ABSTRACT
Objectives: Two important classes of hydrazide-containing fused azaisocytosines were evaluated as possible antioxidants and characterised by UV spectroscopy.
Methods: 2,2-Diphenyl-1-picrylhydazyl (DPPH), nitric oxide (NO), hydrogen peroxide (H2O2) scavenging potencies and reducing power of molecules were evaluated.
Results: The strongest DPPH scavengers were found to be 9, showing the potency superior to that of butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and comparable to that of ascorbic acid (AA), and 6, revealing the antioxidant potency superior to that of BHA, BHT, PG and Trolox. In turn, 3 and 9 were the most promising NO scavengers, exhibiting the potency superior to that of BHA, BHT (3 and 9) and AA (3). The most potent H2O2 scavengers proved to be 10 and 9 showing similar or even better neutralising potency than that of Trolox, BHT and BHA. Simultaneously, the majority of hydrazides revealed higher ferric reducing abilities than that of AA and BHT. Some structure-activity relationships were explored. A possible mechanism for the DPPH radical scavenging ability of hydrazide-containing molecules was proposed.
Discussion: Hydrazides 3, 6 and 9 with an antioxidant potential better or comparable to that of the well-known antioxidants are proposed as new antioxidant candidates.
Introduction
The reactive oxygen species (ROS) and other related free radicals are continuously generated in the human body as by-products of metabolic processes (e.g. mitochondrial electron transport chain of cellular respiration) or from exogenous factors. It is well known that these species are capable of revealing both beneficial and harmful effects in the organism [Citation1,Citation2]. They fulfil essential functions in normal cell processes when are present in required quantities, while they can become toxic when are generated in excess [Citation3]. ROS are unstable and easily react with other molecules to achieve stability, so an overproduction of these species may lead to serious damages of all components of vital cells, including DNA, proteins and lipids [Citation1–3]. These oxidative modifications can cause the disruption of normal cell physiology and ROS are believed to be associated with multiple disease conditions, such as carcinogenesis, inflammation, mutagenesis, genotoxicity and aging [Citation4]. Therefore, the balance between the formation and removal of different types of ROS in the body is kept under strict control by several cellular and extracellular mechanisms. Antioxidants are capable of stabilising or neutralising free radicals before they attack the biological targets in the cell system, hence protecting the molecules from oxidative damage [Citation5]. It is a well-known fact that antioxidants, by acting as free radical scavengers, may be an efficient weapon in the prevention and treatment of many widespread diseases (i.e. cancer, cardiovascular and autoimmune diseases, diabetes, neurodegenerative Alzheimer’s, Parkinson’s and Huntington’s diseases, liver cirrhosis and atherosclerosis) which are believed to be caused or promoted by the oxidative stress (i.e. an imbalance between prooxidants and antioxidants) [Citation1]. For this reason, the design and synthesis of small molecules are directed towards the search and identification of new agents with antioxidant activity to combat cellular oxidative stress. An antioxidant therapy appears as a tool to minimise the molecular damages of vital constituents of living organisms caused by ROS and therefore the search for antioxidant agents has significantly increased during the past decades [Citation3–6].
The hydrazide pharmacophore seems to be attractive in medicinal chemistry for the development of new antioxidant agents containing privileged heterocyclic scaffolds, of which the most active molecules may be of benefit in the prevention of diseases induced by ROS and related ones. This moiety, due its redox and therefore free radical scavenging behaviour, is prone to quick oxidation giving the acyl radical [Citation7,Citation8]. Amos et al. [Citation7] have shown that during oxidation of the corresponding hydrazide an appropriate acyl radical is generated through the diimide intermediate. It is commonly known that an acyl radical is prone to nucleophilic acyl substitution.
