ABSTRACT
Objetive: Arsenic (As) and fluoride (F) are found in groundwater and soils around the world, causing different problems to crops. Because these elements compete against phosphorus (P) in soils and plants, their relationship is complex. The aim of this work was to study the oxidative stress of soybean plants subjected to different concentrations of As and F, and the effect of P.
Methods: The following 10 treatments were carried out in each of two soils with different P content: three As levels (low 10 mg As kg-1, medium 50 mg As kg−1 and high 100 mg As kg−1), three F levels (low 160 mg F kg−1, medium 250 mg F kg−1 and high 500 mg F kg−1) and three As + F levels (same concentrations), and the control treatment (soil with the background As and F concentrations) Lipid peroxidation, chlorophyll, gluthatione contents and antioxidant enzymes activities were determination.
Results: Increased lipid peroxidation and alterations in glutathione content, catalase, superoxide dismutase and peroxidase activities as well as in chlorophyll content revealed that As causes higher oxidative stress in plants grown in soils with low P content.
Conclusion: Stress parameters in F treatments were less affected. Plants grown in soils enriched with P revealed a decrease in the toxic effects caused by As and F.
Introduction
Soybean (Glycine max L. Merrill) is a highly demanded crop due to its nutritive value, for both humans and domestic animals. Thus, it has emerged as the main protein and oil seed crop in the world trade. This crop has the ability to grow under a wide range of environmental conditions and management systems, and the expansion of the cropped area has led to its introduction in marginal lands. Like other crop plants, it is frequently exposed to various environmental stresses, which generally result in decreased yield. Among the abiotic environmental stresses, drought, radiation, temperature, heavy metal toxicity and soil salinity are regarded as major factors that pose a great threat to agricultural yield [Citation1–4]. The presence of arsenic (As) and fluoride (F) in water and soils is an emerging abiotic stress in several areas of the world, and should thus be taken into account [Citation5,Citation6].
Toxic elements like cadmium, lead, mercury, As and aluminum (for simplicity, ‘metals’ henceforth) are hazardous pollutants of the environment worldwide. Their actions on plants cause typical symptoms of toxicity, such as chlorosis, leaf rolling, wilting and stunted growth, limited seed numbers or even death. In turn, plants defend themselves against metal toxicity by activating enzymes that eliminate reactive oxygen compounds and by accumulating compounds that enhance chelation or sequestration of metals (e.g. organic acids, phytochelatins or metallothioneins) [Citation7]. It is widely known that, under normal growth conditions, plants maintain an equilibrium between production and scavenging of reactive oxygen species (ROS), avoiding the damage caused by their accumulation.
To scavenge ROS and protect against oxidative stress, plants have evolved an efficient antioxidant defense system composed of both antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), peroxidases, glutathione (GSH) reductase and ascorbate peroxidase, and antioxidants such as ascorbate, GSH, flavonoids, and lipid-soluble compounds such as carotenoids and tocopherols [Citation8]. Among the mentioned enzymes, SOD is involved in O2− scavenging, whereas CAT, ascorbate peroxidase and GSH reductase are involved in H2O2 decomposition [Citation9].
Phosphorus (P) is an essential element required for plant growth and a chemical analog of As [Citation10] and competes with As in plant uptake [Citation11]. Wu et al. [Citation12] have demonstrated that As is taken up by plants via the phosphate transport system because of the chemical similarity between As and P [Citation13,Citation14]. Arsenates and fluorides also share several physico-chemical properties with phosphates and it is known that phosphates have a significant role in As and F dynamics in soils. Owing to the similarity between these elements, they compete for the same soil adsorption sites. As(V) and P compete for uptake through the same transport systems in As hyperaccumulators [Citation15,Citation16], As-tolerant non-hyperaccumulators [Citation17] and As-sensitive non-accumulators [Citation13]. Phosphates are preferentially adsorbed as compared with As, leading to an increase in As solubility when soils are fertilized with P [Citation18]. Several authors have found higher As accumulation in cultivated plants in the presence of P [Citation19]. Loganathan et al. [Citation20] also found an increase in the availability of F in plant when P content is soils increases via P fertilization.
