ABSTRACT
Objective: Here we investigated the impact of chronic high-intensity interval training (HIIT) and caffeine consumption on the activities of Na+-K+-ATPase and enzymes of the antioxidant system, as well as anxiolytic-like behaviour in the rat brain.
Methods: Animals were divided into groups: control, caffeine (4 mg/kg), caffeine (8 mg/kg), HIIT, HIIT plus caffeine (4 mg/kg) and HIIT plus caffeine (8 mg/kg). Rats were trained three times per week for 6 weeks, and caffeine was administered 30 minutes before training. We assessed the anxiolytic-like behaviour, Na+-K+-ATPase, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities, levels of reduced glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) in the brain.
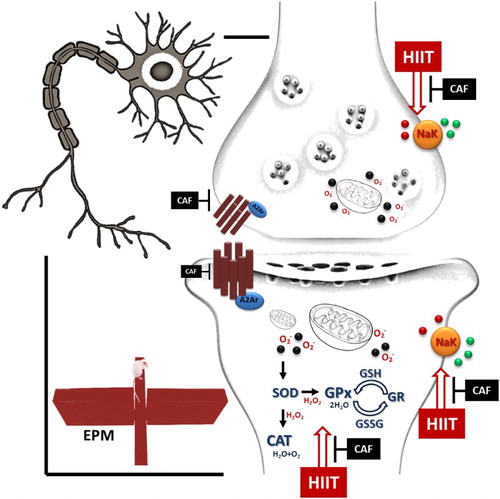
Results and discussion: HIIT-induced anxiolytic-like behaviour increased Na+-K+-ATPase and GPx activities and TBARS levels, altered the activities of SOD and CAT in different brain regions, and decreased GSH levels. Caffeine, however, elicited anxiogenic-like behaviour and blocked HIIT effects. The combination of caffeine and HIIT prevented the increase in SOD activity in the cerebral cortex and GPx activity in three brain regions. Our results show that caffeine promoted anxiogenic behaviour and prevented HIIT-induced changes in the antioxidant system and Na+-K+-ATPase activities.
Introduction
Healthier lifestyles are frequently associated with the regular practice of physical exercise, which disrupts cellular homeostasis by stimulating muscular activity [Citation1]. Researchers have sought to elucidate the benefits of high-intensity interval training (HIIT), a training characterized by brief periods (around 30 seconds) of extreme stress (≥90–100% of VO2 max) intercalated with short periods of recovery [Citation2]. HIIT exercise affects all body systems, increasing muscle glycogen levels [Citation3], aerobic capacity and muscle hypertrophy [Citation4], and also improves brain functions [Citation5]. Studies show that HIIT increases the levels of brain-derived neurotrophic factor (BDNF) and insulin receptors, improving glucose uptake and metabolism [Citation6,Citation7,Citation8]. Moreover, HIIT has been shown to improve blood flow in the brain [Citation9] and activate signalling pathways that promote adult hippocampal neurogenesis [Citation10].
Na+-K+-ATPase plays a key role in the maintenance of intracellular electrolyte homeostasis in virtually all tissues [Citation11]. Reduction of Na+-K+-ATPase levels and activity directly impairs neurotransmitter signalling with deleterious consequences on learning and memory and increases locomotor activity and anxiety [Citation12,Citation13]. Impairment of Na+-K+-ATPase activity and/or mutations in its alpha subunits lead to neuronal dysfunction and may trigger depression, anxiety and bipolar disease [Citation14,Citation15,Citation16].
Intake of ergogenic substances has become a common strategy to enhance sports performance. Caffeine is an alkaloid compound with psychostimulant effects that is mainly consumed through tea and coffee [Citation17], and that acts as a non-selective adenosine receptor antagonist [Citation18], with antioxidant [Citation19,Citation20] and neuroprotective effects [Citation21–24]. In recent decades, athletes have used caffeine to improve their performance [Citation25–27], and several studies have suggested that intake of 3–9 mg/kg of caffeine can slow the process of fatigue during long-term exercise, prolonging activity by 20–50% [Citation27–30]. However, caffeine doses greater than 15 mg/kg trigger several undesired effects, including anxiety, irritability, tachycardia, nausea and seizures [Citation31,Citation32].
Considering that HIIT has beneficial effects on the central nervous system (CNS), we investigated the role of caffeine in combination with HIIT training in anxiolytic-like behaviour and in the activity of Na+-K+-ATPase and oxidative stress biomarkers in the rat brain.
Materials and methods
Animals
Male Wistar rats (100 days old; 250–280 g) from the Central Animal House of the Federal University of Santa Maria (UFSM) were maintained at a constant temperature (23 ± 1°C) on a 12-hour light/dark cycle with free access to food and water. All animal procedures were approved by the Animal Ethics Committee from the UFSM (077/2011).
Protocol of HIIT training
We used swimming as an HIIT exercise. In the first 5 days, all rats were put in shallow water (5 cm deep, 32°C) for 20 minutes before beginning the training. The objective was to give the animals time to adapt to the new environment and reduce their stress without, however, promoting adaptation to the training. After this procedure, animals were put in a circular tank (115 cm diameter, 90 cm depth) with water (32°C) for 12 minutes per day for 10 consecutive days (, ).