A number of nitrogen-containing heterocyclic hydrazides with extremely good scaffolds (from medicinal chemistry viewpoint) are known for their advantageous antioxidant properties. Aleksandrova et al. [Citation9] have recently reported that the presence of a hydrazide moiety in the case of synthesised 1,3-dimethylxanthine-7-acetic acid derivatives significantly enhances their antioxidant activities. Menteşe et al. [Citation10,Citation11] have found that 2-substituted benzimidazol-1-ylacetohydrazides reveal remarkable radical scavenging activities. Khan et al. [Citation12] have disclosed 3-propylpyrazole-5-carbohydrazide as the anticipated drug candidate with the highest antioxidant properties in the class of hydrazide-containing pyrazoles, whereas Bonacorso et al. [Citation5] have confirmed antioxidant properties of 6-pyrazolylhydrazides of nicotinic acid. Furthermore, Hadjipavlou-Litina et al. [Citation13] have proposed inter alia 1-H-indol-3-ylpropanoic acid hydrazide as a molecule with prospective utility in the treatment and prevention of a neurodegenerative disorder (such as Alzheimer’s disease) due to its significant antioxidant activities.
However, the antioxidant properties of hydrazide-containing fused azaisocytosines have been much less investigated in the past. Szuster-Ciesielska et al. [Citation14] have previously demonstrated, for the first time, that a novel partial antagonist of adenosine A2A receptors (e.g. 8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide) reveals strong antioxidative and protective properties in ethanol-activated rat liver stellate cells’ model. This nucleobase-like molecule bearing the privileged fused azaisocytosine-like scaffold as well as the exocyclic carbohydrazide pharmacophore (almost non-toxic for normal cells) has been patented as an useful agent for the prevention and treatment in liver diseases, including hepatic cirrhosis [Citation15].
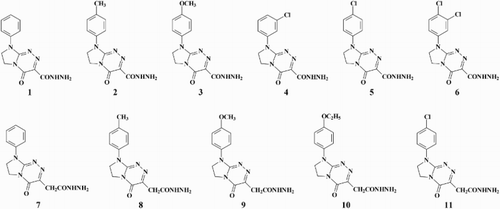
The purpose of the present paper was the exploration of antioxidant properties of biologically active and bioavailable six carbohydrazides (1–6) and five acetohydrazides (7–11) incorporating the privileged fused azaisocytosine template. We have chosen molecules which are unsubstituted or substituted by electron-donating (e.g. alkyl, alkoxy) or electron-withdrawing (e.g. chloro and dichloro) groups at the phenyl ring with the goal of finding the best substitution patterns for antioxidant activities of our structurally related hydrazides. The paper reports here the methodology and results of antioxidant assays. The investigated hydrazide-containing fused azaisocytosines that there were employed were as follows: 4-oxo-8-phenyl-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4] triazine-3-carbohydrazide (1), 8-(4-methylphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (2), 8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c] [1,2,4]triazine-3-carbohydrazide, a non-selective antagonist of adenosine A2A receptors (3), 8-(3-chlorophenyl)-4-oxo-4,6,7, 8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (4), 8-(4-chlorophenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (5), 8-(3,4-dichlorophenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (6), 2-(4-oxo-8-phenyl-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)acetohydrazide (7), 2-[8-(4-methylphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (8), 2-[8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (9), 2-[8-(4-ethoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (10), 2-[8-(4-chlorophenyl)-4-oxo-4,6,7, 8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (11) () [Citation14–21].
Material and methods
Synthesis and characterisation of the investigated hydrazide-containing fused azaisocytosines
The studied compounds have been synthesised and characterised by their spectroscopic data (IR, 1H NMR, 13C NMR, EI-MS), sharp melting points, biological (mostly antitumour, antinociceptive activities and antagonism towards adenosine A2A receptors), physico-chemical, drug-likeness properties and lipophilicity indices in correlation studies with in silico pharmacokinetic descriptors as published and patented previously [Citation14–19,Citation21]. Furthermore, the range of thermal stability of carbohydrazide-bearing compounds, a mechanism of thermal decomposition and major decomposition products of some of the most active and selective agents with prospective medical use have been determined and described recently [Citation20]. However, IR, 1H NMR and 13C NMR characterisation of 8-(4-chlorophenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (5) is the first time presented. Spectroscopic data for 5: IR (KBr) (ν, cm−1): 3347.28, 3312.76 (NH + NH2), 3097.17–2989.20 (CH), 2923.63 (CH2), 2853.21 (CH2), 1685.85 (endocyclic triazine-C = O), 1677.88 (exocyclic hydrazide-C = O), 1615.75 (NH2), 1581.41, 1504.72 (aromatic skeleton), 1541.15 (C = N), 1457.52 (CH2), 1414.83 (CH2), 1091.65 (aromatic C-Cl), 830.45 (1,4-disubstituted benzene); 1H NMR (δ, ppm, DMSO-d6, 300 MHz, TMS): 4.19 (s, 4H, 2CH2), 4.62 (s, 2H, NH2), 7.52 (d, J = 9.0 Hz, 2H, ar: H-2′ and H-6′), 7.89 (d, J = 9.0 Hz, 2H, ar: H-3′ and H-5′), 9.67 (s, 1H, NH); 13C NMR (δ, ppm, DMSO-d6, 75 MHz, TMS): 40.1 (C-6, CH2), 45.2 (C-7, CH2), ar C: [120.8 (2CH), 127.9 (C), 128.3 (2CH), 136.9 (C)], 143.4 (triazine-C-4), 150.5 (C-3), 152.0 (hydrazide-C = O), 160.3 (C-8a).