Chlorophyll is the most abundant pigment in all plant species, and is essential for light harvesting and energy transduction in photosynthesis. Its biosynthesis occurs mainly in the plastid and is synchronized with the formation of other pigments such as carotenoids.
The basic structure of a chlorophyll molecule is a porphyrin ring, coordinated with a central magnesium atom. This structure is responsible for its antioxidative properties, as it occurs with heme molecule, an iron protoporphyrin IX, a very similar molecule that shares the first steps of its biosynthesis.
Antioxidant system responses against different abiotic stresses have been observed in soybean plants [Citation4,Citation21,Citation22]. To our knowledge, there is no information about the effect of P on antioxidant response of soybeans induced by different concentrations of As.
Materials and methods
Plant material and growing conditions
Experiments were performed in a greenhouse where soybean was grown in 5 l pots, containing a substrate composed of 30% of washed sand and 70% of the top horizon of a sandy loam Typic Argiudoll soil. Two soils, classified on the basis of the concentration of bioavailable P and taken from two adjacent plots, were used. One of the soils received P fertilizer in the season previous to sampling, whereas the other did not (control). The final concentration of Bray and Kurtz available P in soils was 21 mg P kg-1 (P+ soil) and 8 mg P kg−1 (P− soil). The available P concentration in the P− soil was deficient for soybean growth and the crop responded to P fertilization. In contrast, the available P concentration available in the P+ soil was above that limit, and thus the crop did not need P application. Other soil properties were identical in the two plots: size particle distribution 13% clay, 12%, silt, 74% sand; 12.6 g kg−1 of organic carbon (Walkley and Black method); pH 7.6 and 0.38 dSm−1 ECS (soil saturation extract). In all cases, the methodologies outlined by Sparks et al. [Citation23] were followed.
The design was factorial (2 × 10) at random with three replications. The factors and treatments concentration are shown in .
Table 1. Factors, treatments and As and F concentrations.
To spike these soils, different concentrations of sodium arsenate and/or sodium F were added to achieve a wide range of total As and total F levels (see below). Then, soils were subjected to wetting/drying cycles for 3 months. This procedure allowed the interaction between the added elements and the soil components, reducing the overestimation of toxic effects occurring when they are in their soluble forms [Citation24].
Soybean seeds (cv Nidera 4613) were pregerminated in the dark for 48 h. Afterward, three of them were sown in each pot and then thinned to one plant per pot 10 days later. To prevent any nutritional deficit, each pot received a 2 g complete fertilizer including macro- and micronutrients, except P. Pots were irrigated with deionized water, maintaining the soil near field capacity throughout the experiment. At 60 days after sowing (R4 stage), the aerial biomass and roots were harvested.
Arsenic and fluoride determination
Leaf and root samples were rinsed with distilled water, dried, ground, sieved, homogenized and collected. As and F content were determined as follows: As was extracted by HNO3/H2O2 acid digestion and measured by atomic adsorption (ICP-AES) (USEPA, 2006) [Citation25], whereas F content was ashed at 400°C and quantified by colorimetry (SPADNS, APHA, 1993) [Citation26].
Determination of thiobarbituric acid reactive substances (TBARS)
Lipid peroxidation was measured on fresh tissues as the amount of TBARS determined by the thiobarbituric acid (TBA) reaction as described by Heath and Packer [Citation27]. Fresh control and treated leaves and roots (0.3 g) were homogenized in 3 ml of 20% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 3500 g for 20 min. Then, 1 ml of 20% TCA containing 0.5% (w/v) TBA and 100 ml 4% butylated hydroxytoluene in ethanol were added to 1 ml of the supernatant. The mixture was heated at 95°C for 30 min and then quickly cooled on ice. The contents were centrifuged at 10,000 g for 15 min and the absorbance was measured at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The concentration of TBARS was calculated using an extinction coefficient of 155 mM−1 cm−1.
