ABSTRACT
Objective: Recent studies have shown that cerebral ischaemia causes not only local, but also systemic oxidative stress. This leads to oxidation of thiol-containing compounds, including low-molecular-weight thiols (cysteine, glutathione, homocysteine and others). Therefore, the aim of this work was to verify the hypothesis that the thiol/disulphide homeostasis of low-molecular-weight thiols is disturbed in the early stages of cerebral ischaemia.
Methods: Two experimental rat models of ischaemia were used: a global model of vascular ischaemia (clamping the common carotid arteries + haemorrhage) and focal ischaemia (middle cerebral artery occlusion). The total levels of thiols and their reduced forms were measured before surgery and after 40 minutes of reperfusion (global) or 3 hours (focal) ischaemia.
Results: The global ischaemia model caused a marked (2.5–4 times, P < 0.01) decrease in the plasma thiol/disulphide redox state, and focal ischaemia caused an even larger decrease (30–80 times, P < 0.001).
Discussion: These results suggest that plasma low-molecular-weight thiols are actively involved in oxidation reactions at early stages of cerebral ischaemia; therefore, their reduced forms or redox state may serve as a sensitive indicator of acute cerebrovascular insufficiency.
Introduction
Oxidative stress plays an important role in the pathophysiology of cardiovascular diseases, particularly in cerebral ischaemia [Citation1–3]. Oxidative modifications of neurons are one of the key factors of their dysfunction and may lead to necrosis or apoptosis during ischaemia and cerebral reperfusion [Citation4,Citation5]. Furthermore, acute ischaemic injury causes systemic oxidative stress, which is associated with inflammatory responses in peripheral arteries [Citation3,Citation6]. As a result, blood plasma vasoconstrictor activity is elevated because of the massive release of reactive oxygen species (ROS) and cytokines [Citation5,Citation7,Citation8]. It has been suggested that this contributes to a hypertensive state and has negative effects on the course and outcome of stroke [Citation9,Citation10].
It has previously been shown that global cerebral ischaemia–reperfusion causes a rapid and continuous (up to 10–30 days) decrease in sulfhydryl (SH) groups and an increase in the products of lipid peroxidation in rat plasma [Citation11]. Concomitantly, the total content of thiol groups may not reflect the full dynamics and intensity of oxidative stress, because of the contribution of albumin, which has a lifetime of about 20 days and an abnormally low pK (∼5) for its free SH group [Citation12]. Despite the fact that a decrease in blood plasma SH groups has also been shown in clinical studies, and that, in some cases, its correlation with the severity, type, volume and outcome of stroke has been demonstrated, conclusions about the diagnostic and prognostic value of this parameter remain contradictory [Citation13–17].
Low-molecular-weight thiols (LMWTs: cysteine [Cys], glutathione [GSH], homocysteine [Hcy] and others) are part of the antioxidant system of blood plasma. They are in a state of dynamic equilibrium between the disulphide and reduced forms. The ratio of reduced form to the total aminothiol content represents its redox status (RS) [Citation18]. The disulphide forms are located predominantly in blood plasma, and the reduced fractions account for only 1–5% of the total aminothiol content in humans [Citation19]. LMWTs have a high turnover rate (1–2 minutes [Citation20,Citation21]) and exhibit reactivity with ROS. Thus, we assume that the LMWT redox status in the blood plasma may be a more sensitive indicator of oxidative stress levels than the total level of SH groups.
Therefore, the aim of this work was to verify the hypothesis that the thiol/disulphide homeostasis of LMWTs is disturbed in the early stages of cerebral ischaemia. We used two models of cerebral ischaemia: focal ischaemia (middle cerebral artery occlusion [MCAO]) and global ischaemia (bilateral occlusion of the common carotid arteries [BCAO]). Plasma total and reduced thiol levels (Cys, GSH and Hcy) were determined and compared with cerebral tissue thiols, hemodynamic changes and histological data.
