ABSTRACT
Objective: This study investigated gender-dependent differences of mitochondrial function and sensitivity to in vitro ROS exposure in rat skeletal muscle at rest and after exercise training.
Methods: Wistar rats underwent running training for 6 weeks. In vitro measurements of hydroxyl radical production, oxygen consumption (under basal and maximal respiration conditions) and ATP production were made on permeabilized fibers. Mitochondrial function was examined after exposure and non-exposure to an in vitro generator system of reactive oxygen species (ROS). Antioxidant enzyme activities and malondialdehyde (MDA) content were also determined.
Results: Compared with sedentary males, females showed a greater resistance of mitochondrial function (oxygen consumption and ATP production) to ROS exposure, and lower MDA content and antioxidant enzyme activities. The training protocol had more beneficial effects in males than females with regard to ROS production and oxidative stress. In contrast to male rats, the susceptibility of mitochondrial function to ROS exposure in trained females was unchanged.
Discussion: Exercise training improves mitochondrial function oxidative capacities in both male and female rats, but is more pronounced in males as a result of different mechanisms. The resistance of mitochondrial function to in vitro oxidative stress exposure and the antioxidant responses are gender- and training-dependent, and may be related to the protective effects of estrogen.
Introduction
Mitochondria play an important role in cell metabolism, not only through ATP production but also by being a main site for the production of reactive oxygen species (ROS) related to electron leakage from the respiratory chain [Citation1,Citation2].
Adaptations in skeletal muscle mitochondria are recognized as a key factor in the beneficial outcomes of exercise training [Citation3,Citation4]. One of the most prominent changes induced by exercise training is upregulation of mitochondrial energy metabolism with increases in mitochondrial biogenesis, oxidative capacity and muscle contraction efficiency [Citation5,Citation6]. Moderate levels of ROS also regulate exercise training-induced muscle changes by modulating gene expression via redox-sensitive transcription pathways [Citation6]. Indeed, physical exercise modulates ROS production, the effects of which can be described by the hormetic curve [Citation7], whereby low or moderate dose stimulation upregulates antioxidant defenses [Citation8] and a high-dose inhibition with oxidative stress and the potential cellular damage it causes [Citation9].
These relationships between mitochondrial energy metabolism and ROS, particularly their adaptation to exercise, are not yet well clarified. These mechanisms are usually studied separately, and the results are highly dependent on many parameters, such as the exercise protocol, animal species, age, and tissue studied. Gender is also one of the many factors that can affect mitochondrial function in skeletal muscle and other tissues [Citation10,Citation11].
Gender differences have been observed in response to oxidative stress, as female rats often show lower oxidative damage than males [Citation12]. Exercise-induced oxidative stress may also show some differences between the sexes in humans and animals [Citation13]. Indeed, oxidative stress responses are also dependent on many other parameters, such as exercise protocol, animal species and strain. For example, Balci and Pepe [Citation14] reported that swimming training in Wistar rats (60 minutes/day, 5 days/week for 8 weeks) decreased lipid peroxidation in female muscle but not in male muscle. In contrast, Liu et al. [Citation15] reported that 8 weeks of moderate running training in Sprague-Dawley rats resulted in greater increases of lipid peroxidation in the skeletal muscle of female rats than in male ones. In a recent study, Farhat et al. [Citation16] reported that moderate training (running 60 minutes/day, 5 days/week for 6 weeks, 70% of Vmax) improved mitochondrial function and changed the susceptibility of mitochondrial function to in vitro ROS exposure in the skeletal muscle of male Wistar rats.
Given the disparity of results in the literature when different factors (gender, training, mitochondrial function, redox status, etc.) are examined separately, the present study took a combined approach. Using Wistar rats, we examined the effects of gender and training on both mitochondrial function, including its sensitivity to ROS exposure, and skeletal muscle redox status.
To achieve this, we used the same moderate exercise training protocol in female Wistar rats as Farhat et al. [Citation16] used in male rats. To the best of our knowledge, this is the first study to explore both hypothetical gender and training differences in the mitochondrial function of rat skeletal muscle and its susceptibility to in vitro ROS exposure.
Methods
To compare the results of female rats with the male rats studied in Farhat et al. [Citation16], the methods used are the same as those in this previous paper.
Animal care
All experimental procedures were approved by the French Ethics Committee (CEFEA no. 74) and the Ministère de l’Enseignement Supérieur et de la Recherche [French Ministry of Higher Education and Research], under the reference ‘02180.03’. Six-week-old (at their arrival) Wistar female rats (n = 20) were obtained from the Janvier Breeding Center (Le Genest-Saint Isle, France). They were housed in a 21 ± 1°C room with a 12:12 hour light:dark photoperiod. Water and food (standard rat chow) were provided ad libitum.
