Abstract
Objective: The aim of this study was to evaluate serum paraoxonase-1 (PON1) activity and its association with oxidative stress in autoimmune thyroid disease (AITD).
Methods: A total of 50 patients with AITD, including 25 with Hashimoto's thyroiditis and 25 with Graves’ disease were enrolled. The control group comprised 27 healthy subjects. Blood samples were obtained in the euthyroid period and 3 months after initiation of medical treatment. Serum samples from patients with AITD and the healthy control group were analyzed for basal PON1, salt-stimulated PON1, and arylesterase (ARE) activities, along with lipid hydroperoxide (LOOH) and total free sulfhydryl (–SH) levels.
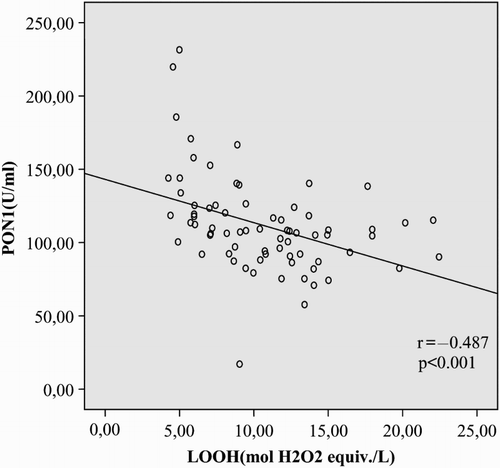
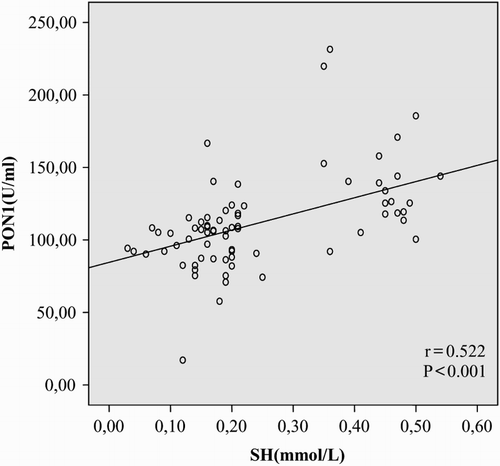
Results: Serum PON1 activities and –SH levels were significantly lower (P < 0.001, for each), whereas LOOH levels were significantly higher (P < 0.001, for each) in patients with AITD, compared to the control group. We observed no significant differences in ARE levels between the patient and healthy control groups (P > 0.05). PON1 activity was positively correlated with –SH (r = 0.522, P < 0.001) and negatively correlated with LOOH (r = −0.487, P < 0.001). PON1 phenotype distribution of the subjects was not significantly different among the three groups (P = 0.961).
Conclusions: Serum PON1 activity is decreased in patients with AITD, and correlated positively with –SH, a well-known antioxidant, and negatively with LOOH, an index of lipid oxidation.
Introduction
Oxidation of iodide to iodine is an important step for thyroid hormone synthesis. This reaction is catalyzed by thyroid peroxidase and involves hydrogen peroxide (H2O2) and oxidized iodine. Therefore, a certain oxidative load level in the thyroid gland is required for thyroid hormone synthesis. Strong antioxidant systems exist to protect thyroid cells against increased levels of oxidants, such as glutathione peroxidase, peroxiredoxin, and catalase.Citation1,Citation2
Oxidative stress occurs when production of reactive oxidant species (ROS) is not controlled by antioxidant systems. Excessive oxidative stress increases inflammation, causes proapoptotic effects, and impairs immune tolerance, thereby contributing to the pathogenesis of various autoimmune diseases, including autoimmune thyroid disease (AITD).Citation3,Citation4
Lipid hydroperoxide (LOOH) is a well-known marker of oxidative stress. The basic form of LOOH is the oxidized low-density lipoprotein (LDL).Citation5,Citation6 Increased levels of lipid peroxidation products have been reported in AITD.Citation7,Citation8
Paraoxonase1 (PON1) is an antioxidant enzyme with paraoxonase (PON) and arylesterase (ARE) activities. PON1 protects LDL from oxidation by binding to high-density lipoprotein (HDL).Citation9 PON1 activity has been shown to be reduced in several autoimmune diseases, including rheumatoid arthritis (RA), mixed connective tissue disease, Sjögren's syndrome, and systemic lupus erythematosus (SLE).Citation10,Citation11,Citation12,Citation13 However, PON1 activity in AITD has not been investigated to date. In the present study, we evaluated PON1 activity in AITD and its association with antioxidant total free sulfhydryl (–SH) and oxidant LOOH levels.