Material and methods
Reagents and equipment
Acetonitrile Ultra Gradient (RCI Labscan, Poland) 99.9%; Iodoacetamide SigmaUltra, N-ethylmaleimide >98%, 5,5′-dithiobis(2-nitrobenzoic acid) or DTNB, trifluoroacetic acid 99.0% (Sigma-Aldrich, U.S.A); 5-sulfosalicylic acid dehydrate (Sigma, South Korea), L-Cys 97% (Aldrich, U.S.A), GSH 99% (Sigma-Aldrich, Japan); sodium citrate dehydrate (Sigma, U.S.A); NaH2PO4·2H2O 98–100.5%, Na2HPO4·2H2O 98–101% (Panreac, Spain); NaOH (Dia-M, Russia); EDTA disodium salt dihydrate 99% (Labteh, Russia); Chloral hydrate >99.5%, d-penicillamine or PA (Sigma, Germany), dl-dithiothreitol >99.5% (Fluka, India), dl-Hcy >95% (Sigma, UK), ethanol 96% (Reahim, Russia).
The Waters ACQUITY system (Waters, Milford, MA, U.S.A) equipped with a PDA λ UV detector (absorption, 330 nm; resolution, 10.8 nm; freq., 5 s−1) and an Agilent Poroshell 120 SB-C18 (2.8 µm, 150 × 2 mm) column (Agilent, Santa Clara, CA, U.S.A) were used for high-performance liquid chromatography (HPLC) analysis. The temperature of the column and samples was held at 50 and 10°C, respectively. A vacuum concentrator (Eppendorf Concentrator plus, Hamburg, Germany) was used for liquid extraction and sample preparation for HPLC.
Blood flow was recorded, and a spectral wavelet analysis of blood flow oscillations was performed using a LAKK-02 blood flow analyzer (version 2.2.0.507; LAZMA, Russia, Moscow), as described previously [Citation22].
Animals and operation technique
The experiment was performed on 36 adult (2–3 months of age) outbred white male rats weighing 260–300 g. The protocol used in the research project and all experimental protocols were approved by the Ethics Committee of the Federal State Budgetary Scientific Institution ‘Institute of General Pathology and Pathophysiology’.
The operations were performed under general anaesthesia (chloral hydrate given intraperitoneally at a dose of 300 mg/kg). To measure systemic blood pressure and to sample blood, both femoral arteries were isolated and cannulated (heparin was administered intra-arterially, 500 U/kg). To register the blood flow using a cylindrical sensor, a metal frame surrounding the craniotomy area was fixed to the skull as a means to hold the head of animal rigidly and the parietal bone was trephined (5 × 3 mm) without impairing the integrity of the dura mater (coordinates: AP, 5 mm; L, 3 mm). Cerebral blood flow recording was started 30 minutes after completion of all surgical procedures at an ambient temperature of 20–21°C.
MCAO
Cerebral focal ischaemia was induced using the MCAO model as described previously [Citation22], with some modifications. A surgical 4-00 polypropylene thread (22 mm long; Ethicon, U.S.A) treated with silicone and poly-l-lysine was inserted retrogradely into the left external carotid artery and then carried through the bifurcation of the common carotid artery and the internal carotid artery to the mouth of the middle cerebral artery to block it. Rats in the sham-operated control groups underwent the same operation without artery occlusion.
BCAO
Global ischaemia was induced as described previously [Citation23], with some modifications. Systemic blood pressure was reduced by 40–45 mm Hg by inducing haemorrhage (∼30% of blood volume, or 2.5 ± 0.2 ml/100 g of body weight). This was followed by bilateral occlusion of the common carotid arteries for 10 minutes and re-infusion of blood and removal of the carotid artery clips. In the control group, the procedure was performed similarly with the exception of blood loss and compression of blood vessels.
Blood and brain tissue samples
Control blood samples (1 ml) were obtained at the beginning of the operation and after 40 minutes of reperfusion (for BCAO) and 3 hours after MCAO. Venous blood was collected into tubes with sodium citrate and centrifuged at 3000×g for 3 minutes. The plasma for the total thiol assay was collected, frozen at −20°C, and stored until analysis. Plasma (100 µl) was added to 25 µl of 5-sulfosalicylic acid solution (230 g/l) immediately after isolation, for reduced LMWT analysis. The samples were mixed thoroughly, frozen and stored at −80°C.
Rats were decapitated after surgery and the brains were removed and promptly placed on ice for 5 minutes. Subsequently, forebrain slices (50–100 mg) were prepared and homogenized in acetonitrile (10 mkl of extragent per 1 mg of tissue). We used 20 mmol/l DTNB with 2.5 µmol/l PA and 1 mmol/l iodoacetamide solutions for reduced and oxidized thiol determination, respectively. Probes were centrifuged 5 minutes at 15,000×g and the supernatant was stored at −80°C.