Training protocol
Maximal aerobic speed (MAS) was evaluated for each rat at 8.5 weeks old on a motor-driven treadmill (0% incline). This protocol consisted of an exercise session in which the speed was progressively increased in increments of 0.2 km/hour and then 0.1 km/hour every 1.5 minutes. MAS was also evaluated after 3 weeks of training to adjust the training intensities.
The rats were randomly assigned to either trained (n = 10) or sedentary (n = 10) groups. The rats in the trained group ran on a treadmill five times per week for 60 minutes/day for 6 weeks, at a speed equivalent to 60–70% of their MAS. The rats in the sedentary group were handled identically to the trained rats, except that the treadmill was left turned off.
Forty-eight hours after the last session of endurance training, the rats were intramuscularly anesthetized (ketamine/xylazine: 100/15 mg/kg). After anesthesia, the gastrocnemius muscle was removed. Some of the tissue was used immediately, and the remainder was frozen in liquid nitrogen and stored at −80°C for later use.
Preparation of permeabilized gastrocnemius muscle fibers
The gastrocnemius muscle was minced into fine pieces (approximately 5 mg/piece). Fibers were placed in buffer A (10 mM EGTA, 20 mM taurine, 3 mM MgCl2, 0.1 mM MES potassium, 0.5 mM dithiothreitol, 15 mM phosphocreatine and 5 mM ATP; pH 7.4) containing saponin (100 µg/ml) to selectively destroy the integrity of the sarcolemma. After 20 minutes of incubation, the fibers were washed twice in buffer A for 10 minutes to completely remove the saponin and metabolites. All incubations and washing procedures were carried out with mild stirring at 4°C [Citation17].
Mitochondrial respiration measurement
The mitochondrial oxygen consumption rate (VO2) of the control and ROS-exposed fibers (around 20 mg) were polarographically measured using a Strathkelvin 928 6-Channel Oxygen System and a Clark-type electrode in a water-jacketed glass chamber maintained at 37°C with magnetic stirring. The measurement was carried out in 2 ml of a respiratory medium (20 mM Tris, 150 mM KCl, 0.08 mM EDTA, 10 mM NaH2PO4, and 7.5 mM MgCl2; pH 7.2). Basal respiration (V0), defined as state 4 in mitochondria preparation, was first assessed by adding the Krebs cycle intermediates pyruvate/malate (12 mM/6 mM). The maximal ADP-stimulated respiration (Vmax), defined as state 3 mitochondria preparation, was then measured by adding 5 mM ADP. The respiratory substrates (pyruvate/malate and ADP) were used at saturating concentrations [Citation18]. The mitochondrial respiration rate is expressed in µmol O2/minute/g of tissue.
Rate of ATP production by permeabilized fibers
ATP synthesis in muscle fibers was measured in the respiratory medium in the presence of respiratory substrates (see above) as described by Ouhabi et al. [Citation19] and Cambier et al. [Citation20]. We verified that there was no ATP production after the addition of pyruvate/malate (V0 conditions). ATP synthesis was initiated by adding 5 mM ADP (Vmax conditions), and this was recorded over 2 minutes by withdrawing a 20 µl aliquot every minute after the ADP addition, quenching in 100 µl dimethyl sulfoxide, and then diluting in 5 ml distilled water. The quantity of ATP was measured by bioluminescence with a Berthold detection system luminometer. Standardization was performed with known quantities of ATP (0−500 nmol) measured under the same conditions. The rate of ATP synthesis (VATP) was calculated using linear regression. Rates are expressed in µmol ATP/minute/g tissue.
Rate of •OH production by permeabilized fibers
Using the remainder of the permeabilized fibers, •OH radical production (V•OH) was determined with an indirect method as previously described by Amérand et al. [Citation18]. Briefly, salicylic acid is used as an •OH radical trapper, and its hydroxylation produces two stable metabolites (2,3- and 2,5-dihydroxybenzoic acid, DHBA), which are further quantified by high-performance liquid chromatography (HPLC) (Knauer Smartline Autosampler 3900) coupled with electrochemical detection (Bioanalytical Systems LC-4C). Incubation of the permeabilized fibers with salicylic acid was performed under the same conditions of temperature and saturated substrate concentrations as the VO2 measurements under Vmax conditions. V•OH production rate is expressed in ng of DHBA/minute/g tissue.