UV spectra of the examined hydrazide-containing fused azaisocytosines
Absorption band maxima in the ultraviolet region of electronic spectra of all the hydrazide-containing compounds (1–11) were determined on U2800 Hitachi spectrophotometer (Japan).
Antioxidant activity of the hydrazide-containing fused azaisocytosines
Reagents and apparatus
2,2-Diphenyl-1-picrylhydrazyl (DPPH), sodium nitroprusside (SNP), sulphanilamide, N-(1-naphthyl)ethylenediamine dihydrochloride (NEDD), orthophosphoric acid, hydrogen peroxide (H2O2), phenol red, horseradish peroxidase (HRP), sodium chloride, sodium hydroxide, potassium ferricyanide, ferric chloride, trichloroacetic acid and antioxidant standards, such as 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ascorbic acid (AA), propyl gallate (PG), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), were purchased as the highest grade available from Sigma-Aldrich (Germany). Solvents such as methanol and dimethyl sulfoxide (DMSO) of HPLC grade were supplied from Roth (Germany). A phosphate buffer was prepared from disodium hydrogen phosphate and sodium dihydrogen phosphate, which were obtained from POCH (Poland). Double distilled water was used.
All spectrophotometric measurements were conducted using U2800 Hitachi spectrophotometer (Japan).
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity assay
The DPPH radical scavenging activities of the synthesised hydrazide-containing compounds (1–11) were performed according to the procedure described by Brand-Williams et al. [Citation22] with some modifications.
In brief, 1 mL of the freshly prepared methanolic DPPH solution (0.1 mM) was added to 0.5 mL of the compound or antioxidant standard (Trolox, AA, PG, BHA and BHT) at different concentrations (250, 125, 62.5, 31.3, 15.6, 7.8 and 3.9 μM) dissolved in DMSO. The reaction mixture was shaken vigorously and afterwards allowed to stand in the dark at an ambient temperature for an appropriate time period. The blank was prepared by mixing 0.5 mL of DMSO with 1 mL of the methanolic DPPH solution. The scavenging ability of the DPPH radical by each sample was determined spectrophotometrically after 30 and 60 min by recording the absorbance at λmax 517 nm.
The DPPH radical scavenging activity of each synthesised compound or antioxidant standard was calculated using the following equation:where Ablank is the absorbance of the DPPH radical solution without the studied compound or antioxidant standard, Asample/standard denotes the absorbance of the DPPH radical solution with the investigated compound or antioxidant standard.
The half maximal inhibitory concentration (IC50) value, which represents the concentration of the tested compound or antioxidant standard required to scavenge 50% of DPPH free radicals, was calculated from a concentration–response curve-fitting model.
The experiments were performed at least in triplicate and the obtained results were averaged.
Nitric oxide radical scavenging assay
Nitric oxide radical scavenging activities of the tested hydrazide-containing compounds (1–11) were determined using the diazotisation reaction described by Griess.
Briefly, 1 mL of SNP (3 mM) in sodium phosphate buffer (0.1 M, pH 7.4) was incubated on light at 25°C for 150 min with the corresponding hydrazide or antioxidant standard (AA, BHT and BHA) at different concentrations (1000, 500, 250, 100, 50 and 25 µM) dissolved in DMSO. After incubation, 1 mL of the sample solution was mixed with an equal volume of Griess reagent (1% sulphanilamide, 0.1% NEDD and 2% orthophosphoric acid dissolved in double distilled water). The control experiment – without the investigated compound or antioxidant standard but with equivalent amounts of DMSO – was conducted in an identical manner. The absorbance of the coloured chromophore (that was formed during diazotisation of nitrate with sulphanilamide and its subsequent coupling with NEDD) was measured spectrophotometrically at λmax 546 nm.
