration of grain filling (days) and plant height (cm). In each case, the value was subtracted from the corresponding value for Soukhouma 2012. A positive value for change in flowering time indicated a delay in flowering under stress. Likewise, a negative value for change in grain fill duration implied a truncation in grain fill duration under stress, and a negative value for change in plant height implied a reduction in plant height under stress. These change parameters were used to assist interpretation of the patterns of grain yield of genotype groups across environment groups, with means compared using l.s.d. with appropriate degrees of freedom for main effects and interactions (Steel & Torrie, Citation1960).
3. Results and discussion
Site mean yield ranged from 2.95 to 4.23 t ha−1 (Table ), while genotype mean yield ranged from 2.97 to 3.93 t ha−1 (Table ), with an overall mean yield of 3.36 t ha−1. The G×E interaction accounted for 20.3% of the total sum of squares for grain yield, which, together with genotype, accounted for 34.5% of the total variation (Table ). Three vectors accounted for 75.1% of G×E, suggesting a high repeatable component, which was consistent with other studies in rice (Botwright Acuna et al., Citation2008; Wade, McLaren et al., Citation1999). Thus, cluster and ordination analysis reduced the matrix from 14 genotypes × 8 environments (112) to 6 genotype groups × 6 environment groups (36), whilst retaining the repeatable variation.
Table 4. Across site ANOVA for G×E interaction studies with 14 genotypes and 8 environments.
Timing of rainfall shortfall relative to flowering has been used to examine and explain genotype response (Jearakongman et al., Citation1995), with change in flowering time (advance or delay) under stress then used as a further refinement in interpreting plant response in the field (Pantuwan et al., Citation2002). Likewise, in the absence of stress, grain fill duration in rice is often observed to be about 28 days (Kropff et al., Citation1994), but grain fill is observed to be truncated under late water deficit. Final plant height is attained by flowering, so water deficit pre-flowering can reduce plant height. Again, any change in plant height relative to a favourable reference environment would be expected to be a sensitive parameter of plant response to stress. A dry start or early onset of water deficit has been observed to shorten both time to flowering and plant height, while a later onset may delay flowering as well as cause some height reduction. Stress after flowering is expected to adversely affect grain filling by shortening the time available to fill grains. These principles are used to assist interpretation of patterns of grain yield for genotype groups across environment groups (Table ), in conjunction with weather data (Table ), environment conditions (Materials and Methods) and the change parameters delay in flowering (Table ), truncation in grain filling (Table ) and reduction in plant height (Table ). The patterns from cluster (Figure 1) and ordination (Figure 2) analysis then illustrate and further clarify these relationships, as discussed below.
Table 5. Grain yield (t ha−1) for 6 genotype groups across 6 environment groups; (l.s.d. = .26, .22, 1.58 for E, G, G×E, respectively; p = .05).
Table 6. Time to flowering (d) for 6 genotype groups across 6 environment groups; (l.s.d. = .5, .5 and 2.0 for E, G and G×E, respectively; p = .05).
Table 7. Duration of grain fill (d) for 6 genotype groups across 6 environment groups; (l.s.d. = .5, .5 and 2.0 for E, G and G×E, respectively; p = .05).
Table 8. Plant height (cm) for 6 genotype groups across 6 environment groups; (l.s.d. = 1, 1 and 5 for E, G and G×E, respectively; p = .05).
Soukhouma 2012 (E2) was the favourable standard, with the highest mean grain yield (Table ), average time to flowering (Table ), average duration of grain filling (Table ) and tallest plants (Table ). Phin 2011 and Phonthong 2011 (E3) encountered relatively favourable conditions, with time to flowering only mildly advanced (Table ), grain fill duration unaffected (Table ) and plant height only mildly reduced (Table ), relative to Soukhouma 2012 (E2). In contrast, Phalanxay 2011 (E1) encountered pre-flowering stress following late onset of rainfall in 2011 (Table ), which reduced time to flowering, plant height and grain yield. Presence of gall midge in the absence of early ponded water may have exacerbated this response.