Materials and methods
The study was approved by the Ethics Committee of Gaziantep University Faculty of Medicine. Informed consent was obtained from all subjects prior to the study.
Patient groups and study protocols
This prospective study was conducted in the Department of Endocrinology at the Medical School of Gaziantep University. A total of 50 patients with autoimmune thyroid disease, including 25 with Graves’ disease (GD) (mean age: 44.00 ± 14.36 years; 17 females, 8 males) and, 25 with Hashimoto's thyroiditis (HT) (mean age: 43.92 ± 12.78 years; 16 females, 9 males) as well as 27 healthy controls (mean age: 41.44 ± 9.53 years; 16 females, 11 males) were enrolled.
Thyroid ultrasonography was performed in all patients by the same physician, regardless of laboratory results (LOGIQ p6, GE Healthcare, USA). Size, nodularity, and paranchymal echogenicity of the thyroid gland were evaluated.
Diagnosis of HT was based on thyroid function tests (low/normal serum free triiodothyronine (T3) and free thyroxine (T4), high thyroid stimulating hormone (TSH) levels), heterogeneous appearance of the thyroid parenchyma in ultrasound, and increased levels of antithyroid peroxidase (anti-TPO) and antithyroid thyroglobulin (anti-TG) antibodies. Levothyroxine therapy was initiated in these patients. Serum samples were obtained during the euthyroid period in month 3 of the treatment.
Diagnosis of GD was based on thyroid function tests (high/normal serum free triiodothyronine (T3) and free thyroxine (T4), low TSH levels), increased levels of TSH receptor antibody (anti-TRAB), anti-TPO, and anti-TG antibodies, heterogeneous appearance of the thyroid parenchyma in thyroid ultrasound, increased diffuse radioactive iodine uptake in thyroid scintigraphy, and the medical history of the patients. Methimazol therapy was initiated in these cases. Serum samples were obtained during the euthyroid period in month 3 of treatment.
Subjects had infectious diseases, inflammatory diseases, hypertension, liver failure, cardiovascular diseases, malignancies, neurodegenerative diseases, renal failure, cerebrovascular diseases, diabetes mellitus, obesity, and metabolic syndrome. Patients from both study and control groups on antioxidant treatments, such as antihypertensive and, lipid-lowering medications, and vitamin E, and smokers were excluded.
Measurements
Age, weight, height, and body mass index (BMI: body weight (kg)/height (m2)) of all subjects were recorded. Blood samples were collected in the morning after an 8 hours fasting period. Serum samples were stored at −80°C until measurement of PON, ARE, –SH, and LOOH. Fasting plasma glucose (FPG), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), total cholesterol, triglyceride (TG), free T3, free T4, TSH, anti-TPO, and anti-TG levels were measured in all subjects.
Free T3, free T4, and TSH levels were measured with the electrochemiluminescence method, using a Cobas Integra 800 model auto-analyzer. The reported reference ranges were as follows: free T3: 2–4, 4 pg/ml, free T4: 0.7–1.48 ng/dl, TSH: 0.35–4.94 uIU/ml (Roche Diagnostics, Germany).
Measurement of LOOH levels
Serum LOOH levels were measured with the ferrous ion oxidation–xylenol orange assay. The principle of the assay depends on oxidation of the ferrous to ferric ion through various oxidants, and the produced ferric ion is measured with xylenol orange. LOOH is reduced by triphenyl phosphine (TPP), a specific reductant for lipids. LOOH levels are determined from the difference with and without TPP pretreatment.Citation14
Measurement of serum –SH levels
Free sulfhydryl serum levels were measured using the method of Ellman.Citation15 For determination of –SH groups, 1 ml of buffer containing 0.1 M Tris, 10 mM EDTA, pH 8.2, and 50 μl serum were added into cuvettes, followed by 50 μl of 10 mM 5,5'-dithio-bis-(2-nitrobenzoic acid) (DTNB) in methanol. Reagent blanks were run for each sample, substituting methanol alone for DTNB in methanol. Following incubation for 15 minutes at room temperature, sample absorbance was read at 412 nm on a Cecil 3000 spectrophotometer. Sample and reagent blanks were subtracted. Sulfhydryl group concentrations were calculated using reduced glutathione as the free sulfhydryl group standard, and the results expressed as millimolar (mM).