Morphological examination
In anesthetized rats, the thorax was opened and perfusion of the brain was performed transcardially through the left ventricle using 20 ml of isotonic sodium chloride followed by 20 ml of 2.5% glutaraldehyde in phosphate-buffered saline. Tissues were sampled from the left primary somatosensory cortex cut at a distance of 1 mm from the bregma in the rostral direction (field S1FL) [Citation24]. Samples were embedded in epoxy resin. One-micrometre sections were cut with an ultramicrotome (Leica EM UC6, Austria), stained with 1% toluidine blue solution and 0.5% solution of borax and analyzed with an Olympus BX51 light microscope. Photography and image analysis were performed using the Imaging Software for Life Science Microscopy Cell F (Olympus, Japan). The sections for electron microscopy (50 nm) were obtained using an ultramicrotome (Leica EM UC6 Austria) and were contrasted using a Leica EM AC20 apparatus (Austria) with uranyl acetate and lead citrate solutions (Ultrastain no. 2, Lot 09/138, Laurylab, France). The sections were then analyzed using an electron microscope (Leo 912AB Omega, Germany).
Biochemical assay
Total plasma thiol levels were measured as described previously [Citation25]. Reduced thiol levels were measured as described previously [Citation26], with some modifications. Before derivatization, the samples were centrifuged for 5 minutes at 15000×g. Next, 40 µl of supernatant was mixed with 40 µl of 20 mmol/l DTNB with 2.5 µmol/l of PA (internal standard) in 0.4 mol/l Na-phosphate buffer (pH 8.0). Then, 10 µl of 1 mol/l NaOH was added, the solution was mixed for 5 seconds and 12.5 µl of 1 mol/l HCl with 20 mmol/l N-ethylmaleimide was added to stop the reaction.
For reduced thiol determination, 100 µl of brain tissue extract was evaporated (30 minutes, 60°C), and the pellet was re-suspended in the same volume of 0.1 mol/l Na-phosphate buffer (pH 7.4). For oxidized thiol determination, 10 µl of 50 mmol/l dithiothreitol and 10 µl of 250 µmol/l PA were added to 100 µl of brain extract. Probes were incubated at 37°C for 15 minutes, and then 200 µl of 25 mmol/l DTNB in 0.2 mol/l Na-phosphate buffer pH 8.0 was added.
The HPLC injection volume was 10 µl and the flow rate was 0.2 ml/minute. Eluent A was 0.1 mol/l NH4Ac with 0.12% (v/v) HCOOH, and eluent B was acetonitrile. Chromatography was performed using a linear gradient elution of B from 2.5 to 10% for 5 minutes. Regeneration was performed with 70% B for 1.5 minutes, and equilibration with 2.5% B for 4 minutes.
Data analysis
Primary processing of chromatograms (detection and integration of peak areas) was performed using MassLynx4.1 (Waters Milford, MA, U.S.A). Calibration data plotting and statistical analysis were performed using Microsoft Excel 2003. Where appropriate, data are presented as the mean with the variation expressed as standard deviation (±). Paired and unpaired t-tests were used to compare the results between initial/after ischaemia and between ischaemia/control groups, respectively. The comparison of group dispersions (homoscedasticity) was performed using the Fisher–Snedecor test at a significance level of α = 0.05. For analyses, a two-sided P value <0.05 was considered statistically significant.
Results
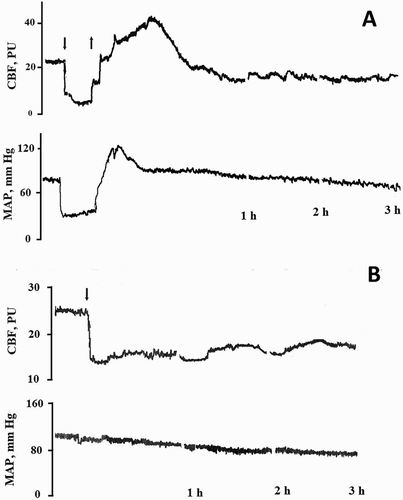
Changes in cerebral blood flow and mean arterial pressure (MAP) are shown in . Cerebral blood flow in the neocortical area declined by nearly half, from 33.4 ± 2.1 to 18.0 ± 1.0 perfusion units after 3 hours of MCAO (P < 0.001; n = 6). MAP declined slowly from 102.8 ± 1.6 to 83.2 ± 1.2 mmHg in this case. BCAO caused a greater decrease in cerebral blood flow and MAP, by about 70%, from 30.8 ± 1.9 to 8.9 ± 0.9 perfusion units and from 85.3 ± 1.2 to 43.4 ± 0.6 mm Hg, respectively (P < 0.001; n = 6).