For the remaining environment groups (E4–E6), post-flowering conditions were generally more important for grain yield (Table ), due to earlier rainfall cessation in 2012 (Table ). In Phin 2012 (E5), the moderate delay in flowering (6 d, Table ), substantial truncation in grain fill duration (7 d, Table ) and a significant reduction in plant height (19 cm, Table ) were consistent with rainfall deficit increasing from heading onwards. Conditions in Phalanxay 2012 (E4) were milder, with apparent delay in flowering there more likely a consequence of its earlier sowing (Table ). The advance in flowering at Moulapamouk 2011 and 2012 (E6) was presumably influenced by warmer temperatures in the south, although the timing data for Moulamamouk (E6) were less reliable, unfortunately, with no data available for harvest date (Table ). Nevertheless, the shorter growth cycle at Moulapamouk seems consistent with some truncation in grain fill duration, as discussed further for the biplots below.
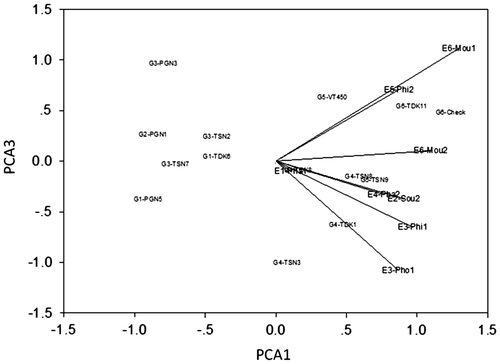
The environmental relationships are reflected in their group positions in the cluster dendograms (Figure 1) and their group locations in the biplots (Figure 2). In the environment dendogram (Figure 1), Phalanxay 2011 (E1) with pre-flowering stress separated from Soukhouma 2012 (E2), and Phin 2011 and Phonthong 2011 (E3), with relatively favourable conditions, which in turn separated from Phalanxay 2012 (E4), Phin 2012 (E5) and Moulapamouk 2011 and 2012 (E6), with various versions of post-flowering stress. In the biplots (Figure ), most environmental groups were positive for PCA1, except Phalanxay 2011 which was neutral. The pre-flowering stress environment of Phalanxay 2011 (E1) was strongly positive for PCA2, consistent with flowering being advanced for positive values of PCA2 and delayed for negative values of PCA2. The tallest plants were observed for Soukhouma 2012 (E2) and Moulapamouk (E6), and the shortest at Phalanxay 2011 (E1) and Phin 2011 (E3). For PCA3 (Figure ), environments with post-flowering stress were neutral to positive and others were negative, implying grain fill duration was truncated for positive values of PCA3. The positions of Moulapamouk 2011 and 2012 (E6) relative to Phalanxay 2012 (E4) and Phin 2012 (E5) are consistent with some truncation of grain fill at Moulapamouk, especially in 2012 (E6), as truncation of grain fill duration was positive for PCA3.
For genotypes, G5 (VT450-2, TSN9) was highest ranking in three environment groups (Table ) and mapped favourably for all environment axes (Figures and ), showing it was high-yielding and widely adapted in all 6 environment groups. G6 (TDK11, TDK37) was also positive for all axes and high yielding, topped the rankings in the other three environment groups, but mapped further from the origin, showing its yields were less stable over environments, as indicated in Phalanxay 2011 (E1) and Phalanxay 2012 (E4) (Table ). This unstable behaviour in G6 is confirmed by its wide variation in delay in flowering, ranging from −17 to +20 days (Table ). Pre-flowering stress favoured the later-flowering group G1 (PNG5, TDK6), which maintained its time to flowering (Table ) and yielded comparably to the high-yielding group G5 (VT450-2 and TSN9) in Phalanxay 2011 (E1) (Table ). Conversely, with mild post-flowering stress, early maturity was favoured, such as G2 (PNG1) in Phalanxay 2012 (E4), whose time to flowering was less delayed than other entries (Table ), so yielded comparably to the high-yielding group G5 (VT450-2 and TSN9) there (Table ). Nevertheless, early flowering was no advantage when post-flowering stress was more severe at Phin 2012 (E5).