PON1 and ARE activities
PON1 and ARE activities were measured with commercially available kits (Relassay, Gaziantep, Turkey). PON1 measurement was performed either in the presence (salt-stimulated) or absence of NaCl. The paraoxon hydrolysis rate (diethyl-p-nitrophenyl phosphate) was measured by monitoring increased absorption at 412 nm at 37°C. The amount of p-nitrophenol generated was calculated from the molar absorption coefficient at pH 8.5, which was 18.290/M/cm.Citation16 PON1 activity was expressed as U/l serum. The coefficient of variation (CV) for individual samples was 1.8%. ARE activity was measured using phenyl acetate as the substrate. Enzymatic activity was calculated from the molar absorption coefficient of the phenol produced, 1310/M/cm. One unit of ARE activity was defined as 1 mmol phenol generated per minute under the above conditions, and expressed as U/l.Citation17 The CV for individual serum samples was 4.1%. Sensitivities of both tests were over 98%.
PON1–Q192R phenotyping
PON1–Q192R phenotype distributions were determined using both paraoxon and phenylacetate as substrates according to the method of Eckerson et al.Citation18 To determine the phenotype of a given participant as QQ (homozygous low activity) or QR (heterozygous moderate activity), the ratio of salt-stimulated PON1 to ARE activity was used to assign phenotypes to individuals. After frequency analysis, subjects with salt-stimulated PON1 to ARE ratio <2.90 were classified as the homozygous AA group (PON1–192QQ; n = 21 for GD, n = 21 for HT, n = 22 for healthy control), those with ratios between 2.90 and 5.07 as the heterozygous AB group (PON1–192QR; n = 4 for GD, n = 4 for HT, n = 5 for healthy control), and those with ratios >5.08 (PON1–192RR; n = 0 for each) as the homozygous BB group.
Statistical analysis
The Shapiro-Wilk test was used to test continuous variables for normality. Measurements of normally distributed variables (BMI, FPG, total-C, HDL-C, LDL-C, TG, free T3, free T4, TSH) are presented as mean ± standard deviation. Variables with non-normal distribution (PON1, ARE, LOOH, –SH) are presented as median and interquartile range (IQR). Student's t-test was used to compare two independent groups of normally distributed variables. For non-normally distributed variables, the Mann–Whitney U test was used to compare two independent groups. Spearman correlation analysis was conducted to identify the associations between the parameters. The statistical significance of differences in phenotype frequency between the groups was tested using the chi-square (χ2) test. SPSS for Windows version 15 software was applied for statistical analyses. The level of significance was set at P ≤ 0.05.
Results
The mean ages of the GD, HT, and healthy control groups and gender distribution were similar (P = 0.970 and P = 0.806, respectively). BMI, free T3, free T4, TSH, FPG, total cholesterol, HDL-C, LDL-C, and TG levels were not significantly different among the groups (P = 0.363, P = 0.208, P = 0.825, P = 0.400, P = 0.785, P = 0.325, P = 0.491, P = 0.070, P = 0.295, respectively; Table ).
Table 1 Demographic and laboratory parameters of HT, GD, and healthy control groups
Serum PON1 activity was markedly lower in the GD and HT groups, compared to the healthy control group (P < 0.001). PON1 activity was not significantly different between the GD and healthy control groups (P = 0.143). Serum salt-stimulated PON1 activities were significantly lower in patients, compared to the healthy control group (P = 0.035 for GD, P = 0.024 for HT), whereas serum ARE levels were not significantly different among the groups (P > 0.05).
PON1 phenotype distribution of the subjects was not notably different among the three groups (P = 0.961).
Serum LOOH levels were significantly higher in the GD and HT groups, compared to the healthy control group (P < 0.001, for each). Moreover, the HT group displayed markedly higher LOOH levels than the GD group (P = 0.008).