Figure 1. Experimental protocol for chronic high-intensity interval training (HIIT) with caffeine intake (4 and 8 mg /kg). Adaptation occurred between day −10 to 0 (with a gradual increase in workload to 10.5% body weight). On day 2 we measured blood lactate content (LT). On day 3 HIIT was started, three times a week, with a 48 hours interval between each session. Workload (%) can be found in the timeline. The last HIIT session was performed at day 45. The elevated plus maze task was performed 48 hours later.
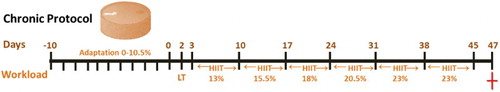
Table 1. High-intensity interval training (HIIT) protocol.
Chronic protocol for HIIT and caffeine intake
Forty-eight rats from different litters were divided into six groups: vehicle, caffeine (4 mg/kg), caffeine (8 mg/kg), HIIT, HIIT plus caffeine (4 mg/kg) and HIIT plus caffeine (8 mg/kg). The exercise groups were trained three times a week (with 48 hours of recovery between sessions) for 6 weeks, with increases in the workload corresponding to 23% of the body weight by the end of the experiment. The animals were submitted to a HIIT protocol consisting of 12 sets of swimming exercises of 25 seconds each, alternated with 35 seconds of recovery [Citation33–35]. Non-trained groups were placed in shallow water at 32 ± 1°C for 12 minutes three times per week.
Treatment with caffeine started after the adaptation to HIIT. Caffeine was dissolved in a 0.9% saline solution (1 ml/kg) and was orally administered 30 minutes before training, 5 days per week at a dose of 4 or 8 mg/kg. The vehicle and HIIT groups received saline. Caffeine was administered for 6 weeks until 48 hours after the last training. After behavioural testing, animals were submitted to euthanasia.
Elevated plus maze task
Anxiolytic-like behaviour was evaluated using the elevated plus maze task [Citation36]. The apparatus consisted of a structure 50 cm above the floor, with four arms of the same size, two of them closed (walls 40 cm) and two open. Initially, each animal was placed on the central platform of the maze in front of an open arm and was given 5 minutes to explore the apparatus. We then recorded the time spent and the number of entries in the centre, open and closed arms, the number of faecal pellets and head dips. The apparatus was thoroughly cleaned with 30% ethanol between each session. The analyses of the elevated plus maze parameters were expressed on average by three blinded researchers to the treatment of animals.
Sample preparation for biochemical analysis
Cerebral cortex, hippocampus and striatum were separated and homogenized in a solution of 10 mM Tris–HCl, 0.1 mM EDTA, pH 7.4, as previously described [Citation37,Citation38]. After centrifugation at 1500g at 4°C for 15 minutes, the supernatant was stored at −80°C until further use.
Na+-K+-ATPase activity
Na+-K+-ATPase activity was measured in the supernatant as previously described [Citation39,Citation40]. The composition of the assay medium was 40 mM Tris–HCl buffer (pH 7.4), 0.1 mM EDTA, 50 mM NaCl, 5 mM KCl and 6 mM MgCl2. Enzyme activity was evaluated in the presence or absence of ouabain (4 mM). The reaction was started by adding ATP (3 mM), and after 30 minutes at 37°C, the reaction was stopped with TCA. The amount of inorganic phosphate (Pi) released was colorimetrically quantified [Citation41]. Na+-K+-ATPase was expressed in nmol of Pi/mg of protein/min.
Superoxide dismutase (SOD) activity
SOD activity was measured in the supernatant with a method based on the autoxidation reaction of adrenaline to adenochrome [Citation42]. Results were expressed as U SOD/mg of protein. One SOD unit was defined as the enzyme amount causing a 50% inhibition of adrenaline autoxidation.
Catalase (CAT) activity
CAT activity was carried out as previously described [Citation43,Citation44]. Activity was determined by following the decomposition of H2O2 The specific activity was reported as units per mg protein. One unit of the enzyme is defined as 1 nmol of H2O2 consumed per minute.
Glutathione peroxidase (GPx) activity
GPx activity was measured using a commercial kit (RANSEL®; Randox Lab, Antrim, United Kingdom). GPx activity was determined in the supernatant using glutathione reductase and NADPH. This method is based on the oxidation of NADPH, indicated by a decrease in the absorbance at 340 nm. Enzymatic activity was expressed as µmol NADPH/min/mg of protein.
Glutathione reduced (GSH) levels
Reduced glutathione (GSH) was determined in the supernatant as previously described [Citation45]. Results were expressed as μmol of GSH/mg of protein.
Thiobarbituric acid reactive substances (TBARS) measurement
TBARS levels were analyzed in the homogenate by a method previously described [Citation46] and slightly modified [Citation47]. The reaction mixture contained 200 μl of sample or standard (MDA, malondialdehyde 0.03 mM), 200 μl of 8.1% sodium dodecylsulphate, 750 μl of acetic acid solution (2.5 M HCl, pH 3.5) and 750 μl of 0.8% TBA. Reaction mixtures were heated at 95°C for 90 minutes, and the absorbance was measured at 532 nm. Results were expressed as µmol MDA/mg of protein.