Malondialdehyde content in gastrocnemius muscle
Malondialdehyde (MDA) content, used as an index of lipid peroxidation, was determined according to the specific TBA–MDA test described by Mortelette et al. [Citation21]. Muscle was homogenized with a Polytron homogenizer in a 2% methanolic solution with 1% butylated hydroxytoluene. In the presence of 1% phosphoric acid, a complex formed between MDA and Thiobarbituric acid (TBA 7.4 mM) after development of the reaction at 100°C for 30 minutes. The TBA/MDA complex was extracted in n-butanol, then evaporated at 37°C, and the residue was re-dissolved in the mobile phase. The TBA/MDA complex was separated by HPLC and detected by UV spectrophotometry (BioTek-Kontron) at 532 nm. It was expressed in nmol/g tissue.
Antioxidant enzyme activities and protein content in gastrocnemius muscle
Enzyme activities were determined by UV spectrophotometry (UVIKON XL model) at 37°C. Samples from frozen tissues were placed in an extraction buffer (75 mM Tris and 5 mM EDTA) at 4°C and pH 7.4 for homogenization with a Polytron homogenizer prior to centrifugation. After centrifugation of the resulting supernatant at 12 000g for 10 minutes at 4°C, the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and the protein content were determined as follows.
− SOD activity was measured at 480 nm using the method that inhibits the adrenaline–adrenochrome reaction [Citation22]. One unit (U) of SOD is equal to the amount of sample needed to cause 50% inhibition relative to the control (100%). SOD activity is expressed in U/mg protein.
− CAT activity was determined at 240 nm through its ability to transform H2O2 into H2O and O2 [Citation23]. The H2O2 assay concentration was 10 mM in 75 mM Tris and 5 mM EDTA buffer at pH 7.4. CAT activity is expressed in μmol H2O2/minute/mg protein.
− GPx activity was assessed at 340 nm with an indirect method adapted from Paglia and Valentine [Citation24]. Briefly, this was determined from the decrease of NADPH induced by a coupled reaction with glutathione reductase. GPx activity was expressed in μmol NADPH oxidized/minute/mg protein.
− Protein content was measured by the colorimetric method using a BC Assay Protein Quantitation Kit (Uptima-Interchim, France, #FT-40840A). Cu2+ was reduced to Cu+ by proteins in an alkaline medium. The BCA (bicinchoninic acid) assay chelates Cu+ ions with very high specificity to form a water soluble purple-colored complex. The absorbance of the this final Cu+ complex is measured with a spectrophotometer at 562 nm and is directly proportional to the protein concentration expressed in mg/g tissue.
In vitro exposure of permeabilized gastrocnemius fibers to ROS
Some of the permeabilized fibers were exposed to exogenous ROS by incubating them with FeCl2/H2O2 according to the Fenton reaction (Fe2+ + H2O2 → Fe3+ + −OH + •OH). We adapted the method to permeabilized rat gastrocnemius fibers [Citation25] as follows: 20 mg of permeabilized fibers (four pieces) were transferred to 1.5 ml of buffer A containing 1 mM FeCl2 and 5 mM H2O2. After 30 minutes of incubation in the dark, the fibers were washed twice for 5 minutes in 1.5 mL of buffer A. The control permeabilized fibers were incubated in buffer A without H2O2 or FeCl2. All incubations and washing procedures were carried out with mild stirring at 4°C. Measurements of maximal oxygen consumption and ATP production rate were then determined.
Data analysis
All data are presented as means ± SEM. Differences between groups were assessed by two-way analysis of variance (ANOVA). When appropriate, the simple effects of training (within gender) and gender (within untrained) were evaluated with Student’s t-tests after testing the distributions for normality (Lilliefors’ test). Statistical significance was set at P < 0.05. R statistical software was used for all statistical analyses.
Results
Apart from those given in , the results on male rats were previously published [Citation16]. These data on male rats are given for comparison with female rats.
Table 1. Effects of gender and training on body weight at the end of the protocol.
Body weight
Body weight in the female rats was significantly lower (P < 0.001) compared with that of the male rats under both trained and untrained conditions (). Training status had a significant effect (P < 0.001) on rat body weight in both male and female animals, but there was no interaction between gender and training.