Determination of chlorophyll content
Leaves (0.5 g, fresh weight) were homogenized with 96% ethanol (1:30 w/v). After centrifugation, the absorbance was measured in the supernatant at 665, 649 and 654 nm, as described by Wintermans and de Mots [Citation28].
Total glutathione assay
Non-protein thiols were extracted by homogenizing 0.3 g of fresh leaves or roots in 3 ml of 0.1 N HCl (pH 2), and 1 g polyvinylpyrrolidone (PVP). After centrifugation at 10000 g for 10 min at 4°C, the supernatants were used for analysis. Total GSH content (i.e. both reduced and oxidized GSH) was determined in the homogenates by spectrophotometry at 412 nm, using yeast GSH reductase, DTNB and NADPH [Citation29].
Antioxidant enzymes: preparations and assays
Extracts for the determination of CAT (EC 1.11.1.6), SOD (EC 1.15.1.1) and guaiacol peroxidase (GPOX, EC 1.11.1.7) activities were prepared from 0.3 g of fresh leaves and roots homogenized under ice-cold conditions in 3 ml of extraction buffer, containing 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g PVP and 0.5% (v/v) Triton X-100 at 4°C. The homogenates were centrifuged at 10,000 g for 20 min and the supernatant was used for the assays. CAT activity was determined in the homogenates by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2) and 2 mM H2O2. The pseudo-first order reaction constant (k’ = k.[CAT]) of the decrease in H2O2 absorption was determined and the CAT content in pmol mg−1 protein was calculated using k = 4.7 × 107 M−1 s−1 [Citation30]. Total SOD activity was assayed by the inhibition of the photochemical decrease of nitro blue tetrazolium (NBT), as described by Becana et al. [Citation31]. The reaction mixture consisted of 50–150 μl of enzyme extract and 3.5 ml O2− generating solution, which contained 14.3 mM methionine, 82.5 μM NBT and 2.2 μM riboflavin. Extracts were brought to a final volume of 0.3 ml with 50 mM K-phosphate (pH 7.8) and 0.1 mM Na2EDTA. Test tubes were shaken and placed 30 cm from a light bank consisting of six 15-W fluorescent lamps. The reaction was allowed to run for 10 min and stopped by switching the lights off. The decrease in NBT was followed by reading absorbance at 560 nm. Blanks and controls were run in the same way but without illumination and enzyme, respectively. One unit of SOD was defined as the amount of enzyme which inhibited 50% of NBT decrease under the assay conditions. GPOX activity was determined in the homogenates by measuring the increase in absorption at 470 nm due to the formation of tetraguaiacol (ε: 26.6 mM−1 cm−1), 50 mM K-phosphate buffer pH 7, 0.1 mM EDTA, 10 mM guaiacol and 10 mM H2O2.
Protein determination
Protein concentration was evaluated by the method of Bradford [Citation32], using bovine serum albumin as a standard.
Statistics
The results obtained were evaluated using an analysis of variance (ANOVA) test. When significant differences were found, a comparison of means by the Tukey’s multiple range test was applied.
Results
Effects of As and F on plants
The aerial biomass of plants (data not shown) subjected to As decreased by 28.6%, whereas the aerial biomass of plants subjected to F was statistically not different from that of controls. The root biomass was more affected than the aerial biomass. When plants were subjected to As treatments, the root biomass decreased by 70% compared with the control, whereas when they were subjected to F treatments, the root biomass decreased by 23.7%. Results obtained from plants subjected to both As and F resembled those obtained with As alone. As and F concentrations in plant tissues increased in a dose-dependent manner. As concentration increased from 2.4 to 15.4 mg kg−1 in roots and from 0.8 to 7.2 mg kg−1 in leaves. F concentration in roots increased from 1.9 mg kg− to 23.1 mg kg−, whereas that in leaves increased from 2.4 mg kg− to 20.6 mg kg−1 was similar between organs. As concentration was always higher in roots than in leaves, whereas F concentration P treatments.