Statistical analysis
Results were analyzed by one or two-way analysis of variance (ANOVA), followed by the Tukey post hoc test (Graph Pad Prism 5.0). Differences between groups were considered to be significant when P < 0.05. Data were expressed as mean ± standard error medium (SEM).
Results and discussion
HIIT induces anxiolytic-like behaviour and caffeine blocks this effect
Anxiety disorders are the most common mental illness in the general population, with a prevalence of approximately 25% [Citation48,Citation49]. Clinical symptoms are often accompanied by cognitive impairment, suggesting that interactions between the affective state and cognition may underlie the debilitating nature of pathological anxiety [Citation50]. Physical activity has been proposed as an alternative to improve mental health due to its beneficial effects on anxiety, depression and cognition [Citation50,Citation51]. Our results show that chronic HIIT induced an anxiolytic-like behaviour in rats, as it decreased the number of entries in the closed arms (F2,49 = 3.921, P < 0.001, (A)) and increased the number of entries in the open arms (F2,49 = 3.799, P < 0.001, (B)) of the elevated plus maze. Caffeine per se (4 and 8 mg/kg) did not change the number of entries in both the open and closed arms but prevented HIIT-induced anxiolytic-like behaviour ((A,B)). Furthermore, both doses of caffeine (without HIIT) increased the time spent in the closed arm (F2,49 = 2.048, P < 0.001, (C)) and reduced that in the open arms (F2,49 = 0.3178, P < 0.001, (D)). HIIT prevented caffeine-induced anxiogenic-like behaviour induced by increasing the time spent in the open arms ((D)).
Figure 2. Effects of chronic high-intensity interval training (HIIT) and caffeine (4 and 8 mg/kg) in the elevated plus maze task. (A) Number of closed-arm entries, (B) number of open-arm entries, (C) time spent in the closed arms, (D) time spent in the open arms, (E) time spent in the centre and (F) total number os entries to arms. Data are expressed as mean ± SEM. P < 0.05 represents a significant difference. * Indicates significant difference compared to the vehicle group. # indicates significant difference compared to the HIIT group (ANOVA one-way followed by post hoc Tukey, n = 8–10).
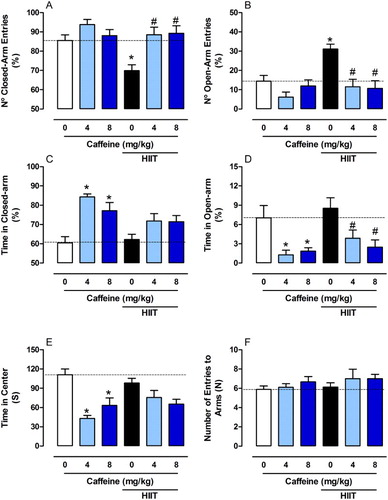
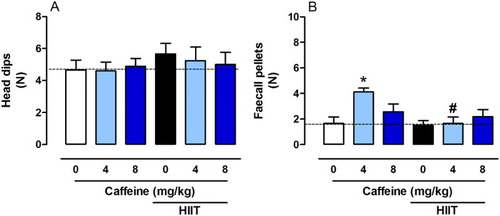
The anxiogenic behaviour induced by caffeine (either 4 or 8 mg/kg) without HIIT was also demonstrated by a reduction in the time spent in the centre (F2,49 = 1.713, P < 0.001, (E)). No significant differences were observed between groups in the number of crossings between the arms (F2,49 = 0.222, P > 0.05, (F)) and in head dips between groups (F2,49 = 0.2360, P > 0.05, (A)). Caffeine (4 mg/kg) increased the number of faecal pellets. However, when caffeine was associated with exercise, this effect was not observed (F2,49 = 3.464, P < 0.05, (B)). Caffeine intake showed an opposite effect to HIIT by inducing an anxiogenic-like behaviour, blocking the beneficial effects of HIIT in anxiety. Our findings are in line with previous studies showing the psychostimulant effect of caffeine in anxiety [Citation52–54].
Figure 3. Effects of chronic high-intensity interval training (HIIT) and caffeine (4 and 8 mg/kg) on behaviour in the elevated plus maze task. (A) Head dips and (B) Number of faecal pellets. Data are expressed as mean ± SEM. P < 0.05 represents a significant difference. * Indicates significant difference compared to the vehicle group. # indicates significant difference compared to the HIIT group (ANOVA one-way followed by post hoc Tukey, n = 8–10).