The 14 genotypes were grouped in cluster and ordination analysis by their responses to the environmental challenges, with PCA1 to PCA3 summarizing the patterns of genotype adaptation across the environment groups, based on the projection of each genotype or genotype group on the respective environmental vectors (Botwright Acuna & Wade, Citation2013; Yan, Citation2002). In the genotype dendogram (Figure ), the lower-yielding groups (G1–G3) separated strongly from the higher yielding groups (G4–G6). This is clearly illustrated in the biplot (Figure ), in which G1–G3 are negative for PCA1, and G4–G6 are positive for PCA1. G4–G6 had generally positive intercepts with vectors for all three axes, consistent with their higher performance, with G4 stable close to the origin, and G6 unstable further away from the origin. In contrast, G1–G3 had negative intercepts with all vectors, consistent with their poorer performance. Consequently, PCA1 was interpreted to represent water-limited yield potential, based on the separation of the genotype groups. PCA2 was interpreted to represent pre-flowering stress, based on its associations with change in flowering time. PCA3 was interpreted to represent post-flowering stress, associated with truncation of grain filling.
Figure 1a. Environment groupings applied to standardized yield data for 14 lowland rice genotypes.
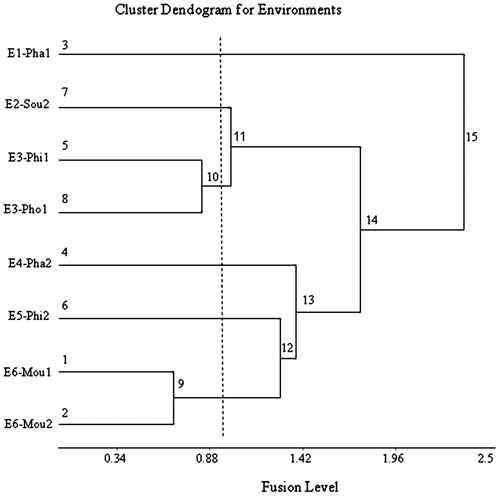
Figure 1b. Genotype groupings applied to standardized yield data for 14 lowland rice genotypes over 8 environments.
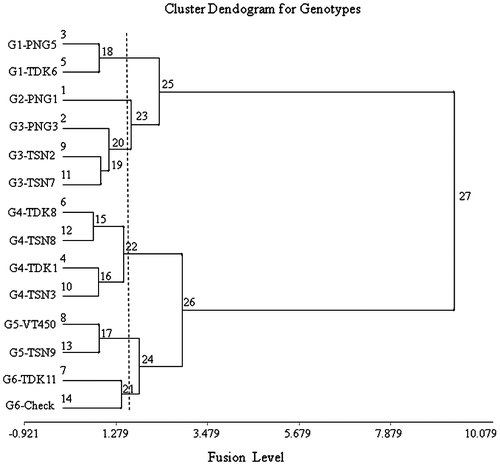
Figure 2a. Principal component analysis (location standardized) for the environment × genotype interaction for PCA1 and PCA2 for grain yield for 8 environments and 14 lowland rice genotypes.
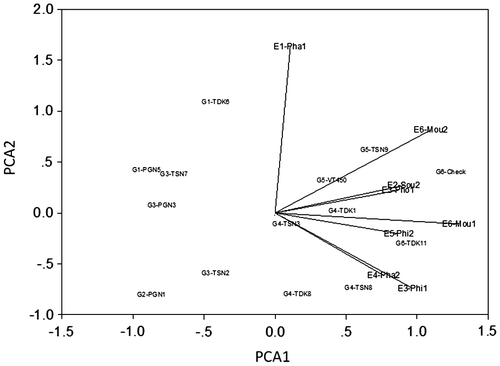
Figure 2b. Principal component analysis (location standardized) for the environment × genotype interaction for PCA1 and PCA3 for grain yield for 8 environments and 14 lowland rice genotypes.