Effects of As and F on lipid peroxidation
shows the results of TBARS determination in plants grown in both P+ and P− soils. Results from leaves and roots revealed that all concentrations of As led to an increase in TBARS formation in a dose-dependent manner with respect to controls in the P+ soil. TBARS content also was significantly affected by the different concentrations of F as well as plants treated with both F and As. also shows the similar results obtained in roots and leaves of plants grown in the P− soil. However, most concentrations of TBARS in leaves and in roots were statistically different than in P+ soil. Particularly TBARS concentration was generally higher in leaves of plants subjected to the imposed treatments when grown on the P− soil.
Table 2. Determination of TBARS on soybean roots and leaves treated with low, medium and high As and/or F concentration with both P conditions.
Effects of As and F on chlorophyll content
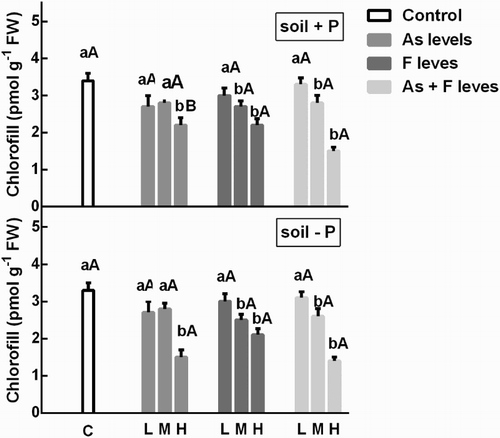
The chlorophyll content decreased with respect to controls in plants treated with As, F and As + F in both the P+ and the P− soils in a dose-dependent manner (). This effect of As and F was similar in both soils, except by the significant decrease observed in the chlorophyll content in plants grown in soils treated in the P− soil with the highest concentration of As.
Figure 1. Chlorophyll content on soybean leaves treatment with low, medium and high As and/or F concentration with both P conditions. Experiments were carried out as described in the ‘Materials and methods’ section. Data are mean values of three independent experiments ± S.E. Each value represents three replicates. Different lowercase letters within columns indicate significant differences with respect to controls (p < 0.05). Different capital letters within rows indicate significant differences between roots or leaves in Soil P+ and Soil P−(p < 0.05), according to the Tukey’s multiple range test.
Effects of As and F on GSH content
shows the results obtained in plants grown in both soils. The As in all the concentrations decreased the GSH content in the roots and leaves and this effect was more pronounced at high As levels, with respect to controls. Conversely, F affected the GSH concentration less and only high F concentrations caused a significant decrease in this parameter with respect to controls. Moreover, As + F led to similar or even more pronounced decrease to that caused by As alone. In a no defined picture, the decrease in GSH content in both tissues was something more accentuated in plants grown in the P− soil than in those grown in the P+ soil ().
Table 3. Glutathione content on soybean leaves treated with low, medium and high As and/or F concentration with both P conditions.
Effects of As and F on CAT, SOD and GPOX activities
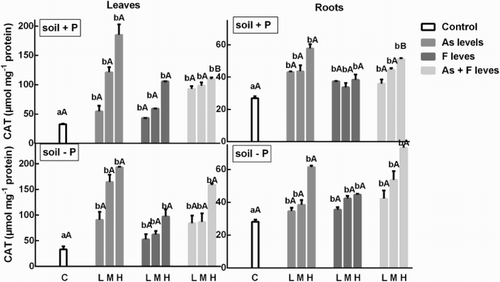
Arsenic enhanced CAT activity in the roots in the concentrations tested in the P+ soil (). Conversely, F showed no effect with respect to controls and when the effect of As + F was analyzed, no difference with respect to As alone was observed. In contrast, in leaves, all treatments increased CAT activities in a dose-dependent manner, except in the As + F treatments. The results obtained in roots and leaves of plants grown in the P− soil were similar to those observed in the P+ soil (). There is not a clear differentiation in the response of the enzyme in each soil.
Figure 2. Determination of CAT activity on soybean roots and leaves’ treatment with low, medium and high As and/or F concentration with both P conditions. Experiments were carried out as described in the ‘Materials and methods’ section. Data are mean values of three independent experiments ± S.E. Each value represents three replicates. Different lowercase letters within columns indicate significant differences with respect to controls (p < 0.05). Different capital letters within rows indicate significant differences between roots or leaves in Soil P+ and Soil P−(p < 0.05), according to the Tukey’s multiple range test.