Figure 1. Example of experimental curves of cerebral blood flow (CBF) and mean arterial pressure (MAP) at global (A) and focal (B) cerebral ischaemia in rats. Arrow down – the beginning of ischaemia, arrow up – the end of ischaemia.
Morphological examination in light and electron microscopy not found noticeable differences in the samples obtained from sham-operated animals in comparison with the samples after global and focal ischaemia ( and ).
Figure 2. Images of rat brain tissues. (A) Control (sham-operated); (B) bilateral occlusion of the common carotid arteries (BCAO); (C) middle cerebral artery occlusion (MCAO). The scale bar is 15 μm.
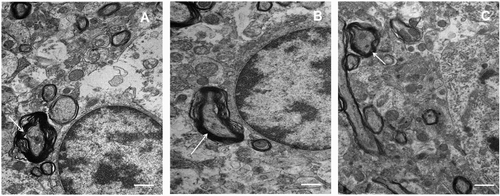
Figure 3. Electron microscopic examination of somatosensory cortex with global and focal ischaemia. Splitting myelin sheaths (arrow) is present in all samples. The scale bar is 2 μm. (A) Control (sham-operated); (B) bilateral occlusion of the common carotid arteries (BCAO); (C) middle cerebral artery occlusion (MCAO).
Brain tissue LMWT levels are presented in . The level of Hcy in the brain was too low to allow quantitative determination. As seen in , reduced GSH and RS of Cys were approximately equal in all groups. The reduction of GSH and RS of Cys was approximately equal in all groups. Oxidized GSH level was significantly increased after MCAO and BCAO; thus, its RS decreased by 5–7 times. During focal ischaemia, brain Cys levels increased by about 20–30%, but the RS remained unchanged.
Table 1. Brain tissue low-molecular-weight thiol (LMWT) levels (nmol/g).
Total and reduced plasma LMWT levels and their RS are shown in . No significant differences were observed in the total LMWT baseline levels between all groups. In addition, no differences were seen between the initial LMWT levels and the levels recorded after operations in the control groups. A small decrease in total Cys (20–30%, P < 0.01) and a less significant increase in total GSH levels were found after MCAO and BCAO. The variability of total GSH was increased significantly in both groups (ВСАО, 7–30 µmol/l; МСАО, 7.9–22 µmol/l).
Table 2. LMWTs in rat plasma before and after focal medial cerebral arterial occlusion and global cerebral ischaemia.
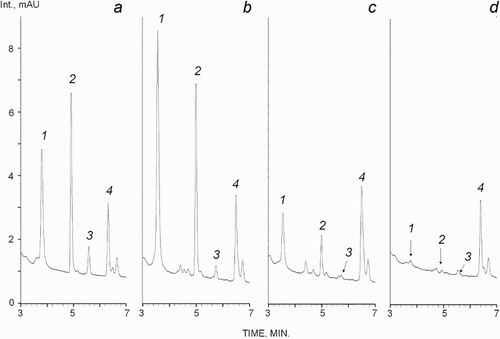
Reduced LMWT forms were decreased dramatically after BCAO and MCAO, but not in the control groups (). Typical chromatograms of plasma reduced thiols are shown in . Thus, changes in thiol RS were determined by their reduced (but not oxidized) forms in global and focal cerebral ischaemia. As shown in , thiol RS decreased markedly, by about 30–80 times, after MCAO. This effect was smaller, but still significant, in the global cerebral ischaemia model. Thiol RS was decreased 2–4.5 times on average in this case.