Nitric oxide scavenging ability of each hydrazide or antioxidant standard was calculated as follows:where Ablank is the absorbance of the NO radical solution without the tested compound or antioxidant standard, Asample/standard denotes the absorbance of the NO radical solution with the examined compound or antioxidant standard.
The half maximal inhibitory concentration (IC50) value, which represents the concentration of the tested compound or antioxidant standard required to scavenge 50% of NO radicals, was calculated from a concentration-dependent inhibition curve.
Each experiment was done at least in triplicate and the average result was taken.
Hydrogen peroxide scavenging assay
The H2O2 scavenging abilities of the synthesised hydrazides (1–11) were determined according to the method of Pick and Keisari [Citation23].
In this assay, the reaction mixture containing 0.1 mL of the investigated compound or antioxidant standard (Trolox, BHT and BHA) at different concentrations (1000, 500, 250, 100, 50 and 25 µM) dissolved in DMSO, 0.1 mL of H2O2 (0.002%), 0.1 mL of sodium chloride (0.1 M) and 0.7 mL of sodium phosphate buffer (0.1 M, pH 7.4) was incubated in the dark at 37°C for 20 min. Then, 1 mL of phenol red dye (0.2 mg mL−1) with HRP (0.1 mg mL−1) in sodium phosphate buffer (0.1 M, pH 7.4) was added and the reaction was carried out at 37°C for 15 min. Finally, 0.1 mL of sodium hydroxide (1 M) was added to stop the reaction. The blank solution – without the investigated compound or antioxidant standard but with equivalent amounts of DMSO – was conducted in an identical manner. The absorbance of each reaction mixture was recorded after 10 min at λmax 610 nm.
The percentage of H2O2 scavenging by each hydrazide or antioxidant standard was calculated according to the formula given below:where Ablank is the absorbance of the reaction mixture without the investigated compound or antioxidant standard, Asample/standard denotes the absorbance of the reaction mixture with the examined compound or antioxidant standard.
The half maximal inhibitory concentration (IC50) value, which represents the concentration of the tested compound or antioxidant standard required to scavenge 50% of H2O2, was calculated from a concentration-dependent inhibition curve.
Each assay was conducted at least in triplicate and the obtained results were averaged.
Ferric ions reducing power assay
The Fe3+ reducing power of the investigated hydrazides (1–11) was determined by the method of Oyaizu [Citation24].
Briefly, 1 mL of the tested compound or antioxidant standard (AA and BHT) at different concentrations (1000, 500, 250, 100, 50 and 25 µM) dissolved in DMSO was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min. The reaction was stopped by adding 2.5 mL of 10% trichloroacetic acid and next the mixture was centrifuged at 3000 rpm for 10 min. Finally, 2.5 mL of the upper layer of solution was collected and mixed with 2.5 mL of a double distilled water and 0.5 mL of 0.1% ferric chloride. The absorbance of each solution was measured at λmax 700 nm, against a blank (containing all the reagents, except the examined compound or antioxidant standard).
The assays were carried out at least in triplicate and the results were averaged.
Statistical analysis
The results of antioxidant evaluations were expressed as mean ± standard deviation. Statistical analyses were performed using STATISTICA 12.5PL software (StatSoft, Poland).
Results and discussion
Ultraviolet characteristic of the tested hydrazide-containing fused azaisocytosines
For the first time, all the hydrazide-containing compounds (1–11) were studied for their absorption bands in an ultraviolet range.
The characteristic absorption bands of the diluted methanolic solutions of hydrazides 1–11 are compiled in . The registered two absorption bands are assigned to characteristic π →π* and n → π* electronic transitions. The most likely is that a conjugated π system with C = O and C = N (having a lone pair of electrons at the nitrogen atoms) chromophores, within the investigated molecules (1–11) absorbs UV light at the band maximum (of a lower intensity) in the wavelength range of 321–346 nm due to the n → π* (R) transition. The second UV band maximum (of a higher intensity) in the shorter wavelength range (251–263 nm) is assigned to the π →π* (K) transition within all the studied hydrazides.