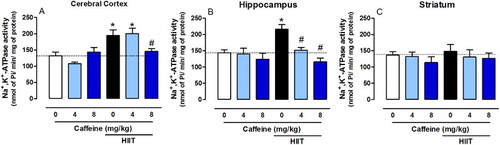
Caffeine prevents the increase in Na+-K+-ATPase activity induced by HIIT
Na+-K+-ATPase activity is inhibited in various neuropathological conditions [Citation14,Citation55,Citation56]. Indeed, impairment of ion homeostasis, triggered by reduced Na+-K+-ATPase activity, is found in patients with depression [Citation57] and the inhibitor ouabain-induced symptoms of bipolar disorder in rats [Citation58]. Reduced Na+-K+-ATPase activity has also been associated with increased anxiety [Citation13,Citation59]. In our study, HIIT increased Na+-K+-ATPase activity in the cerebral cortex (F2,24 = 7.252, P < 0.001, (A)), hippocampus (F2,24 = 5.282, P < 0.01, (B)) and striatum (F2,24 = 0.1093, P < 0.01, (C)), whereas caffeine (8 mg/kg) prevented this effect in the cerebral cortex and caffeine (either 4 or 8 mg/kg) prevent this effect in the hippocampus.
Figure 4. Effects of chronic high-intensity interval training (HIIT) and caffeine (4 and 8 mg/kg) in Na+-K+-ATPase activity in the cortex (A), hippocampus (B) and striatum (C). Data are expressed as mean ± SEM. P < 0.05 represents a significant difference. * Indicates significant difference compared to the vehicle group. # indicates significant difference compared to the HIIT group (ANOVA one-way followed by post hoc Tukey, n = 6–8).
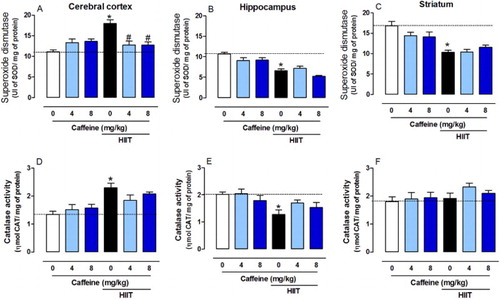
Chronic HIIT alters SOD and CAT activity
Considering that brain is highly sensitive to reactive oxygen species (ROS) and that Na+-K+-ATPase activity is known to be affected by the redox state of the cell [Citation40], we analyzed the activity of key antioxidant enzymes. HIIT increased SOD activity in the cerebral cortex (F2,39 = 6.89, P < 0.01, (A)) and both caffeine doses prevented this effect. However, HIIT decreased SOD activity in the hippocampus (F2,39 = 0.7495, P < 0.001, (B)) and striatum (F2,39 = 0.7495, P < 0.001, (C)). HIIT also increased the CAT activity in the cerebral cortex (F2,39 = 5.138, P < 0.001, (D)) but reduced it in the hippocampus (F2,39 = 1.207, P < 0.01, (E)), and caffeine was not able to prevent these effects. No significant differences were observed in the striatum among groups (F2,39 = 0.460, P > 0.05, (F)). Studies have shown that caffeine intake provides neuroprotection against several disorders such as Alzheimer’s and Parkinson’s diseases [Citation60–62]. This may be due to A2A receptor modulation, as previous work shows that blocking these receptors reduces ROS production and cell death [Citation63,Citation64].
Figure 5. Effects of chronic high-intensity interval training (HIIT) and caffeine (4 and 8 mg/kg) in superoxide dismutase (SOD) and catalase (CAT) activities. SOD activity in the cortex (A), hippocampus (B) and striatum (C). CAT activity in the cortex (D), hippocampus (E) and striatum (F). Data are expressed as mean ± SEM. P < 0.05 represents a significant difference. * Indicates significant difference compared to the vehicle group. # indicates significant difference compared to the HIIT group (ANOVA one-way followed by post hoc Tukey, n = 6–8).
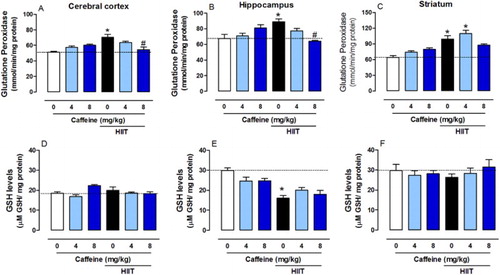
Caffeine prevents HIIT-induced changes in GPx activity
HIIT increased GPx activity in the cerebral cortex (F2,39 = 16.64, P < 0.0001), hippocampus (F2,39 = 10.12, P < 0.001) and striatum (F2,39 = 5.679, P < 0.0001) and caffeine (8 mg/kg but not 4 mg/kg) prevented this effect in the cerebral cortex and hippocampus ((A–C)). HIIT reduced the GSH content in the hippocampus, which caffeine did not restore (F2,39 = 3.245, P < 0.0001, (E)). There were no significant differences between groups in the cerebral cortex (F2,39 = 4.967, P > 0.05) and striatum (F2,39 = 0.8749, P > 0.05) ((D,F)).
Figure 6. Effects of chronic high-intensity interval training (HIIT) and caffeine (4 and 8 mg/kg) in glutathione peroxidase (GPx) activity and in reduced glutathione levels (GSH). GPx activity in the cortex (A), hippocampus (B) and striatum (C). GSH levels in the cortex (D), hippocampus (E) and striatum (F). Data are expressed as mean ± SEM. P < 0.05 represents a significant difference. * Indicates significant difference compared to the vehicle group. # indicates significant difference compared to the HIIT group (ANOVA one-way followed by post hoc Tukey, n = 6–8).