Mitochondrial function
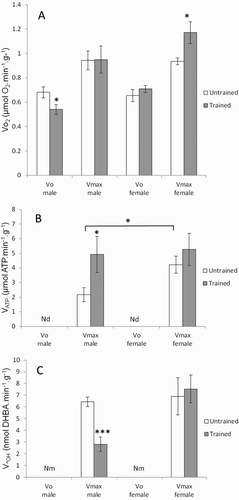
The effects of gender and endurance training on mitochondrial function in permeabilized gastrocnemius muscle fibers are shown in . The VO2 was measured under two conditions: V0 (basal respiration) and Vmax (maximal ADP-stimulated respiration), while VATP and V•OH were only measured under Vmax conditions (see the section ‘Methods’). Basal respiration (V0) showed a significant interaction between gender and endurance training (P < 0.05) ((a)). Training decreased the V0 in male rats (P < 0.05) and increased the maximal respiration (Vmax) in female rats (P < 0.05). In sedentary rats, the VATP was significantly higher (P < 0.05) in female rats than in male rats ((b)). An endurance training effect was observed on VATP (P < 0.05), with a non-significant change in female rats. No gender effect was observed for the V•OH (P = 0.052), and endurance training did not decrease the V•OH in female rats, unlike in male rats (P < 0.001) ((c)).
Figure 1. Effects of gender and training on (a) oxygen consumption VO2, (b) ATP production VATP, and (c) •OH production V•OH rates in permeabilized gastrocnemius muscle fibers of untrained (white histogram) and trained Wistar rats (gray histogram). VO2 and VATP were measured in the absence (V0) and in the presence (Vmax) of ADP and V•OH was measured only in the presence of ADP. Values are means ± SEM. A two-way ANOVA and Student’s t-tests were used to compare trained vs. untrained and female vs. male rats. *P < 0.05, **P < 0.01, ***P < 0.001. Nd = not detected and Nm = not measured.
Mitochondrial function after in vitro ROS exposure
As expected, mitochondrial function was markedly affected after treatment with the ROS generation system. The effects of gender and endurance training on the susceptibility of mitochondrial function to in vitro ROS exposure are shown in . Gender had a significant effect on respiratory rate after ROS exposure under V0 and Vmax conditions, as well as on VATP (P < 0.0001, P < 0.01, and P < 0.01, respectively; ). The interaction between gender and endurance training for VATP was significant (P < 0.05). Following ROS exposure, changes in the three aforementioned parameters were significantly lower in female rats than in male rats (V0, P < 0.01; Vmax, P < 0.05; VATP, P < 0.001). In contrast to male rats, endurance training in female rats did not influence the sensitivity of Vmax and VATP to ROS exposure.
Figure 2. Effects of gender and training on the vulnerability of mitochondrial function to ROS exposure. Oxygen consumption VO2 was measured under V0 and Vmax conditions, and VATP under Vmax conditions, in permeabilized gastrocnemius muscle fibers of untrained (white histogram) and trained rats (gray histogram). VO2(max) and VATP were measured after 30 minutes of incubation in buffer A containing H2O2 + FeCl2 for treated fibers and in buffer A alone for untreated fibers. The change in respiratory and ATP rates is expressed relative to fibers without H2O2 and FeCl2 according to this formula: [(VTreated−VUntreated)/VUntreated] × 100. Values are means ± SEM. A two-way ANOVA and the Student’s t-tests were used to compare trained vs. untrained and female vs. male rats. *P < 0.05, **P < 0.01, ***P < 0.001.
![Figure 2. Effects of gender and training on the vulnerability of mitochondrial function to ROS exposure. Oxygen consumption VO2 was measured under V0 and Vmax conditions, and VATP under Vmax conditions, in permeabilized gastrocnemius muscle fibers of untrained (white histogram) and trained rats (gray histogram). VO2(max) and VATP were measured after 30 minutes of incubation in buffer A containing H2O2 + FeCl2 for treated fibers and in buffer A alone for untreated fibers. The change in respiratory and ATP rates is expressed relative to fibers without H2O2 and FeCl2 according to this formula: [(VTreated−VUntreated)/VUntreated] × 100. Values are means ± SEM. A two-way ANOVA and the Student’s t-tests were used to compare trained vs. untrained and female vs. male rats. *P < 0.05, **P < 0.01, ***P < 0.001.](/cms/asset/ff375dce-888a-4d74-ba45-ae67c203a0bc/yrer_a_1296637_f0002_b.gif)
Antioxidant enzyme activities and MDA content of gastrocnemius muscle
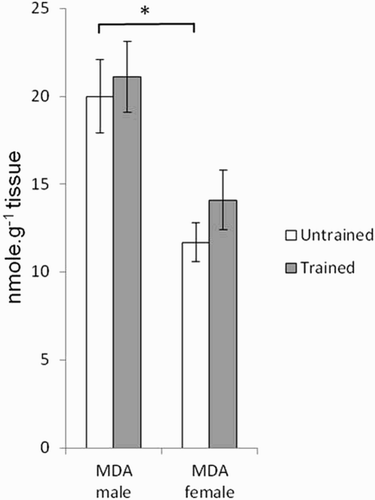
and show the effects of gender and endurance training, respectively, on antioxidant enzyme activities and on MDA content. Gender effects were observed for SOD (P < 0.001), CAT (P < 0.0001), and MDA (P < 0.0001). Interactions between gender and endurance training were also observed for SOD (P < 0.01) and CAT (P < 0.05). Female rats presented SOD and CAT activities, as well as MDA contents, that were significantly lower (P < 0.001) than those in male rats. Endurance training did not change antioxidant enzyme activities in female rats while in males it brought a reduction.