Serum –SH levels were significantly lower in the GD and HT groups, compared to the healthy control group (P < 0.001, for each). We observed no notable differences between the GD and HT groups in terms of –SH levels (P = 0.869).
In correlation analysis, PON1 activity was positively correlated with –SH (r = 0.522, P < 0.001) and negatively correlated with LOOH in patients with AITD (r = −0.487, P < 0.001; Figs. and ).
Discussion
In our experiments, PON1 activity was significantly reduced in AITD patients, compared to the control group (P < 0.001). Additionally, PON1 activity was positively correlated with the antioxidant –SH and negatively correlated with the oxidant LOOH (r = 0.522, P < 0.001, r = −0.487, P < 0.001). To our knowledge, the present study is the first to report reduced PON1 activity in AITD.
H2O2 is required for thyroid hormone biosynthesis in thyrocytes.Citation19 Thyroglobulin fragmentation is observed upon exposure of thyroid cell culture to high concentrations of H2O2. Extended exposure of thyrocytes to H2O2 and/or impaired antioxidant systems are suggested to alter the antigenicity of thyroglobulin and thyroid peroxidase, in turn, contributing to AITD development.Citation20
Lipids are the most sensitive molecules to the effects of free radicals. Free radicals react with polyunsaturated fatty acids in the cell membrane, causing oxidative degradation known as lipid peroxidation, a process of self-sustaining chain reactions.Citation21,Citation22 A number of studies in the literature have demonstrated increased lipid peroxidation products in AITD.Citation4,Citation7
LOOH, a product of lipid peroxidation, is used as an indicator of the lipid peroxidation level.Citation21,Citation23 Data from the present study revealed increased LOOH levels in AITD patients and decreased levels of the antioxidant –SH (P < 0.001, for each), compared to healthy controls.
PON1 contributes significantly to the antioxidant capacity of HDL.Citation23 Activity of the enzymes reduced in several autoimmune diseases, such as RA, mixed connective tissue disease, SLE, and primary antiphospholipid syndrome.Citation10,Citation11,Citation13 To date, PON1 activity has not been evaluated in AITD. Here, we observed significantly lower PON1 activity in AITD patients, compared to the healthy control group (P < 0.001). The comparable lipid levels among the groups in our study suggest that reduction of PON1 in AITD occurs independently of HDL. We propose that the reduced levels of PON1 may be attributable to use of the enzyme by thyrocytes for protection from increased oxidative stress. A study by Deakin et al. on hamster ovaries and human endothelial cells demonstrated that PON1 passes through the cell membrane and outer environment easily, reduces oxidative stress of the target cell to a significant extent, and facilitates cells to gain function. The group suggested that PON1 is not fixed to HDL and may be used by cells exposed to oxidative stress.Citation24
PON1 levels in our study were significantly lower in AITD, compared to the healthy control group, whereas no differences among groups were observed in ARE levels. Previously, we showed reduced PON activity in Sjögren's syndrome, compared to the control group, but no differences in ARE activity, which was suggested to result from PON1 gene polymorphisms.Citation12 PON1 activity was higher in individuals with the Q192R polymorphism resulting in a glutamine to arginine substitution at position 192, whereas ARE activity was higher in the QQ genotype.Citation25 The ratio of salt-stimulated PON1 to ARE activity is used to define phenotypes.Citation26 We observed no significant differences between PON1 192Q and R polymorphism distribution among the GD, HT, and healthy control groups. Based on these data, we propose that the use of PON1 to prevent oxidative stress-induced thyroid cell damage is the underlying cause of low PON1 activity.
In conclusion, serum activity of PON1 is decreased in AITD. Additionally, reduced activity of PON1 is positively correlated with levels of the antioxidant, –SH, and negatively correlated with the oxidant, LOOH. Our results collectively suggest that PON1 contributes to the pathogenesis of AITD. Extensive longer-term studies are required to validate the current findings.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethics approval None.
References
- Zarković M. The role of oxidative stress on the pathogenesis of graves’ disease. J Thyroid Res 2012. doi:10.1155/2012/302537.