Table 1. Two characteristic absorption bands (with extinction coefficients) of the diluted methanolic solutions (10%) of analysed hydrazide-containing compounds (1–11).
From the data listed in , it follows that the examined carbohydrazide-containing compounds (1–6) reveal λ’max of the n → π* band in the higher UV regions of the electromagnetic spectrum than structurally related acetohydrazide-bearing molecules (7–11). This may be rationally explained by a higher degree of conjugation of double bonds within their structures.
The DPPH radical scavenging activities of the examined compounds
A lot of methods are available to investigate the antioxidant activities of synthetic and natural compounds. One of the best-known, frequently employed, accurate and rapid method is the DPPH assay based on the reduction of DPPH free radical by the examined compounds. This test provides an information about the free radical scavenging ability of antioxidant compounds. DPPH radical has an unpaired electron and in the solution it shows a strong absorption maximum at λ 517 nm in a visible spectroscopy (a deep purple colour). In the presence of a free radical scavenger the odd electron is paired off, the absorption decreases and thus the decolouration from purple to yellow is observed. The capacity of compound to scavenge free radicals is quantified by the decrease of absorbance after DPPH reduction, which is stoichiometric with respect to the number of electrons taken up [Citation11,Citation25].
The DPPH radical scavenging abilities of hydrazide-containing fused azaisocytosines (1–11) and antioxidant standards at different concentrations at two time points (after the 30- and 60-min incubation periods) are presented in and (a,b). The antiradical activities are expressed as the inhibition percentage for scavenging DPPH radicals and IC50 values.
Figure 2. DPPH radical scavenging activities of the investigated heterocyclic hydrazides (1–11) after the 30-min (A) and the 60-min (B) incubation periods.
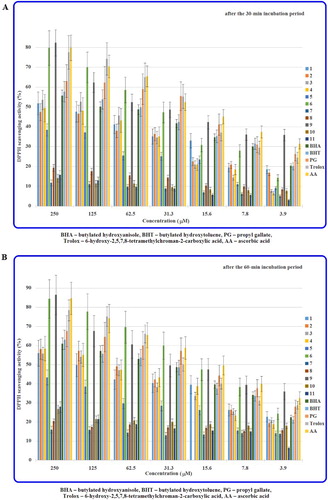
Table 2. DPPH radical, nitric oxide and H2O2 scavenging activities (expressed as the IC50 values) of the investigated heterocyclic hydrazides (1–11).
The majority of the investigated heterobicyclic hydrazides revealed concentration- and time-dependent DPPH scavenging activities. 8-(3,4-Dichlorophenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (6) proved to be the most potent in a class of carbohydrazides (1–6), whereas 2-[8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (9) revealed the most promising antiradical activities in a series of acetohydrazides (7–11). It is noteworthy that the antiradical potencies of these structures (6 and 9) were superior to that of BHA, BHT and PG, while the potency of 9 was better than that of Trolox and similar to that of AA. These results suggest an excellent antioxidant profile of these compounds.
The results of antiradical action of closely related para-methoxy analogues of carbohydrazide and acetohydrazide (3 and 9, respectively) allowed us to evaluate the effect of methylene linkage (between the C-3 and a hydrazide pharmacophore) on structure–activity relationships. In this case the presence of –CH2– bridge had a pivotal role in increasing the DPPH radical scavenging ability. Noteworthy is that the antiradical potency of an analogue containing the methylene linkage (9) proved to be nearly 4.5-fold and almost 5-fold superior, after the 30- and 60-min incubation periods, respectively, to that of the biologically important antagonist of adenosine A2A receptors (3). Notwithstanding, it has been proved surprisingly that owing to the presence of that methylene linkage the DPPH radical scavenging abilities of the remaining counterpart analogues (7, 8, 11) from the acetohydrazide class are considerably decreased.