Despite the importance of the genotype by environment interaction, the widely adapted group G5 (VT450-2 and TSN9) was able to contribute similar yields to groups with specific adaptation in their favoured environments, e.g. G1 (PNG5 and TDK6) in E1 (Phalanxay 2011), and G2 (PNG1) in E4 (Phalanxay 2012). This suggests, even for the lower yielding environments encountered here, the broadly adapted group G5 (VT450-2 and TSN9) was still a better choice. While the other high-yielding group G6 (TDK11 and TDK37) may have had a small yield advantage over G5 in more favourable environments with only mild stresses, its unstable phenology under more severe stresses would make it a risky choice unless water and nutrients could be assured. Since yields of rainfed lowland rice in Lao PDR are often less than 2.50 t ha−1 (Linquist & Sengxua, Citation2001), however, and there are seasons in which little or no yield is recorded, yield stability should be a prime consideration, which was associated here with more stable time to flowering over environments in G5 (Table ). More stable phenology, or only a short delay in flowering under stress, was also considered desirable recently for intermittent drought in northeast Thailand (Monkham et al., Citationin press). Thus, VT450-2 and TSN9 (G5) would be the preferred genotypes for rainfed lowland conditions in southern Lao PDR. These results are consistent with adaptation to rainfall deficit being desirable for yield stability, so selection for improved drought tolerance should be of benefit (Fukai et al., Citation1999; Xangsayasane et al., Citation2014), with the intent to combine yield potential and grain yield under drought (Atlin et al., Citation2006; Blum, Citation2009, 2011; Venuprasad et al., Citation2008).
In conclusion, choice of cultivars with stable phenology and high yield in water-limited environments, such as VT450-2 and TSN9, would be desirable for rainfed lowland conditions in southern Lao PDR, for improved farmer livelihood and food security. The principles discussed here have wide application in rainfed environments, especially for rainfed lowland rice in South-East Asia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Australian Centre for International Agricultural Research (ACIAR) [grant number CSE/2009/004], Developing Improved Farming and Marketing Systems in Rainfed Regions of Southern Lao PDR.
Acknowledgements
We thank the Provincial Governments of Savannakhet and Champassak, and the National Agricultural and Forestry Research Institute for providing land, facilities and labour for the successful conduct of these experiments, and farmers who participated for the use of their land and contributions to management.
References
- Atlin, G., Lafitte, H. R., Tao, D., Laza, M., Amante, M., & Courtois, B. (2006). Developing rice cultivars for high-fertility upland systems in the Asian tropics. Field Crops Research, 97, 43–52.10.1016/j.fcr.2005.08.014
- Blum, A. (2009). Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Research, 112, 119–123.10.1016/j.fcr.2009.03.009
- Blum, A. (2011). Plant breeding for water-limited environments. New York, NY: Springer.10.1007/978-1-4419-7491-4
- Botwright Acuna, T. L., Lafitte, H. R., & Wade, L. J. (2008). Genotype by environment interactions for yield in upland rice backcross lines in diverse hydrological environments. Field Crops Research, 108, 117–125.10.1016/j.fcr.2008.04.003
- Botwright Acuna, T. L., & Wade, L. J. (2013). Use of genotype x environment interactions to understand rooting depth and the ability of wheat to penetrate hard soils. Annals of Botany, 112, 359–368.10.1093/aob/mcs251
- Cooper, M. (1999). Concepts and strategies for plant adaptation research in rainfed lowland rice. Field Crops Research, 64, 13–34.10.1016/S0378-4290(99)00048-9
- DeLacy, I. H., Basford, K. E., Cooper, M., Bull, J. K., & McLaren, C. G. (1996). Analysis of multi-environment trials – An historical perspective. In M. Cooper & G. L. Hammer (Eds.), Plant adaptation and crop improvement (pp. 39–124). Wallingford: CAB International.
- Eliste, P., Santos, N., & Pravongviengkham, P. (2012). Lao PDR rice policy study. Vientiane: Food and Agriculture Organisation of the United Nations.
- Fukai, S., Pantuwan, G., Jongdee, B., & Cooper, M. (1999). Screening for drought tolerance in rainfed lowland rice. Field Crops Research, 64, 61–74.10.1016/S0378-4290(99)00051-9
- Inthapanya, P., Boualaphanh, C., Hatsadong, & Schiller, J. M. (2006). The history of lowland rice variety improvement in Laos. In J.M. Schiller, M. B. Chanphengxay, B. Linquist, & S. Appa Rao (Eds.), Rice in Laos (457 p.). Los Banos: International Rice Research Institute.