Figure 3. Effects of gender and training on SOD (U/mg protein), CAT (H2O2/minute/mg protein), and Gpx(μmol NADPH oxidized/minute/mg protein) in gastrocnemius muscle of untrained (white histogram) and trained rats (gray histogram). Values are means ± SEM. A two-way ANOVA and the Student’s t-tests were used to compare trained vs. untrained and female vs. male rats, **P < 0.01, ***P < 0.001.
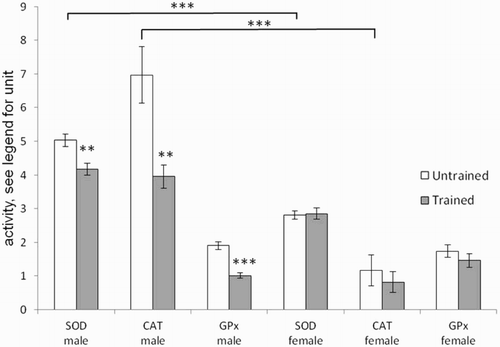
Figure 4. Effects of gender and training on MDA (lipid peroxidation index) content in gastrocnemius muscle of untrained (white histogram) and trained rats (gray histogram). Values are means ± SEM. A two-way ANOVA and the Student’s t-tests were used to compare trained vs. untrained and female vs. male rats, *P < 0.05.
Discussion
The present study aimed to examine the effects of gender and exercise training on both Wistar rat mitochondrial function, including its sensitivity to ROS exposure, mitochondrial ROS release, and skeletal muscle redox status. Most of the time, these physiological mechanisms are studied separately. Additionally, in the literature, hypotheses concerning resistance of mitochondria to oxidative stress are often proposed only on the basis of oxidative markers and antioxidant status. In the present paper, resistance of mitochondrial function to oxidative stress is directly tested on fresh permeabilized muscle fibers.
The first finding shows that, compared with sedentary male Wistar rats (as reported in a recent study [Citation16]), the mitochondrial function of sedentary female rats shows greater resistance to in vitro ROS exposure but also lower skeletal muscle TBARS content and antioxidant enzyme activities. The second finding is that the training protocol used has more beneficial effects on male than on female Wistar rats with regard to mitochondrial function and muscle oxidative stress. Otherwise, in contrast to male rats, susceptibility of the mitochondrial function to ROS exposure was unchanged in trained female rats compared to sedentary ones.
Effects of gender in sedentary rats
Sexual dimorphism in mitochondrial function, including oxidative capacity and mitochondrial biogenesis, has been demonstrated in many rat tissues, such as adipose tissue, liver, and skeletal muscle [Citation10,Citation11]. Gender differences in skeletal muscle have also been described for substrate metabolism, molecular mechanisms, and insulin sensitivity [Citation26]. Redox status also shows gender differences, as female rats often suffer less oxidative damage than males. Català-Niell et al. [Citation12] reported that muscle mitochondria from females produce significantly less hydrogen peroxide and have more effective antioxidant capacities than those from males.
Our results show that mitochondrial function did not differ between untrained male and female rats. The permeabilized fibers showed similar maximal ADP-stimulated (Vmax) and basal (V0) respiration rates (VO2) ((a)). The generation of reactive oxygen species, as indicated by in vitro •OH production (V•OH) in skeletal muscle fibers, did not change significantly according to gender in untrained rats ((c)). In parallel, ATP production (VATP) was significantly higher in females, suggesting a tendency for more efficient oxidative phosphorylation (VATP/VO2(max) = 4.54 ± 0.96 in females vs. 2.32 ± 1.08 in males; (b)). In accordance with our findings, other authors have reported higher oxidative capacity in the gastrocnemius muscle of female Wistar rats. Català-Niell et al. [Citation12] demonstrated higher mitochondrial respiration at state 4 and state 3. Colom et al. [Citation10] found higher enzymatic activities for some electron transport chain complexes in the mitochondria of females compared with males. Gomez-Perez et al. [Citation27] showed a higher cytochrome C oxidase protein content in female rats than in males.