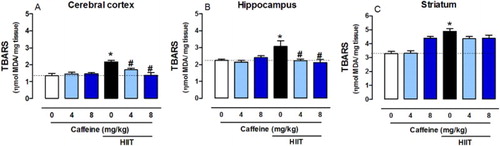
Caffeine prevents HIIT-induced increases in TBARS levels
HIIT increased TBARS levels in the cerebral cortex (F2,36 = 8.8416, P < 0.001), hippocampus (F2,36 = 5.383, P < 0.01) and striatum (F2,36 = 9.704, P < 0.01). Caffeine (4 and 8 mg/kg) was able to prevent this alteration only in the cerebral cortex and hippocampus ((A–C)).
Figure 7. Effects of chronic high-intensity interval training (HIIT) and caffeine (4 and 8 mg/kg) in malondialdehyde (MDA) levels in the cortex (A), hippocampus (B) and striatum (C). Data are expressed as mean ± SEM. P < 0.05 represents a significant difference. * indicates significant difference compared to the vehicle group. # Indicates significant difference compared to the HIIT group (ANOVA one-way followed by post hoc Tukey, n = 6–8).
Neurochemical and behavioural changes induced by chronic HIIT and caffeine intake
HIIT decreased anxiety and increased the activity of antioxidant enzymes, we observed an increase in TBARS levels, which suggests lipoperoxidation. In the brain, the polyunsaturated fatty acid high content and increased oxygen use account for the susceptibility to free radical damage. Chronic HIIT induces an adaptive response in the brain redox system by stimulating the activity of the antioxidant enzymes SOD, CAT and GPx. It is possible that HIIT-induced increase in cerebral blood flow leads to an increase in tissue oxygen supply and glycolytic metabolism, resulting in elevated production of ROS in the mitochondria. Physical exercise also induces an increase in the number of mitochondria, thereby promoting ROS production. In this context, improvement of the antioxidant enzyme activity can be an adaptive response induced by HIIT. Also, previous work has shown that HIIT increases BDNF and GDNF in the rat brain, which is associated with higher concentrations of H2O2 and TNF-α, produced during exercise [Citation65]. After HIIT, an increase in the hippocampal TNFα and BDNF levels have also been reported as being associated with oxidative stress, especially H2O2 levels, and the hypoxic condition during exercise [Citation65,Citation66]. These reports can explain the neurochemical alterations in the enzymatic activity of the antioxidant system, which may be a response to increased H2O2 production. Since caffeine is a neuroprotective and antioxidant molecule, chronic caffeine intake prevented ROS production and the adaptive response of the redox system induced by chronic HIIT. It is interesting to note that different responses in the redox state in the brain regions can be associated with: (i) the regional differences observed in the population of glutamatergic neurons, especially of the NMDAr type, since an imbalance in the Ca+ currents evoked by these receptors induce a series of neurotoxic events; (ii) the distribution across the neuroaxis may also be important because hydrophilic compounds, such as ascorbic acid, present rapid and widespread distributions in the CNS in rodent models, including substantial penetration into brain parenchyma, as well as cerebrospinal fluid bordering regions [Citation67]. Moreover, in the rat brain, the cerebral cortex has a lower basal level of activity of some antioxidant enzymes, such as SOD, CAT and GPx [Citation68,Citation69].
We conclude that HIIT induces changes in antioxidant enzymes and increases Na+-K+-ATPase activity and anxiolytic-like behaviour (). It is not clear whether caffeine has a beneficial or harmful role when combined with HIIT, since it prevented the effects induced by physical exercise. Differences in metabolism, diet, the frequency of caffeine intake and type of exercise are factors that can determine how each individual will react to combinations of caffeine and HIIT.
Acknowledgements
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Fundação de Amparo à Pesquisa do Rio Grande do Sul (DOCFIX/CAPES-FAPERGS).
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributor
Juliano M. Vieira, a PhD student in biochemistry, investigates the protocols of high intermittent intensity exercise (HIIT), and is a researcher in the area of physical performance and supplementation with ergogenic compounds.
Fabiano B. Carvalho, a PhD in biochemistry, investigates nootropic and neuroprotective properties of natural or synthetic compounds in experimental models of neurodegenerative diseases.
Jessié M. Gutierres, a PhD, is a researcher with experience in endurance exercise training, aging and experimental models for neurodegenerative diseases.
Mayara S. P. Soares, a PhD student in Biochemistry, investigates the oxidative stress parameters in neurological and metabolic disorders.
Pathise S. Oliveira, a PhD student in biochemistry, has experience in the area of metabolic diseases, natural compounds and oxidative stress in the brain.
Maribel A. Rubin, a PhD, is a researcher with experience in the field of pharmacology, with emphasis on neuropsychopharmacology, acting mainly on the following topics: memory, polyamines, and analgesia.
Vera M. Morsch, a PhD, is researcher with experience in the field of pharmacology, with emphasis on toxicology, acting mainly in the following subjects: NTPDase, 5-nucleotidase and acetylcholinesterase.