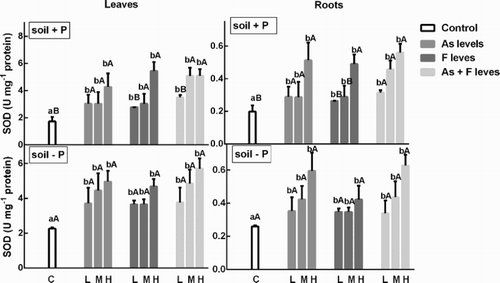
In general terms, the SOD activity increased in a dose-dependent manner as As and F increased. The roots showed no changes in the enzyme activity with respect to controls, in both the P+ and the P− soils (). In contrast, in leaves of soybean tested in the P− soils, SOD activity increased with respect to controls with As, F and As + F treatments at all the concentrations ().
Figure 3. Determination of SOD activity on soybean roots and leaves’ treatment with low, medium and high As and/or F concentration with both P conditions. Experiments were carried out as described in the ‘Materials and methods’ section. Data are mean values of three independent experiments ± S.E. Each value represents three replicates. Different lowercase letters within columns indicate significant differences with respect to controls (p < 0.05). Different capital letters within rows indicate significant differences between roots or leaves in Soil P+ and Soil P−(p < 0.05), according to the Tukey’s multiple range test.
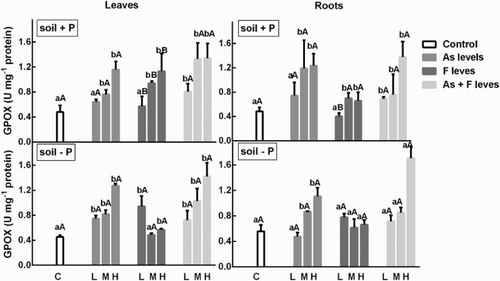
Arsenic enhanced GPOX activity in the roots and leaves with respect to controls. In general, the response was dose-dependent. On the other hand, roots from plants grown in the P− soil showed that F had no effect on roots and that GPOX decreased in leaves as its concentration increased (). Except in this case, in general, there were no differences between soils on the enzyme activity.
Figure 4. Determination of GPOX activity on soybean roots and leaves’ treatment with low, medium and high As and/or F concentration with both P conditions. Experiments were carried out as described in the ‘Materials and methods’ section. Data are mean values of three independent experiments ± S.E. Each value represents three replicates. Different lowercase letters within columns indicate significant differences with respect to controls (p < 0.05). Different capital letters within rows indicate significant differences between roots or leaves in Soil P+ and Soil P−(p < 0.05), according to the Tukey’s multiple range test.
Discussion
In this work, GSH levels and roots decrease in a dose-dependent manner. These results are in agreement with those reported that physiological processes are susceptible to As toxicity. Cellular membranes become damaged in the plants exposed to As [Citation33]. Moreover, As-induced oxidative stress causes many toxic effects in plants, such as decrease in the photosynthetic rate [Citation34], decrease in photosynthetic pigments [Citation35], GSH depletion [Citation36] and decrease in soluble protein content [Citation37].
In the present study, we demonstrated that As causes membrane damage at low, medium and high concentrations as well as a decrease in chlorophyll content. These effects were still more accentuated in plants in Pteris vittata by Stoeva and Bineva [Citation34].
Phytochelatins are synthesized enzymatically from GSH by phytochelatin synthase in a metal-dependent manner. When complexed with phytochelatins, cations are less toxic and can be sequestered in the vacuoles. As a consequence, in the presence of As, phytochelatin’s biosynthesis increases diminishing the GSH pool [Citation38] Taking into account that GSH is involved in phytochelatin synthesis, a decrease in the former could be a consequence of the increase of the latter because of the complexation of As by phytochelatins. This is an important mechanism of detoxification [Citation39]. Moreover, the activities of antioxidant enzymes (CAT, SOD, GPOX) were enhanced in roots and in the leaves as it has been previously observed in rice [Citation40] and wheat [Citation41]. The increase in SOD activity was also detected in Holcus lanatus [Citation37]. Bustingorri et al. [Citation22] observed an increase in CAT and GPOX activities in soybean plant but with higher concentration of As and F.