Figure 4. Chromatograms of reduced plasma thiols. Thiols are numbered: 1, cysteine (Cys); 2, glutathione (GSH); 3, homocysteine (Hcy); and 4, penicillamine (PA). The retention times were: 3.55 (Cys), 5.0 (GSH), 5.74 (Hcy) and 6.5 minutes (PA). (a) Model solution (Cys and GSH, 6.5 µmol/l; Hcy, 1.25 µmol/l; and PA; 2.5 µmol/l); (b) control rat blood plasma (Cys, 9.5 µmol/l; GSH, 5.4 µmol/l; Hcy, 0.33 µmol/l); (c) plasma after BCAO (Cys, 1.8 µmol/l; GSH, 0.94 µmol/l; Hcy, 0.1 µmol/l); (d) plasma after MCAO (Cys, 0.35 µmol/l; GSH, 0.12 µmol/l; Hcy, 0.05 µmol/l).
Discussion
Currently, there are many derivatization agents for LMWT determination [Citation27]. However, only a few of these are suitable for reduced thiol analysis. Previously, Katrusiak et al. [Citation26] proposed a simple and sensitive HPLC-UV method with DTNB derivatization. However, the reaction between DTNB and thiols leads to the formation of 2-nitro-5-thiobenzoate (TNB–), which slowly reacts with the oxidized forms of thiols. This can lead to non-reproducible results in blood plasma. To solve this problem, we used N-ethylmaleimide, which rapidly and irreversibly blocks the TNB–. Our preliminary experiments showed that this reagent significantly suppressed by-product reactions [Citation28].
According to the literature, the levels of total plasma LMWT in rats are 75–200 µmol/l (Cys), 5–22 µmol/l (GSH) and 3.2–14.4 µmol/l (Hcy) [Citation29–31]. It should be noted that age and diet (B9, fatty acid consumption) have a great impact on the total LMWT levels [Citation30,Citation32]. The levels of reduced forms of Cys, GSH and Hcy are about 17, 11 and >0.5 µmol/l, respectively, in rat plasma [Citation32,Citation33]. We obtained similar results, and the total level of Hcy was near the lower threshold, indicating the presence of a sufficient amount of folate in the diet of rats.
We used two models of acute cerebral ischaemia caused by hypoperfusion: a short-time (10 minutes) global and a continuous (3 hours) focal ischaemia. In the first case, ischaemia was caused by the combined effect of hypoperfusion and hypovolemia. As shown previously, the organism increases the production of antioxidants (ascorbic acid, uric acid) in response to brain ischaemia [Citation34]. The lowering of total plasma Cys and the uptrend of total GSH at MCAO and BCAO may be caused by the activation of GSH synthesis aimed at ROS neutralization. Concomitantly, the utilization of Cys increases. RS and reduced LMWT in blood plasma were dramatically decreased in both cases, but this effect was most pronounced in the case of focal ischaemia.
MCAO and BCAO induce a decrease in GSH RS in brain tissues. However, unlike what is observed in blood plasma, this effect is caused by the increase in oxidized GSH levels, rather than by the decrease in its reduced fraction. Cys RS does not occur in the brain. These results indicate that GSH homeostasis is disturbed in cerebral ischaemia; however, there is a temporary adaptation of its metabolism to hypoxia. More severe ischaemia models associated with the stopping of blood flow cause not only an increase in oxidized GSH, but also a decrease in its reduced fraction [Citation35–37].
In both cases, histological examination did not reveal characteristics of necrosis or apoptosis of neurons; this finding is consistent with previous studies [Citation38]. This indicates that the decrease in LMWT levels in plasma is connected to the brain response to ischaemia and precedes stroke formation. Apparently, this effect is driven by the activation of the sympathetic–adrenal system; i.e. it is based on mechanisms that are common in a wide range of systemic biochemical changes observed during brain injury.
It is obvious that the decline in reduced LMWT was associated with ROS release in the blood rather than with thiol redistribution between cells and plasma. The lack of correlation between the changes of thiols in plasma and brain in MCAO and BCAO suggests that different mechanisms are involved in the regulation of these redox systems. Previously, it was hypothesized that the main factor that contributes to reducing plasma SH is the formation of ROS in the brain tissue in the reperfusion phase [Citation11]. However, it was later shown that endothelial dysfunction of peripheral vessels plays the leading role in the generalization of oxidative stress during cerebral ischaemia [Citation6]. Key enzymes have been identified that are responsible for the production of ROS (cyclooxigenase-2, arginase and endothelial NO synthase) [Citation6–8]. Despite the fact that thiols are part of the antioxidant system, as noted above, their oxidation products are cytotoxic. In particular, Hcy and Cys acidic derivatives can activate neuronal and endothelium receptors, causing a so-called ischaemic cascade in neurons [Citation39,Citation40]. Therefore, the vasoactive and oxidative properties of acute stroke serum potentially contribute to the pathogenesis of reperfusion brain damage.