Maria Rosa Schetinger, a PhD, is a researcher with experience in the field of pharmacology, with emphasis on enzymes, acting mainly in the following subjects: NTPDase, 5-nucleotidase and acetylcholinesterase.
Roselia Maria Spanevello, a PhD, is a researcher with experience in the field of biochemistry, with emphasis on enzymology. It works with the enzymes of the purinergic system, oxidative stress and cholinergic parameters in experimental models and in human pathologies.
ORCID
Juliano M. Vieira http://orcid.org/0000-0002-8619-9502
Fabiano B. Carvalho http://orcid.org/0000-0002-7927-1342
Jessié M. Gutierres http://orcid.org/0000-0002-9574-4007
Mayara P. Soares http://orcid.org/0000-0002-7047-643X
Pathise S. Oliveira http://orcid.org/0000-0002-8321-7402
Maribel A. Rubin http://orcid.org/0000-0002-2763-9619
Vera M. Morsch http://orcid.org/0000-0002-5381-4556
Maria Rosa Schetinger http://orcid.org/0000-0002-5240-8935
Roselia M. Spanevello http://orcid.org/0000-0002-5117-2000
References
- Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol. 2006;97:643–663. doi: 10.1007/s00421-006-0238-1
- Gibala MJ, Little JP, van Essen M, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901–911. doi: 10.1113/jphysiol.2006.112094
- Sano A, Koshinaka K, Abe N, et al. The effect of high-intensity intermittent swimming on post-exercise glycogen supercompensation in rat skeletal muscle. J Physiol Sci. 2012;62:1–9. doi: 10.1007/s12576-011-0170-y
- Osawa Y, Azuma K, Tabata S, et al. Effects of 16-week high-intensity interval training using upper and lower body ergometers on aerobic fitness and morphological changes in healthy men: a preliminary study. Open Access J Sports Med. 2014;5:257–265. doi: 10.2147/OAJSM.S68932
- Matsui T, Ishikawa T, Ito H, et al. Brain glycogen supercompensation following exhaustive exercise. J Physiol. 2012;590:607–616. doi: 10.1113/jphysiol.2011.217919
- Saucedo Marquez CM, Vanaudenaerde B, Troosters T, et al. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol. 2015;119:1363–1373. doi: 10.1152/japplphysiol.00126.2015
- Tonoli C, Heyman E, Roelands B, et al. BDNF, IGF-I, glucose and insulin during continuous and interval exercise in Type 1 diabetes. Int J Sports Med. 2015;36:955–959. doi: 10.1055/s-0035-1548886
- Cardoso AM, Bagatini MD, Martins CC, et al. Exercise training prevents ecto-nucleotidases alterations in platelets of hypertensive rats. Mol Cel Biochem. 2012;371:147–156. doi: 10.1007/s11010-012-1431-7
- Lucas SJ, Cotter JD, Brassard P, et al. High-intensity interval exercise and cerebrovascular health: curiosity, cause, and consequence. J Cerebr Blood Flow Met. 2015;35:902–911. doi: 10.1038/jcbfm.2015.49
- Nokia MS, Lensu S, Ahtiainen JP, et al. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol. 2016;594:1855–1873. doi: 10.1113/JP271552
- Skou JC, Esmann M. The Na,K-ATPase. J Bioenerg Biomembr. 1992;24:249–261.
- Dos Reis EA, de Oliveira LS, Lamers ML, et al. Arginine administration inhibits hippocampal Na(+),K(+)-ATPase activity and impairs retention of an inhibitory avoidance task in rats. Brain Res. 2002;951:151–157. doi: 10.1016/S0006-8993(02)03077-9
- Moseley AE, Williams MT, Schaefer TL, et al. Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007
- Crema L, Schlabitz M, Tagliari B, et al. Na+, K+ ATPase activity is reduced in amygdala of rats with chronic stress-induced anxiety-like behaviour. Neurochem Res. 2010;35:1787–1795. doi: 10.1007/s11064-010-0245-9
- Kirshenbaum GS, Clapcote SJ, Duffy S, et al. Mania-like behaviour induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase alpha3 sodium pump. Proc Nat Acad Sci USA. 2011;108:18144–18149. doi: 10.1073/pnas.1108416108
- Carvalho FB, Gutierres JM, Bueno A, et al. Anthocyanins control neuroinflammation and consequent memory dysfunction in mice exposed to lipopolysaccharide. Mol Neurobiol. 2016.
- McCusker RR, Goldberger BA, Cone EJ. Caffeine content of energy drinks, carbonated sodas, and other beverages. J Anal Toxicol. 2006;30:112–114. doi: 10.1093/jat/30.2.112
- Ribeiro JA, Sebastiao AM. Caffeine and adenosine. JAD. 2010;20:3–15. doi: 10.3233/JAD-2010-1379
- Abreu RV, Silva-Oliveira EM, Moraes MF, et al. Chronic coffee and caffeine ingestion effects on the cognitive function and antioxidant system of rat brains. Pharmacol Biochem Behav. 2011;99:659–664. doi: 10.1016/j.pbb.2011.06.010
- Schmatz R, Mann TR, Spanevello R, et al. Moderate red wine and grape juice consumption modulates the hydrolysis of the adenine nucleotides and decreases platelet aggregation in streptozotocin-induced diabetic rats. Cell Biochem Biophys. 2013;65:129–143. doi: 10.1007/s12013-012-9407-5
- Fletcher DK, Bishop NC. Effect of a high and low dose of caffeine on antigen-stimulated activation of human natural killer cells after prolonged cycling. Int J Sport Nutr Exer Metabol. 2011;21:155–165. doi: 10.1123/ijsnem.21.2.155
- Dall'Igna OP, Porciuncula LO, Souza DO, et al. Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br J Pharmacol. 2003;138:1207–1209. doi: 10.1038/sj.bjp.0705185
- Chen JF, Xu K, Petzer JP, et al. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21: RC143.