It is worthy to note that in the P− soil, As caused a more accentuated increase in lipid peroxidation and a decrease in chlorophyll content. Phosphates and arsenates compete for the same transport system [Citation14], and when P content is lower, As can enter the cell more easily [Citation42–44].
The responses obtained in the F treatments showed some difference from those obtained in the As treatments: F caused less oxidative damage in leaves and roots of soybean than As. These responses could be due to the fact that the amount of plant available F in the soil solution depends strongly on the solubility of mineral phases, soil type and pH. F is taken up passively by plants. Previous reports have indicated that F can enter the plant easily.
Our results show that the damage in the leaves was greater than that in roots. These results were supported by the response of antioxidant enzymes (CAT and GPOX). In the roots, the antioxidant system was less affected in the treatments with F alone. In the leaves, antioxidant enzyme activities were altered with respect to controls according to As concentrations. Data here presented indicated that F is not so toxic as As, and that higher levels of P in the soil do not alter its behavior, and this fact could indicate that P is not involved in F toxicity.
When As and F were simultaneously applied, the toxic effects were not additive and the detrimental effects were caused mainly by As. Plants grown in P+ treatments showed less membrane damage than those grown in P− treatments. The presence of P caused a smaller increase in TBARS levels and no differences in the activities of antioxidant enzymes except for CAT in the leaves, which decreased in the P+ soil with respect to the P− soil. Moreover, the P nutrition status in the plant itself and P availability in the rhizosphere can strongly affect As bioaccumulation in plants [Citation15,Citation16]. The results here presented show that in soybean plants As causes oxidative damage in a dose-dependent manner, as observed by the increase in TBARS formation and the alterations in the non-enzymatic and enzymatic defense system. On the other hand, this oxidative insult was lower in the plants grown in the P+ soil [Citation45,Citation46]. F also caused oxidative stress but to a lesser extent. Moreover, the available P concentration in the soils did not affect F action. When the effect of both elements was analyzed, the deleterious effects of the oxidative damage were due mainly by As.
Considering the economic interest of soybean and its subsequent expansion of As-contaminated areas, the concentration of available P in the soil is of the utmost interest. Data here presented indicate that high available P concentration in the soils could be effective in reducing the toxic effects of As on plants.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Carolina Bustingorri received the Agricultural Engineer, Faculty of Agronomy, University of Buenos Aires in 2010. She is PhD student at National Scientific and Technical Research Council (CONICET). Her main research activity is devoted to study about fertilization of soils and abiotic stress in plant.
Guillermo Noriega received his PhD in Chemistry in 1998 at the University of Buenos Aires. He is currently a professor of Biochemistry at the College of Exact and Natural Sciences, University of Buenos Aires, and professional technician at the National Scientific Research Council (CONICET). His main research activity is devoted to study the heme metabolism and its relationship to oxidative stress.
Raúl S. Lavado received his PhD in Edaphology and Plant Biology at the University of Granada, Spain, in 1971. He is currently a professor of the College of Agronomy at the University of Buenos Aires and a senior researcher at CONICET. His main research activity is devoted to study about fertilization of soils and their interaction with crops.
Karina Balestrasse received his PhD in Biological Chemistry in 2003 at the University of Buenos Aires. She is currently a professor of the College of Agronomy at the University of Buenos Aires and an independent researcher at CONICET. Her main research activity is devoted to study about oxidative stress and growth regulation in plants.