Thus, cerebral ischaemia induces rapid and significant changes in the LMWT thiol/disulphide homeostasis, not only in the area of ischaemia, but also in the systemic blood circulation. Despite the fact that the levels of reduced LMWTs in plasma do not reflect the intracellular concentration of reduced GSH and are not associated with organic neuronal damage, they were highly sensitive to cerebral ischaemia per se. This finding allows us to suggest the redox status of plasma LMWT as an early indicator of the global oxidative stress caused by cerebral ischaemia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr Alexander Vladimirovich Ivanov is a biochemist. He is an expert in oxidative stress in stroke. His field of research is aminothiols redox status. He has written over 20 articles in Russian and International scientific journals.
Dr Valery Vasil’evich Alexandrin is a pathophysiologist and has been working at the Institute of General Pathology and Pathophysiology since 1982. His field of research is the development and investigation of animal models of stroke. He has written over 60 articles in Russian and International scientific journals.
Dr Alexander Alexandrovich Paltsyn is a morphologist and histologist. He is the chief research scientist in the Department of Molecular and Cell Pathophysiology of the Institute of General Pathology and Pathophysiology. He has written over 90 articles in Russian and International scientific journals.
Ksenya Alexandrovna Nikiforova is a postgraduate student at the Institute of General Pathology and Pathophysiology, where she is gaining experience and undergoing practice connected with research methods used in medicine.
Dr Edward Danielevich Virus is an analytical chemist. His field of research is the development of analytical methods in the field of medical chromatography and mass spectrometry. He has written over 40 articles in Russian and International scientific journals.
Prof Boris Petrovich Luzyanin is the head of laboratory of metabolomics and functional proteomics at the Institute of General Pathology and Pathophysiology. He is an expert in analytical chemistry of aminothiols.
Prof Marina Yurievna Maksimova is a doctor of neurology. She is the head of Cerebrovascular Diseases Unit of Research Center of Neurology. She is an expert in oxidative stress in Cerebrovascular Diseases.
Prof Mikhail Aleksanrovich Piradov is a doctor of neurology, corresponding member of Russian academy of sciences, the director of Research Сenter of Neurology.
Prof Aslan Amirkhanovich Kubatiev is a doctor of medical sciences, professor, academician of the Russian Academy of Sciences (RAS) and Russian academy of medical sciences (RAMS), member of RAMS presidium, laureate of the RF State prize. He is the director and head of the department of molecular and cell pathophysiology at the Institute of general pathology and pathophysiology RAMS, chief of the department of general pathology and pathophysiology of Russian medical academy of postgraduate education of the RF Ministry of Health, chairman of RAMS Scientific Council for general pathology and pathophysiology. He is the president of Russian scientific society of pathophysiologists, chief editor of the journal ‘Pathogenesis’. He is a specialist in general, molecular and cell pathophysiology, nanobiology, nanopathology, physiology and pathology of haemostasis.
ORCID
Alexander Vladimirovich Ivanov http://orcid.org/0000-0002-2424-6115
Edward Danielevich Virus http://orcid.org/0000-0001-9371-6494
Mikhail Aleksanrovich Piradov http://orcid.org/0000-0002-6338-0392
Additional information
Funding
References
- Sanderson TH, Reynolds CA, Kumar R, et al. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013;47(1):9–23. doi: 10.1007/s12035-012-8344-z
- Frijhoff J, Winyard PG, Zarkovic N, et al. Clinical relevance of biomarkers of oxidative stress. Antioxod Redox Signal. 2015;23:1113–1129. doi: 10.1089/ars.2015.6430
- Bonaventura A, Liberale L, Vecchie A, et al. Update on inflammatory biomarkers and treatments in ischemic stroke. Int J Mol Sci. 2016;17(12):1967. doi: 10.3390/ijms17121967
- Lehotský J, Murín R, Strapková A, et al. Time course of ischemia/reperfusion-induced oxidative modification of neural proteins in rat forebrain. Gen Physiol Biophys. 2004;23(4):401–415.