It has been confirmed that 4-methoxy as well as 3,4-dichloro substitution at the phenyl ring sharply enhances the antioxidant activities of the investigated carbohydrazide-containing molecules. Introducing the appendant electron-attracting chloro group to 3-Cl and 4-Cl substituted carbohydrazides (4 and 5, respectively) was successful and allowed us to identify the most potent 3,4-dichloro-substituted carbohydrazide molecule (6). Moving the chloro group from para to meta position of the phenyl produced the more antiradical active 3-chloro-substituted carbohydrazide (4), when compared to 5. Replacement of the electron-withdrawing 4-Cl group by the electron-donating 4-CH3 substituent at the phenyl ring improved the scavenging abilities of 2, when compared to 5. However, replacing 4-CH3 substituent in 2 by more electron-donating 4-CH3O group at the phenyl ring in 3 resulted in an increase of the DPPH radical scavenging activity after 30 min of incubation and in a decrease of that effect after 60 min.
It has been found that the substitution at the phenyl moiety generally enhances the antioxidant activities of heterobicyclic acetohydrazides (8–11), when compared to the parent compound (7). Replacement of the para-methoxy group in 9 by para-ethoxy substitution was not preferable due to a considerable decrease in DPPH radical scavenging properties of 10 (when compared to 9). Replacement of the CH3 group by Cl substituent in the para position at the phenyl moiety decreased the antiradical ability of 11 (when compared to 8).
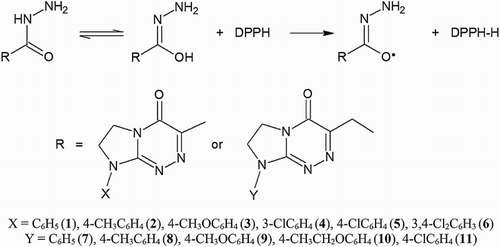
The DPPH radical scavenging activity of the investigated hydrazides may be based on the hydrogen atom transfer (HAT) mechanism leading to the neutralisation of this radical (). Because of the possible keto–enol tautomerism of 1–11, the enol form is capable of donating the hydrogen atom to the DPPH radical yielding the DPPH-H, e.g. 1,1-diphenyl-2-(2,4,6-trinitrophenyl)hydrazine.
The NO radical scavenging activities of the investigated compounds
In order to test the ability of different substances as NO-scavengers the co-incubation of compounds with spontaneous NO-donors with long lifetime, such as SNP, diethylenetriamine/nitric oxide adduct or S-nitroso-N-acetylpenicillamine, is conducted [Citation26]. In the case of our hydrazide-containing compounds (1–11) we used SNP, which in a solution at physiological pH in the presence of light irradiation liberates nitric oxide. Upon spontaneous interaction with atmospheric oxygen NO is transformed into nitrite ion, which with the Griess reagent yields a purple azo-compound. Scavengers of nitric oxide compete with oxygen leading to the reduced production of nitrite [Citation27,Citation28]. The changes in the nitrite levels (a stable oxidative products of NO) were used as a measure of the potential NO scavenging activities of hydrazides.
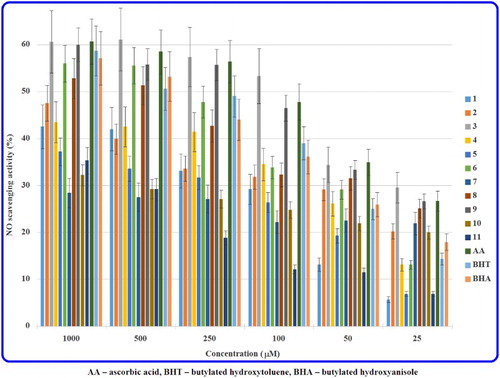
The nitric oxide radical scavenging abilities of the hydrazide-containing fused azaisocytosines (1–11) and antioxidant standards at different concentrations are shown in and . The antiradical activities are expressed as the per cent of NO scavenging effect as well as IC50 values.