- Evans SM, Pinto Pereira LM, Addae JI. Neuroprotection by caffeine and pentoxifylline during experimental cerebral ischaemia. West Indian Med J. 1999;48:23–25.
- Dodd SL, Herb RA, Powers SK. Caffeine and exercise performance. An update. Sports Med. 1993;15:14–23. doi: 10.2165/00007256-199315010-00003
- Graham TE, Spriet LL. Performance and metabolic responses to a high caffeine dose during prolonged exercise. J Appl Physiol. 1991;71:2292–2298.
- Graham TE, Hibbert E, Sathasivam P. Metabolic and exercise endurance effects of coffee and caffeine ingestion. J Appl Physiol. 1998;85:883–889.
- Greer F, McLean C, Graham TE. Caffeine, performance, and metabolism during repeated Wingate exercise tests. J Appl Physiol. 1998;85:1502–1508.
- Spriet LL, MacLean DA, Dyck DJ, et al. Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Am J Physiol. 1992;262.
- Davis JM, Zhao Z, Stock HS, et al. Central nervous system effects of caffeine and adenosine on fatigue. Am J Physiol Regul Integ Comp Physiol. 2003;284:399–404. doi: 10.1152/ajpregu.00386.2002
- Bracco D, Ferrarra JM, Arnaud MJ, et al. Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am J Physiol. 1995;269:671–678.
- Fredholm BB, Battig K, Holmen J, et al. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133.
- Gobatto CA, de Mello MA, Sibuya CY, et al. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:21–27. doi: 10.1016/S1095-6433(01)00362-2
- De Araujo GG, Papoti M, Manchado Fde B, et al. Protocols for hyperlactatemia induction in the lactate minimum test adapted to swimming rats. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:888–892. doi: 10.1016/j.cbpa.2007.09.002
- Koshinaka K, Sano A, Howlett KF, et al. Effect of high-intensity intermittent swimming on postexercise insulin sensitivity in rat epitrochlearis muscle. Metab Clin Exp. 2008;57:749–756. doi: 10.1016/j.metabol.2008.01.014
- Gutierres JM, Carvalho FB, Schetinger MR, et al. Anthocyanins restore behavioural and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014;96:7–17. doi: 10.1016/j.lfs.2013.11.014
- Gutierres JM, Carvalho FB, Rosa MM, et al. Protective effect of α-tocopherol on memory deficits and Na+,K+-ATPase and acetylcholinesterase activities in rats with diet-induced hypercholesterolemia. Biomed Aging Pathol. 2012;2:73–80. doi: 10.1016/j.biomag.2012.03.004
- Gutierres JM, Kaizer RR, Schmatz R, et al. alpha-Tocopherol regulates ectonucleotidase activities in synaptosomes from rats fed a high-fat diet. Cell Biochem Funct. 2012;30:286–292. doi: 10.1002/cbf.2797
- Carvalho FB, Mello CF, Marisco PC, et al. Spermidine decreases Na(+),K(+)-ATPase activity through NMDA receptor and protein kinase G activation in the hippocampus of rats. Eur J Pharmacol. 2012;684:79–86. doi: 10.1016/j.ejphar.2012.03.046
- Carvalho FB, Gutierres JM, Bohnert C, et al. Anthocyanins suppress the secretion of proinflammatory mediators and oxidative stress, and restore ion pump activities in demyelination. J Nutr Biochem. 2015;26:378–390. doi: 10.1016/j.jnutbio.2014.11.006
- Fiske CH, Subbarow Y. The nature of the “inorganic phosphate” in voluntary muscle. Science. 1927;65:401–403. doi: 10.1126/science.65.1686.401
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3
- Furian AF, Oliveira MS, Royes LF, et al. GM1 ganglioside induces vasodilation and increases catalase content in the brain. Free Rad Biol Med. 2007;43:924–932. doi: 10.1016/j.freeradbiomed.2007.05.035
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. Epub 1959/05/01. doi: 10.1016/0003-9861(59)90090-6
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3
- Rossato JI, Zeni G, Mello CF, et al. Ebselen blocks the quinolinic acid-induced production of thiobarbituric acid reactive species but does not prevent the behavioural alterations produced by intra-striatal quinolinic acid administration in the rat. Neurosci Lett. 2002;318:137–140. doi: 10.1016/S0304-3940(01)02504-6
- Castaneda AE, Tuulio-Henriksson A, Marttunen M, et al. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disorders. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006
- Zanini D, Schmatz R, Pimentel VC, et al. Lung cancer alters the hydrolysis of nucleotides and nucleosides in platelets. Biomed Pharmacother. 2012;66:40–45. doi: 10.1016/j.biopha.2011.09.003