- Inthavong, T., Fukai, S., & Tsubo, M. (2011). Spatial variations in water availability, soil fertility and grain yield in rainfed lowland rice: A case study from Savannakhet Province, Lao PDR. Plant Production Science, 14, 184–195. doi:10.1626/pps.14.184
- Jearakongman, S., Rajatasereekul, S., Naklang, K., Romyen, P., Fukai, S., Skulkhu, E., … Nathabutr, K. (1995). Growth and grain yield of contrasting rice cultivars grown under different conditions of water availability. Field Crops Research, 44, 139–150.10.1016/0378-4290(95)00050-X
- Kropff, M. J., Van Laar, H. H., & Matthews, R. B. (Eds). (1994). ORYZA1: An ecophysiological model for irrigated rice production. Proceedings on simulation and systems analysis for rice production (110 p.). Manila: International Rice Research Institute.
- Linquist, B., & Sengxua, P. (2001). Nutrient management in rainfed lowland rice in Lao PDR. Los Banos: IRRI.
- McLean, J. L., Dawe, D. L., Hardy, B., & Hettel, G. P. (2002). Rice almanac (3rd ed.). Wallingford: CABI.
- Manivong, V., Cramb, R., & Newby, J. C. (2014). Rice and remittances: Crop intensification versus labour migration in southern laos. Human Ecology, 42(3), 367–379. doi:10.1007/s10745-014-9656-6
- Monkham, T., Jongdee, B., Pantuwan, G., Mitchell, J. H., Sanitchon, J., & Fukai, S. (in press). On-farm multi-location evaluation of occurrence of drought types and rice genotypes selected from controlled-water on-station experiments in northeast Thailand. Field Crops Research.
- Muthayya, S., Sugimoto, J. D., Montgomery, S., & Maberly, G. F. (2014). An overview of global rice production, supply, trade and consumption. Annals of the New York Academy of Science, 1324, 7–14. doi:10.1111/nyas.12540
- Pantuwan, G., Fukai, S., Cooper, M., Rajatasereekul, S., & O’Toole, J. C. (2002). Yield responses of rice (Oryza sativa L.) genotypes to different types of drought under rainfed lowlands. Part 2. Selection of drought resistant genotypes. Field Crops Research, 73, 169–180.10.1016/S0378-4290(01)00195-2
- Steel, R. G. D., & Torrie, J. H. (1960). Principles and procedures of statistics (481 p.). New York, NY: McGraw Hill Book Company.
- Tilman, D., Balzer, C., Hill, J., & Befort, B. L. (2011). Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Science United States of America, 108, 20260–20264. doi:10.1073/pnas.1116437108
- Timmer, C. P. (2014). Food Security in Asia and the Pacific: The Rapidly Changing Role of Rice. Asia Pacific Policy Studies, 1, 73–90. doi:10.1002/app5.6
- Venuprasad, R., Sta. Cruz, M. T., Amante, M., Magbanua, R., Kumar, A., & Atlin, G. N. (2008). Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crops Research, 107, 232–244.10.1016/j.fcr.2008.02.004
- Wade, L. J., Fukai, S., Samson, B. K., Ali, A., & Mazid, M. A. (1999). Rainfed lowland rice: Physical environment and cultivar requirements. Field Crops Research, 64, 3–12.10.1016/S0378-4290(99)00047-7
- Wade, L. J., McLaren, C. G., Quintana, L., Harnpichitvitaya, D., Rajatasereekul, S., Sarawgi, A. K., … Sarkarung, S. (1999). Genotype by environment interaction across diverse rainfed lowland rice environments. Field Crops Research, 64, 35–50.10.1016/S0378-4290(99)00049-0
- Ward, J. H. (1963). Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association, 58, 236–244.10.1080/01621459.1963.10500845
- Wheeler, T., & von Braun, J. (2013). Climate change impacts on global food security. Science, 341, 508–513. doi:10.1126/science.1239402
- World Food Program. (2013). Food and nutrition security atlas of Lao PDR. Retrieved from http://documents.wfp.org/stellent/groups/public/documents/ena/wfp260762.pdf
- Xangsayasane, P., Jongdee, B., Pantuwan, G., Fukai, S., Mitchell, J. H., Inthapanya, P., & Jothiyangkoon, D. (2014). Genotypic performance under intermittent and terminal drought screening in rainfed lowland rice. Field Crops Research, 156, 281–292.10.1016/j.fcr.2013.10.017
- Yan, W. (2002). Singular-value partitioning in biplot analysis in multi-environment trial data. Agronomy Journal, 94, 990–996.10.2134/agronj2002.0990