The majority of the studied heterobicyclic carbohydrazides and acetohydrazides showed a concentration-dependent NO scavenging ability. Among a carbohydrazide class the most potent NO scavenging properties are exhibited by molecules 3 and 6, bearing the para-methoxy and 3,4-dichloro substitution, respectively, at the phenyl ring. The potency of the most active 8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (3) proved to be nearly 2.7-fold, 2.2-fold and 1.3-fold stronger to that of commonly known antioxidants, such as BHA, BHT and AA, respectively. Simultaneously, in our previous in vivo, in vitro and in silico studies this molecule (3) proved to be relatively low toxic for mice, completely non-toxic for HSF, GMK cells [Citation16], low toxic for human hepatocyte (HepG2) [Citation15] and rat liver stellate (CFSC-2G) cells [Citation15,Citation16]. Moreover, it possessed lipophilicity indices in different reversed-phases imitating biosystems significantly correlated with some bioactivity descriptors relevant to the satisfactory pharmacokinetic profile in vivo [Citation19]. An excellent antioxidant profile of 3 combined with its low toxicity and beneficial pharmacokinetic descriptors makes this thermally stable carbohydrazide [Citation20] a possible drug candidate for prevention of free radical-related disorders. However, antiradical potencies of the second in view of activity 8-(3,4-dichlorophenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (6) were higher to that of BHA, comparable to that of BHT and lower to that of AA.
It has been found that the substitution at the phenyl ring improved the antioxidant properties of the majority of the investigated carbohydrazides (2–4 and 6). The methoxy instead of methyl substitution in the para position at the phenyl ring was preferred as this replacement considerably increases the NO scavenging activities of 3 (when compared to 2). The presence of methyl instead of chloro group in the para position of the phenyl ring was favoured as this bioisosteric replacement increases the antiradical abilities of 2 (when compared to 5). Introducing the appendant chlorine atom to both monochloro-substituted derivatives (4 and 5) resulted in a significant increase in the antioxidant activity of 3,4-dichlorosubstitued compound (6). However, moving the chloro group from the para to meta position at the phenyl ring improved the NO scavenging abilities of 4 (when compared to 5).
Noteworthy is that 2-[8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (9) proved to be an excellent NO radical scavenger in a class of acetohydrazides (7–11), revealing the potency superior to that of BHA and BHT and similar to that of AA. Furthermore, it has been confirmed that the substitution at the phenyl ring improves the antioxidant activities of all the tested acetohydrazide-containing molecules (8–11). The 4-methoxy group in 9 instead of 4-methyl substitution in 8 was preferred as the prominent increase in NO scavenging abilities of the modified structure (9) is noticed. The 4-methyl substituent instead of 4-chloro group was favoured as the bioisostere 8 is more antioxidant active than 11. Replacement of the 4-methoxy by 4-ethoxy substitution at the phenyl moiety was not preferable due to a considerable decrease in the antiradical properties of 10 (when compared to 9).
Nitric oxide is known to be an ubiquitous free radical, which is distributed in tissue or organ systems. Endogenously generated NO is a very unstable radical playing a pivotal role in the regulation of many physiological processes in the organism. It acts as a key inter- and intracellular messenger molecule involved in smooth muscle relaxation, inhibition of platelet aggregation, neuronal signalling and regulation of cell-mediated toxicity [Citation29,Citation30]. Although NO is involved in numerous relevant biological processes, the abnormal excessive release of this free radical is detrimental to the cells and tissues as it can lead to the development of certain diseases. Several studies have shown that chronic expression of NO radical is associated with inflammation, cancer and other pathological conditions [Citation28–30]. Nitric oxide can cause endothelial dysfunction leading to a series of events that have adverse impact on the myocardium. Further, it leads to myocardial ischaemia and disorder in myocardial reperfusion by promoting aggregation of platelets [Citation27].
The NO radical scavenging capacity is helpful to arrest the chain of reactions initiated by overproduction of NO that are harmful to human health. Therefore, the hydrazide-containing NO scavengers (especially 3 and 9) may be useful for preventing radical-related pathological damages and they are valuable targeted molecules from a pharmacological point of view [Citation28,Citation30].
The H2O2 scavenging abilities of the studied compounds
H2O2 molecules – products of superoxide radical (O2•−) dismutation – are considered non-radical reactive oxygen metabolites. They are freely dissolved in aqueous solution and can easily cross the cellular membranes. Their deleterious effects are related to the direct or indirect influence on biomolecules. The direct action of H2O2 is originated from its oxidising properties, however indirect one is resulted from its easy conversion to the highly reactive hydroxyl radicals (OH•). H2O2, directly, can degrade haem proteins and liberate iron ions. Adding H2O2 to cell culture may lead to transition metal ion-dependent OH•-mediated DNA damage. Moreover, this reactive oxygen metabolite can penetrate biological membranes and may slowly oxidise nucleic acids, lipids, sulfhydryl groups and keto acids [Citation31]. Based on these facts, the protection of biological systems against an excessive amounts of H2O2, especially in the presence of transition metal ions, seems to be very important.