With regard to antioxidant enzymes, the activities of SOD and CAT in muscle are lower in untrained female rats than in males (). A decrease in antioxidant enzyme activities could make female skeletal muscle more vulnerable to oxidative stress, yet we observed lower lipid peroxidation (MDA content) in female rats than in males (). Furthermore, mitochondrial function showed greater resistance to oxidative stress in females than in males. As the major site for ROS production, the mitochondrion is particularly prone to oxidative damage [Citation28]. As expected, when permeabilized fibers were exposed to a ROS system generator, the Vmax and V0 respiration rates, as well as the VATP, decreased by 20–60%. All three of the aforementioned parameters were significantly less affected in female rats than in males. The better resistance of mitochondrial function in female rats can be considered surprising considering the data obtained for SOD and CAT in skeletal muscle. However, the measurement of SOD concerned the total activity, so it would be interesting to measure the activity of Mn-SOD located at the mitochondrial matrix. The better oxidative resistance of mitochondrial function is, therefore likely due to mitochondrial antioxidant capacities involving other antioxidant components than those determined here. Indeed, during saponification of permeabilized fibers, the cytosolic antioxidant enzyme content is lost, but other antioxidant components remain present and protect mitochondria, e.g. vitamin E in the membrane, mitochondrial glutathion, or antioxidant enzymes such as Mn-SOD.
Greater antioxidant protection in females is often reported in the literature and is related to gender dimorphism of the life span, in which females have a greater longevity than males (27 and 23 months, respectively, for Wistar rats) [Citation29]. In the same study, mitochondria from females were found to high levels of reduced glutathione than those from males. Oxidative damage to mitochondrial DNA was also fourfold higher in males than in females in hepatic mitochondria. Many studies have attributed the higher antioxidant protection in females to sex hormones, particularly the estrogen 17β-estradiol (also known as E2) [Citation30]. Indeed, estrogen would act by activating MAP kinase, which in turn would activate nuclear factor NF-κB, thereby inducing an increase in the expression of genes encoding antioxidant enzymes (SOD and Gpx). Moreover, Borrás et al. [Citation31] showed that estrogen, a lipophilic hormone that modifies membrane fluidity, increases the efficiency of the respiratory chain, thus increasing the membrane potential and decreasing hydrogen peroxide production in isolated mitochondria. E2 is also known to activate the expression of specific mitochondrial proteins and of genes controlling biogenesis [Citation32]. It would thus have protective effects on mitochondrial function against oxidative stress [Citation33].
Effects of exercise training
The most important finding here is that the exercise training protocol used induced more effects in male rats than in females.
Concerning mitochondrial function, both male and female rats showed changes in the respiration rate of permeabilized fibers but this took different forms. In males, the VO2 decreased under V0 conditions and conditions of maximal mitochondrial functioning (Vmax). ATP production (VATP) increased with a simultaneous decrease in •OH production [Citation16]. In females, the VO2 increased under Vmax conditions, and the other parameters remained stable.
These results show that exercise training leads to an improvement in mitochondrial function regardless of gender, but it is likely that different mechanisms are involved in males and females. Farhat et al. [Citation16] reported that the increased Vmax/V0 for VO2 in males suggested an improvement in oxidative phosphorylation. V0 is due to back leakage of protons into the mitochondrial matrix through other ion inner membrane channels rather than through F0–F1 ATPase. In females, the VATP/VO2 ratio under Vmax conditions was not modified because the ATP rate tended to increase. This means that muscle aerobic capacity increased without improvement in oxidative phosphorylation. Gender-based information on the training effect on skeletal muscle mitochondrial function is relatively scarce in the literature. Previous studies using female rats showed that training improved oxidative capacities. In female Sprague-Dawley rats, endurance training of about 20 days increased maximal mitochondrial respiration in the soleus muscle but not in the gastrocnemius muscle [Citation34]. In the same rat strain, Manabe et al. [Citation35] showed that training induces increased muscular glycogen content in female rats.
Exercise-induced oxidative stress may show some gender differences in humans and animals [Citation13]. In the present study, the effect of training on reactive oxygen species production also differed according to gender. The V•OH decreased in males after training, while production remained unchanged in females. This suggests that training would also reduce electron leakage in male mitochondria and therefore result in greater utilization efficiency of electrons for ATP production.