- Dominguez C, Delgado P, Vilches A, et al. Oxidative stress after thrombolysis-induced reperfusion in human stroke. Stroke. 2010;41(4):653–660. doi: 10.1161/STROKEAHA.109.571935
- Martinez-Revelles S, Jimenez-Altayo F, Caracuel L, et al. Endothelial dysfunction in rat mesenteric resistance artery after transient middle cerebral artery occlusion. J Pharmacol Exp Ther. 2008;325(2):363–369. doi: 10.1124/jpet.107.134619
- Canavero I, Sherburne HA, Tremble SM, et al. Effects of acute stroke serum on non-ischemic cerebral and mesenteric vascular function. Transl Stroke Res. 2016;7(2):156–165. doi: 10.1007/s12975-016-0449-7
- Villalba N, Sackheim AM, Nunez IA, et al. Traumatic brain injury causes endothelial dysfunction in the systemic microcirculation through arginase-1-dependent uncoupling of endothelial nitric oxide synthase. J Neurotrauma. 2017;34(1):192–203. doi: 10.1089/neu.2015.4340
- Urrutia VC, Wityk RJ. Blood pressure management in acute stroke. Neurol Clin. 2008;26(2):565–583. doi: 10.1016/j.ncl.2008.02.002
- Varon J. Diagnosis and management of labile blood pressure during acute cerebrovascular accidents and other hypertensive crises. Am J Emerg Med. 2007;25(8):949–959. doi: 10.1016/j.ajem.2007.02.032
- Frassetto SS, Schetinger MR, Webber A, et al. Ischemic preconditioning reduces peripheral oxidative damage associated with brain ischemia in rats. Brazil J Med Bio Res. 1999;32(10):1295–1302.
- Sengupta S, Chen H, Togawa T, et al. Albumin thiolate anion is an intermediate in the formation of albumin-S–S-homocysteine. JBC. 2001;276(32):30111–30117. doi: 10.1074/jbc.M104324200
- Tsai NW, Hung SH, Huang CR. The association between circulating endothelial progenitor cells and outcome in different subtypes of acute ischemic stroke. Clin Chim Acta. 2014;427:6–10. doi: 10.1016/j.cca.2013.09.029
- Sapojnikova N, Asatiani N, Kartvelishvili T, et al. Plasma antioxidant activity as a marker for a favourable outcome in acute ischemic stroke. In: MA El-Missiry, editor. Antioxidant enzyme. Rijeka: InTech; 2012. p. 141–168.
- Içme F, Erel Ö, Avci A, et al. The relation between oxidative stress parameters, ischemic stroke, and hemorrhagic stroke. Turk J Med Sci. 2015;45:947–953. doi: 10.3906/sag-1402-96
- Musumeci M, Sotgiu S, Persichilli S, et al. Role of SH levels and markers of immune response in the stroke. Dis Markers. 2013;35(3):141–147. doi: 10.1155/2013/246205
- Bektas H, Vural G, Gumusyayla S, et al. Dynamic thiol-disulfide homeostasis in acute ischemic stroke patients. Acta Neurol Belg. 2016;116(4):489–494. doi: 10.1007/s13760-016-0598-1
- Ueland PM, Mansoor MA, Guttormsen AB, et al. Reduced, oxidized and protein-bound forms of homocysteine and other aminothiols in plasma comprise the redox thiol status – a possible element of the extracellular antioxidant defense system. J Nutr. 1996;126(4):1281–1284S.
- Williams RH, Maggiore JA, Reynolds RD, et al. Novel approach for the determination of the redox status of homocysteine and other aminothiols in plasma from healthy subjects and patients with ischemic stroke. Clin Chem. 2001;47(6):1031–1039.
- Wendel A, Cikryt P. The level and half-life of glutathione in human plasma. FEBS Letters. 1980;120(2):209–211. doi: 10.1016/0014-5793(80)80299-7
- Ookhtens M, Mittur AV, Erhart NA. Changes in plasma glutathione concentrations, turnover, and disposal in developing rats. Am J Physiol. 1994;266:979–988.