Additional information
Funding
References
- López Lecube M, Noriega G, Santa Cruz D, et al. Indole acetic acid is responsible for the protection against oxidative stress caused by drought in soybean plants: the role of heme oxygenase induction. Redox Rep. 2014;19(6):242–250. doi: 10.1179/1351000214Y.0000000095
- Hasanuzzaman M, Nahar K, Roychowdhury R, et al. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci. 2013;14(5):9643–9684. doi: 10.3390/ijms14059643
- Noriega G, Caggiano E, Lecube ML, et al. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals. 2012;25(6):1155–1165. doi: 10.1007/s10534-012-9577-z
- Zilli C, Balestrasse K, Yannarelli G, et al. Heme oxygenase up-regulation under salt stress protects nitrogen metabolism in nodules of soybean plants. Environ Exp Bot. 2008;64:83–89. doi: 10.1016/j.envexpbot.2008.03.005
- Bustingorri C, Lavado R. Soybean as affected by high concentrations of arsenic and fluoride in irrigation water in controlled conditions. Agric Water Manag. 2014;144:134–139. doi: 10.1016/j.agwat.2014.06.004
- Brammer H, Ravenscroft P. Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Inter. 2009;35:647–654. doi: 10.1016/j.envint.2008.10.004
- Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Ann Rev Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154
- Molassiotis A, Sotiropoulos T, Tanou G, et al. Boron induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 (Malus domestica Borkh). Env Exp Bot. 2006;56:54–62. doi: 10.1016/j.envexpbot.2005.01.002
- Namdjoyan S, Khavari-Nejad RA, Bernard F, et al. Antioxidant defense mechanisms in response to cadmium treatments in two safflower cultivars. Russ J Plant Physiol. 2011;58:467–477. doi: 10.1134/S1021443711030149
- Adriano D. Trace elements in terrestrial environments: biogeochemistry, bioavailability and risks of metals. 2nd ed. New York: Springer; 2001.
- Meharg A, Macnair M. Polymorphism and physiology of arsenate tolerance in Holcus lanatus L. from an uncontaminated site. Plant Soil. 1992;146:219–225. doi: 10.1007/BF00012015
- Wu F, Liu X, Wu S, et al. Effects of mycorrhizal inoculation of upland rice on uptake kinetics of arsenate and arsenite Fuyong. Plant Nutr Soil Sci. 2015;178:333–338. doi: 10.1002/jpln.201400461
- Esteban E, Carpena R, Meharg A. High-affinity phosphate/arsenate transport in white lupin Lupinus albus) is relatively insensitive to phosphate status. New Phytol. 2003;158:165–173. doi: 10.1046/j.1469-8137.2003.00713.x
- Lessl JT, Guan DX, Sessa E, et al. Transfer of arsenic and phosphorus from soils to the fronds and spores of arsenic hyperaccumulator Pteris vittata and three non-hyperaccumulators. Plant Soil. 2015;390:49–60. doi: 10.1007/s11104-014-2376-2
- Wang J R, Zhao F, Meharg A. Mechanism of arsenic hyperaccumulation in Pteris vittata L.: uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol. 2002;130:1552–1561. doi: 10.1104/pp.008185
- Tu S, Ma L. Interactive effects of pH, arsenic and phosphorus on uptake of As and P and growth of the arsenic hyperaccumulator Pteris vittata L. under hydroponic conditions. Environ Exp Bot. 2003;50:243–251. doi: 10.1016/S0098-8472(03)00040-6
- Bleeker P, Schat H, Vooijs R, et al. Mechanisms of arsenate tolerance in Cytisus striatus. New Phytol. 2003;157:33–38. doi: 10.1046/j.1469-8137.2003.00542.x
- Signes-Pastor A, Burló F, Mitra K, et al. Arsenic biogeochemistry as affected by P fertilizer addition, redox potential and pH in a west Bengal (India) soil. Geoderma. 2007;137:504–510. doi: 10.1016/j.geoderma.2006.10.012
- Fayiga A, Ma L. Using phosphate rock to immobilize metals in soil and increase arsenic uptake by hyper-accumulator Pteris vittata. Sci Total Environ. 2006;359:17–25. doi: 10.1016/j.scitotenv.2005.06.001
- Loganathan P, Liu Q, Hedley M, et al. Chemical fractionation of fluorine in soils with a long-term phosphate fertiliser history. Aust J Soil Res. 2007;45:390–396. doi: 10.1071/SR07030
- Balestrasse K, Gardey L, Gallego S, et al. Response of antioxidant defence system in soybean nodules and roots subjected to cadmium stress. Aust J Plant Physiol. 2001;28:497–504.