- Rogers J, Vo U, Buret LS, et al. Dissociating the therapeutic effects of environmental enrichment and exercise in a mouse model of anxiety with cognitive impairment. Transcult Psychiatry. 2016;6:e794. doi: 10.1038/tp.2016.52
- Hallgren M, Herring MP, Owen N, et al. Exercise, physical activity, and sedentary behavior in the treatment of depression: broadening the scientific perspectives and clinical opportunities. Front Psychiatry. 2016;7:36. doi: 10.3389/fpsyt.2016.00036
- Hughes RN, Hancock NJ, Henwood GA, et al. Evidence for anxiolytic effects of acute caffeine on anxiety-related behaviour in male and female rats tested with and without bright light. Behav Brain Res. 2014;271:7–15. doi: 10.1016/j.bbr.2014.05.038
- Park KS, Oh JH, Yoo HS, et al. (-)-Epigallocatechin-3-O-gallate (EGCG) reverses caffeine-induced anxiogenic-like effects. Neurosci Lett. 2010;481:131–134. doi: 10.1016/j.neulet.2010.06.072
- Stefanello N, Schmatz R, Pereira LB, et al. Effects of chlorogenic acid, caffeine, and coffee on behavioural and biochemical parameters of diabetic rats. Mol Cel Biochem. 2014;388:277–286. doi: 10.1007/s11010-013-1919-9
- Zhang LN, Sun YJ, Pan S, et al. Na(+)-K(+)-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol. 2013;27:96–103. doi: 10.1111/fcp.12000
- Silveira PP, Portella AK, Benetti Cda S, et al. Association between Na(+),K(+)-ATPase activity and the vulnerability/resilience to mood disorders induced by early life experience. Neurochem Res. 2011;36:2075–2082. doi: 10.1007/s11064-011-0531-1
- Gamaro GD, Streck EL, Matte C, et al. Reduction of hippocampal Na+, K+-ATPase activity in rats subjected to an experimental model of depression. Neurochem Res. 2003;28:1339–1344. doi: 10.1023/A:1024988113978
- Riegel RE, Valvassori SS, Elias G, et al. Animal model of mania induced by ouabain: evidence of oxidative stress in submitochondrial particles of the rat brain. Neurochem Int. 2009;55:491–495. doi: 10.1016/j.neuint.2009.05.003
- Schaefer TL, Lingrel JB, Moseley AE, et al. Targeted mutations in the Na, K-ATPase alpha 2 isoform confer ouabain resistance and result in abnormal behaviour in mice. Synapse. 2011;65:520–531. doi: 10.1002/syn.20870
- Panza F, Solfrizzi V, Barulli MR, et al. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J Nutr Health Aging. 2015;19:313–328. doi: 10.1007/s12603-014-0563-8
- Chen JF. Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol. 2014;119:257–307. doi: 10.1016/B978-0-12-801022-8.00012-X
- Basurto-Islas G, Blanchard J, Tung YC, et al. Therapeutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol Aging. 2014;35:2701–2712. doi: 10.1016/j.neurobiolaging.2014.06.012
- Behan WM, Stone TW. Enhanced neuronal damage by co-administration of quinolinic acid and free radicals, and protection by adenosine A2A receptor antagonists. Br J Pharmacol. 2002;135:1435–1442. doi: 10.1038/sj.bjp.0704613
- Leite MR, Wilhelm EA, Jesse CR, et al. Protective effect of caffeine and a selective A2A receptor antagonist on impairment of memory and oxidative stress of aged rats. Exp Gerontol. 2011;46:309–315. doi: 10.1016/j.exger.2010.11.034
- Afzalpour ME, Chadorneshin HT, Foadoddini M, et al. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol Behav. 2015;147:78–83. doi: 10.1016/j.physbeh.2015.04.012
- De Almeida AA, Gomes da Silva S, Fernandes J, et al. Differential effects of exercise intensities in hippocampal BDNF, inflammatory cytokines and cell proliferation in rats during the postnatal brain development. Neurosci Lett. 2013;553:1–6. doi: 10.1016/j.neulet.2013.08.015
- Kuo A, Smith MT. Theoretical and practical applications of the intracerebroventricular route for CSF sampling and drug administration in CNS drug discovery research: a mini review. J Neurosci Methods. 2014;233:166–171. doi: 10.1016/j.jneumeth.2014.06.006
- Hassoun EA, Al-Ghafri M, Abushaban A. The role of antioxidant enzymes in TCDD-induced oxidative stress in various brain regions of rats after subchronic exposure. Free Rad Biol Med. 2003;35:1028–1036. doi: 10.1016/S0891-5849(03)00458-1
- Brannan TS, Maker HS, Raes I, et al. Regional distribution of glutathione reductase in the adult rat brain. Brain Res. 1980;200:474–477. doi: 10.1016/0006-8993(80)90936-1