- Koizumi J. Experimental studies of ischemic brain edema, I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. J Stroke. 1986;8:1–8. doi: 10.3995/jstroke.8.1
- Grogaard B, Schurer L, Gerdin B, et al. Delayed hypoperfusion after incomplete forebrain ischemia in the rat. The role of polymorphonuclear leukocytes. J Cereb Blood Flow Metab. 1989;9(4):500–505. doi: 10.1038/jcbfm.1989.73
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. New York (NY): Elsevier; 2007.
- Ivanov AV, Virus ED, Luzyanin BP, et al. Capillary electrophoresis coupled with 1,1′-thiocarbonyldiimidazole derivatization for the rapid detection of total homocysteine and cysteine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1004:30–36. doi: 10.1016/j.jchromb.2015.09.036
- Katrusiak AE, Paterson PG, Kamencic H. Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl–glycine, homocysteine and glutathione in plasma and cell extracts. J Chromatogr B Biomed Sci Appl. 2001;758(2):207–212. doi: 10.1016/S0378-4347(01)00182-7
- Isokawa M, Kanamori T, Funatsu T, et al. Analytical methods involving separation techniques for determination of low-molecular-weight biothiols in human plasma and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:103–115. doi: 10.1016/j.jchromb.2013.12.041
- Kubatiev AA, Ivanov AV, Luzyanin BP. Analytical methods of homocysteine determination. Moscow: Nauka; 2016. p. 63–66.
- Iciek M, Chwatko G, Lorenc-Koci E, et al. Plasma levels of total, free and protein bound thiols as well as sulfane sulfur in different age groups of rats. Acta Biochim Pol. 2004;51(3):815–824.
- Wilson FA, Holtrop G, Calder AG, et al. Effects of methyl-deficient diets on methionine and homocysteine metabolism in the pregnant rat. Am J Physiol Endocrinol Metab. 2012;302(12):E1531–E1540. doi: 10.1152/ajpendo.00668.2011
- Yeh YY, Yeh SM. Homocysteine-lowering action is another potential cardiovascular protective factor of aged garlic extract. J Nutr. 2006;136(3):745–749.
- Nkabyo YS, Gu LH, Jones DP, et al. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J Nutr. 2006;136(5):1242–1248.
- Priora R, Summa D, Frosali S, et al. Administration of minor polar compound-enriched extra virgin olive oil decreases platelet aggregation and the plasma concentration of reduced homocysteine in rats. J Nutr. 2008;138(1):36–41.
- Sivonová M, Kaplán P, Duracková Z, et al. Time course of peripheral oxidative stress as consequence of global ischaemic brain injury in rats. Cell Mol Neurobiol. 2008;28(3):431–441. doi: 10.1007/s10571-007-9246-x
- Uemura Y, Miller JM, Matson WR, et al. Neurochemical analysis of focal ischemia in rats. Stroke. 1991;22(12):1548–1553. doi: 10.1161/01.STR.22.12.1548
- Cooper AJ, Pulsinelli WA, Duffy TE. Glutathione and ascorbate during ischemia and postischemic reperfusion in rat brain. J Neurochem. 1980;35(5):1242–1245. doi: 10.1111/j.1471-4159.1980.tb07882.x
- Park EM, Choi J-H, Park JS, et al. Measurement of glutathione oxidation and 8-hydroxy-2′-deoxyguanosine accumulation in the gerbil hippocampus following global ischemia. Brain Res Protoc. 2000;6:25–32. doi: 10.1016/S1385-299X(00)00033-7
- Persson L, Hardemark HG, Bolander HG, et al. Neurologic and neuropathologic outcome after middle cerebral artery occlusion in rats. Stroke. 1989;20(5):641–645. doi: 10.1161/01.STR.20.5.641
- Santhosh-Kumar CR, Deutsch JC, Kolhouse JC, et al. Measurement of excitatory sulfur amino acids, cysteine sulfinic acid, cysteic acid, homocysteine sulfinic acid, and homocysteic acid in serum by stable isotope dilution gas chromatography–mass spectrometry and selected ion monitoring. Anal Biochem. 1994;220(2):249–256. doi: 10.1006/abio.1994.1335
- Shi Q, Savage JE, Hufeisen SJ, et al. L-homocysteine sulfinic acid and other acidic homocysteine derivatives are potent and selective metabotropic glutamate receptor agonists. J Pharmacol Exp Ther. 2003;305(1):131–142. doi: 10.1124/jpet.102.047092