These results are consistent with enzymatic antioxidant responses to exercise training. No modification was seen in female rats, whereas SOD, CAT, and Gpx activity decreased in males. This decrease in male antioxidant activity, not often reported in the literature, was quite consistent in relation to the in vitro decrease in •OH production [Citation16]. It has been hypothesized that it is an adaptation to diminish ROS production related to training.
In contrast to males, the absence of increase in MDA in females reflects a well-regulated pro/antioxidant balance. Other studies have reported that endurance training induced a disturbance of the estrous cycle in female Sprague-Dawley rats [Citation36], which may cause a decrease in plasma estrogen output [Citation37]. This process is compensated for, however, by an increased expression of estrogen receptors (Erα or estrogen receptor alpha) in skeletal muscle, which maintains a constant estrogen effect [Citation38].
To our knowledge, the effect of gender on mitochondrial function sensitivity to ROS exposure after exercise training has never been addressed in the literature. Our results show that gender differences do exist. No change occurred in female rats, while the susceptibility of VO2(max) to ROS in male rats was significantly increased and that of VATP was significantly reduced [Citation16]. These results suggest that exercise training causes no alteration of mitochondrial function in females, but leads to better resistance of oxidative phosphorylation to ROS in males. Indeed, some compounds involved in mitochondrial function are known to be particularly sensitive to ROS, such as ATP/ADP translocase, F0–F1 ATP synthase, UCP2 and UCP3 [Citation20], and their sensitivity could be modified with training. It has therefore been suggested that non-enzymatic antioxidants could be involved in oxidative protection in trained male rats, because antioxidant enzymatic levels are lower than in sedentary rats [Citation16]. In females, the absence of change in mitochondrial function susceptibility to ROS after exercise training may be related to a protective effect of estrogen, as mentioned by Lemoine et al. [Citation38] or to non-enzymatic antioxidants, such as vitamins or reduced glutathione (GSH).
It is very difficult to compare our data with other studies due to the use of different protocols, strains or tissues, but some previous papers have also reported gender-dependent training effects. Balci and Pepe [Citation14] showed that swimming training led to a decrease in lipid peroxidation and an increase in GSH in the hearts of female Wistar rats but not in males. Another study showed the exact opposite, whereby moderate endurance training of 8 weeks induced an increase in lipid peroxidation in the hearts and skeletal muscle of female Sprague-Dawley rats. Finally, the effects of exercise training on myocardial tolerance to ischemia have been reported in Sprague-Dawley rats. In sedentary rats, female hearts showed greater cardioprotection against ischemia reperfusion injury than male hearts. As in the present study, exercise training was more beneficial to male hearts, with improved tolerance to ischemia, but no changes in the females [Citation39].
In conclusion, evidence from the present study suggests that gender is an important factor of changes in mitochondrial function. It also demonstrates, for the first time, gender-based differences in susceptibility to ROS exposure after endurance training. This confirms the importance of taking gender into account and not drawing conclusions from data that are usually obtained on males alone. Untrained female Wistar rats were shown to have mitochondrial function that was more resistant to oxidative stress than that of sedentary male rats. Although the training protocol improved mitochondrial function in both genders, the effects were overall greater and more beneficial in males with regard to mitochondrial energy efficiency, decreased radical production and greater resistance of mitochondrial function to ROS. The training protocol had less of an impact on the studied parameters in females but had no deleterious effects. The results may be related to estrogens acting as protective elements against oxidative stress but also to gender dimorphism of the lifespan.
Acknowledgements
We would like to thank the Bureau de Traduction de l'Université (BTU) of the University of Western Brittany, Brest, France, for their assistance with the improvement of the English in this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Firas Farhat http://orcid.org/0000-0002-3138-8162
Aline Amérand http://orcid.org/0000-0003-1589-875X
References
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95.
- Yaniv Y, Juhaszova M, Nuss HB, et al. Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives. Ann N Y Acad Sci. 2010;1188:133–142.
- Ventura-Clapier R, Mettauer B, Bigard X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc Res. 2007;73:10–18.
- Zoll J, Sanchez H, N’Guessan B, et al. Physical activity changes the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2002;543:191–200.
- Daussin FN, Rasseneur L, Bouitbir J, et al. Different timing of changes in mitochondrial functions following endurance training. Med Sci Sports Exerc. 2012;44:217–224.
- Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132.
- Nikolaidis MG, Kerksick CM, Lamprecht M, et al. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid Med Cell Longev. 2012; 2012:707941.
- Pepe H, Balci SS, Revan S, et al. Comparison of oxidative stress and antioxidant capacity before and after running exercises in both sexes. Gend Med. 2009;6:587–595.
- Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126.
- Colom B, Alcolea MP, Valle A, et al. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2007;19:205–212.
- Valle A, Català-Niell A, Colom B, et al. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab. 2005;289:E15–E22.
- Català-Niell A, Estrany ME, Proenza AM, et al. Skeletal muscle and liver oxidative metabolism in response to a voluntary isocaloric intake of a high fat diet in male and female rats. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2008;22:327–336.
- Stupka N, Lowther S, Chorneyko K, et al. Gender differences in muscle inflammation after eccentric exercise. J Appl Physiol Bethesda Md: 1985. 2000;89:2325–2332.
- Balcı SS, Pepe H. Effects of gender, endurance training and acute exhaustive exercise on oxidative stress in the heart and skeletal muscle of the rat. Chin J Physiol. 2012;55:236–244.
- Liu J, Yeo HC, Overvik-Douki E, et al. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol Bethesda Md: 1985. 2000;89:21–28.
- Farhat F, Dupas J, Amérand A, et al. Effect of exercise training on oxidative stress and mitochondrial function in rat heart and gastrocnemius muscle. Redox Rep. 2015;20:60–68.
- Mortelette H, Moisan C, Sébert P, et al. Fish as a model in investigations about the relationship between oxygen consumption and hydroxyl radical production in permeabilized muscle fibers. Mitochondrion. 2010;10:555–558.
- Amérand A, Vettier A, Sébert P, et al. In vitro effect of hydrostatic pressure exposure on hydroxyl radical production in fish red muscle. Redox Rep Commun Free Radic Res. 2005;10:25–28.
- Ouhabi R, Boue-Grabot M, Mazat JP. Mitochondrial ATP synthesis in permeabilized cells: assessment of the ATP/O values in situ. Anal Biochem. 1998;263:169–175.
- Cambier S, Bénard G, Mesmer-Dudons N, et al. At environmental doses, dietary methylmercury inhibits mitochondrial energy metabolism in skeletal muscles of the zebra fish (Danio rerio). Int J Biochem Cell Biol. 2009;41:791–799.
- Mortelette H, Amérand A, Sébert P, et al. Effect of exercise training on respiration and reactive oxygen species metabolism in eel red muscle. Respir Physiol Neurobiol. 2010;172:201–205.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflüg Arch Eur J Physiol. 2001;442:420–425.
- Lundsgaard A-M, Kiens B. Gender differences in skeletal muscle substrate metabolism – molecular mechanisms and insulin sensitivity. Front Endocrinol. 2014;5.
- Gómez-Pérez Y, Capllonch-Amer G, Gianotti M, et al. Long-term high-fat-diet feeding induces skeletal muscle mitochondrial biogenesis in rats in a sex-dependent and muscle-type specific manner. Nutr Metab. 2012;9:15.
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344.
- Borrás C, Sastre J, García-Sala D, et al. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552.
- Barp J, Araújo ASR, Fernandes TRG, et al. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz J Med Biol Res Rev. 2002;35:1075–1081.
- Borrás C, Gambini J, López-Grueso R, et al. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim Biophys Acta BBA - Mol Basis Dis. 2010;1802:205–211.
- Vina J, Gambini J, Lopez-Grueso R, et al. Females live longer than males: role of oxidative stress. Curr Pharm Des. 2011;17:3959–3965.
- Capllonch-Amer G, Sbert-Roig M, Galmés-Pascual BM, et al. Estradiol stimulates mitochondrial biogenesis and adiponectin expression in skeletal muscle. J Endocrinol. 2014;221:391–403.
- Burelle Y, Hochachka PW. Endurance training induces muscle-specific changes in mitochondrial function in skinned muscle fibers. J Appl Physiol Bethesda Md: 1985. 2002;92:2429–2438.
- Manabe Y, Gollisch KSC, Holton L, et al. Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content. FEBS J. 2013;280:916–926.
- Carlberg KA, Fregly MJ. Disruption of estrous cycles in exercise-trained rats. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1985;179:21–24.
- Mosavat M, Mohamed M, Mirsanjari MO. Effect of exercise on reproductive hormones in female athletes. Int J Sport Exerc Sci. 2013;5(1):7–12.
- Lemoine S, Granier P, Tiffoche C, et al. Effect of endurance training on oestrogen receptor alpha transcripts in rat skeletal muscle. Acta Physiol Scand. 2002;174:283–289.
- Thorp DB, Haist JV, Leppard J, et al. Exercise training improves myocardial tolerance to ischemia in male but not in female rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R363–R371.