- Virion A, Michot JL, Deme D, Kaniewski J, Pommier J. NADPH-dependent H2O2 generation and peroxidase activity in thyroid particular fraction. Mol Cell Endocrinol 1984;36(1–2):95–105. doi: 10.1016/0303-7207(84)90088-1
- Marcocci C, Leo M, Altea MA. Oxidative stress in graves’ disease. Eur Thyroid J 2012;1(2):80–7. doi: 10.1159/000337976
- Rostami R, Aghasi MR, Mohammadi A, Nourooz-Zadeh J. Enhanced oxidative stress in Hashimoto's thyroiditis: inter-relationships to biomarkers of thyroid function. Clin Biochem 2013;46(4–5):308–12. doi: 10.1016/j.clinbiochem.2012.11.021
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res 1998;39:1529–42.
- Miyamoto S, Di Mascio P. Lipid hydroperoxides as a source of singlet molecular oxygen. Subcell Biochem 2014;77:3–20. doi: 10.1007/978-94-007-7920-4_1
- Lassoued S, Mseddi M, Mnif F, Abid M, Guermazi F, Masmoudi H, et al. A comparative study of the oxidative profile in Graves’ disease, Hashimoto's thyroiditis, and papillary thyroid cancer. Biol Trace Elem Res 2010;138(1–3):107–15. doi: 10.1007/s12011-010-8625-1
- Akarsu E, Buyukhatipoglu H, Aktaran S, Kurtul N. Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 2011;74(1):118–24. doi: 10.1111/j.1365-2265.2010.03904.x
- Rajkovic MG, Rumora L, Barisic K. The paraoxonase 1, 2 and 3 in humans. Biochem Med (Zagreb) 2011;21:122–30. doi: 10.11613/BM.2011.020
- Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, et al. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem 2005;38(10):951–5. doi: 10.1016/j.clinbiochem.2005.06.010
- Bodolay E, Seres I, Szodoray P, Csípo I, Jakab Z, Vegh J, et al. Evaluation of paraoxonase activity in patients with mixed connective tissue disease. J Rheumatol 2008;35(2):237–43.
- Szántó A, Harangi M, Seres I, Paragh G, Zeher M. Decreased human paraoxonase-1 activity in patients with Sjögren's syndrome. Int Immunol 2010;22(7):605–9. doi: 10.1093/intimm/dxq045
- Delgado Alves J, Ames PR, Donohue S, Stanyer L, Nourooz-Zadeh J, Ravirajan C, et al. Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum 2002;46(10):2686–94. doi: 10.1002/art.10542
- Arab K, Steghens JP. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Anal Biochem 2004;325(1):158–63. doi: 10.1016/j.ab.2003.10.022
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70–7. doi: 10.1016/0003-9861(59)90090-6
- Eckerson HW, Wyte MC, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet 1983;35(6):1126–38.
- Haagen L, Brock A. A new automated method for phenotyping arylesterase (E.C.3.1.1.2.) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 1992;30(7):391–5.
- Eckerson HW, Wytw CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet 1983;35(6):1126–38.
- Carvalho DP, Dupuy C. Role of the NADPH oxidases DUOX and NOX4 in thyroid oxidative stress. Eur Thyroid J 2013;2(3):160–7. doi: 10.1159/000354745
- Baser H, Can U, Baser S, Yerlikaya FH, Aslan U, Hidayetoglu BT. Assessment of oxidative status and its association with thyroid autoantibodies in patients with euthyroid autoimmune thyroiditis. Endocrine 2015;48(3):916–23. doi: 10.1007/s12020-014-0399-3
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 2009;47(5):469–84. doi: 10.1016/j.freeradbiomed.2009.05.032
- Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids 2009;157(1):1–11. doi: 10.1016/j.chemphyslip.2008.09.004
- Yoshida Y, Umeno A, Shichiri M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J Clin Biochem Nutr 2013;52:9–16. doi: 10.3164/jcbn.12-112
- Deakin SP, Bioletto S, Bochaton-Piallat ML, James RW. HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic Biol Med 2011;50(1):102–9. doi: 10.1016/j.freeradbiomed.2010.09.002
- Précourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, Seidman E, et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis 2011;214(1):20–36. doi: 10.1016/j.atherosclerosis.2010.08.076
- Turgay F, Sisman AR, Aksu AC. Effects of anaerobic training on paraoxonase-1 enzyme (PON1) activities of high density lipoprotein subgroups and its relationship with PON1–Q192R phenotype. J Atheroscler Tromb 2015;22(3):313–26. doi: 10.5551/jat.25809