- Bustingorri C, Balestrasse K, Lavado R. Effects of high arsenic and fluoride soil concentrations on soybean plants. Phyton. 2015;84:407–415.
- Sparks D, Page A, Helmke P, et al. Chemical methods. Madison (WI): ASA-SSSA Book Series; 1996.
- Basta N, Ryan, JA, Chaney RL. Trace element chemistry in residual-treated soil: key concepts and metal bioavailability. J Environ Qual. 2005;34:49–63. doi: 10.2134/jeq2005.0049dup
- APHA. Standard methods for the examination of water and wastes. Washington (DC): American Public Health Association; 1993.
- USEPA. Test methods for evaluating solid waste. Washington (DC): United States Environmental Protection Agency; 2006.
- Heath R, Packer L. Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1
- Wintermans J, de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6
- Anderson M. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–554. doi: 10.1016/S0076-6879(85)13073-9
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605.
- Becana M, Aparicio-Tejo P, Irigoyen J, et al. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol. 1986;82:1169–1171. doi: 10.1104/pp.82.4.1169
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3
- Singh N, Ma L, Srivastava M, et al. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 2006;170:274–282. doi: 10.1016/j.plantsci.2005.08.013
- Stoeva N, Bineva T. Oxidative changes and photosynthesis in oat plants grown in as contaminated soil. Bulg J Plant Physiol. 2003;29:87–95.
- Mascher R, Lippmann B, Holzinger S, et al. Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci. 2002;163:961–969. doi: 10.1016/S0168-9452(02)00245-5
- Li Y, Dankher O, Carreira L, et al. The shoot-specific expression of γ-glutamylcysteine synthetase directs the long distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiol. 2006;141:288–298. doi: 10.1104/pp.105.074815
- Hartley-Whitaker J, Ainsworth G, Meharg A. Copper and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. 2001;24:713–722. doi: 10.1046/j.0016-8025.2001.00721.x
- Leopold I, Günther D, Schmidt J, et al. Phytochelatins and heavy metal tolerance. Phytochemistry. 1999;50:1323–1328. doi: 10.1016/S0031-9422(98)00347-1
- Jozefczak M, Remans T, Vangronsveld J, et al. Is a key player in metal-induced oxidative stress defenses. Int J Mol Sci. 2012;13(3):3145–3175. doi: 10.3390/ijms13033145
- Panaullah G, Alam T, Hossain M, et al. Arsenic toxicity to rice (Oryza sativa L). Plant Soil. 2009;317:31–39. doi: 10.1007/s11104-008-9786-y
- Pigna M, Cozzolino V, Violante A, et al. Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Poll. 2009;197:371–380. doi: 10.1007/s11270-008-9818-5
- Stoeva N, Berova M, Zlatev Z. Effect of arsenic on some physiological parameters in bean plants. Biol Plantarum. 2005;49(2):293–296. doi: 10.1007/s10535-005-3296-z
- Mallick S, Sinam G, Sinha S. Study on arsenate tolerant and sensitive cultivars of Zea mays L. Differential detoxification mechanism and effect on nutrients status. Ecotoxicol Environ Saf. 2011;74:1316–1324. doi: 10.1016/j.ecoenv.2011.02.012
- Gomes M, Carvalho M, Carvalho G, et al. Phosphorus improves arsenic phytoremediation by Anadenanthera peregrina by alleviating induced oxidative stress. Int J Phytoremediation. 2013;15(7):633–646. doi: 10.1080/15226514.2012.723064
- Gunes A, Pilbeam D, Inal A. Effect of arsenic–phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil. 2009;314(1):211–220. doi: 10.1007/s11104-008-9719-9
- Pigna M, Cozzolino V, Giandonato Caporale A, et al. Effect of P fertilization on As nutrition in wheat. J Soil Sci Plant Nutr. 2010;10(4):428–442. doi: 10.4067/S0718-95162010000200004