In order to test the ability of H2O2 scavenging by the examined hydrazide-containing fused azaisocytosines (1–11) we used the specific biochemical assay [Citation23] based on HRP-mediated and H2O2-dependent oxidation of phenol red, resulting in the formation of a product which exhibits a maximum absorbance at 610 nm.
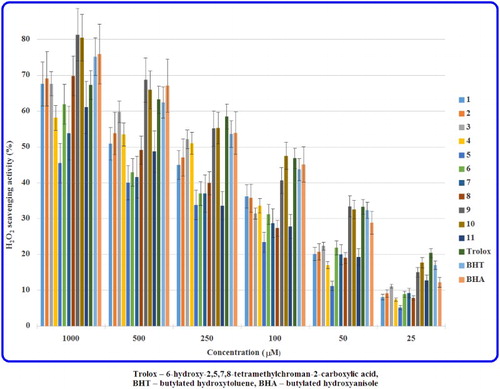
The H2O2 neutralising abilities, expressed as the per cent of H2O2 scavenging effect as well as IC50 values, of the hydrazides (1–11) and antioxidant standards at different concentrations are shown in and .
Many of the tested hydrazide-containing molecules were capable of scavenging H2O2 in a concentration-dependent manner. Among all the studied compounds the most potent scavengers proved to be two acetohydrazides – 2-[8-(4-ethoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (10) and 2-[8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide (9). The structure 10 exhibited similar neutralising potency to that of a well-known antioxidant standard – Trolox and better activity than that of BHT and BHA. However, the molecule 9 revealed H2O2 scavenging potency of the same order of magnitude as that of BHT. Moreover, in this assay, moderate scavenging abilities showed compound 3, that is the para-methoxy derivative belonging to the carbohydrazide class.
Based on the experimental results, some structure–activity relationships were found. It has been proved that the electron-donating alkoxy substitutions in the para position at the phenyl ring had a pivotal role in H2O2 scavenging abilities, as compounds containing these moieties (10 with 4-ethoxy, 9 and 3 with 4-methoxy) are the most active of all the hydrazides that were evaluated. However, replacing the 4-methoxy group in 3 and 9 by 4-methyl substituent in 2 and 8 was not preferable owing to the decrease in antioxidant properties. Simultaneously, introducing the electron-attracting chloro group (in 4, 5 and 11) or groups (in 6) to the parent compound (1 and 7) in both hydrazide classes resulted in a decrease or even a lack of scavenging activities in comparison to ethoxy (10), methoxy (3 and 9) and methyl (2 and 8) substituted derivatives.
The reducing power of the tested compounds
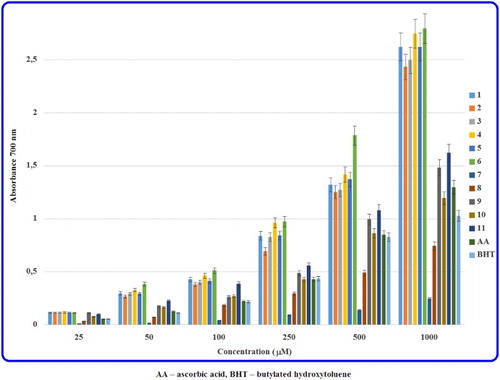
Generally, the antioxidant activity of a compound is directly correlated to its reducing ability. Therefore, the reducing capacity of a molecule may serve as a significant indicator of its potential antiradical action. Apart from the HAT, another important strategy to neutralise free radicals is the single electron transfer. In the reducing power assay, substances having the reductive potential are able to reduce Fe3+/ferricyanide complex to ferrous form by donating an electron. Next, ferrocyanides react with ferric ions forming an intense Prussian blue complex which has a maximum absorbance at λ 700 nm. An increased absorbance of this complex indicates an increased reducing power of the examined compound [Citation32–34].
The reducing abilities of the hydrazide-containing fused azaisocytosines (1–11) and antioxidant standards at different concentrations are shown in . It was found that the reductive potential of all the synthesised molecules is concentration-dependent. It is noteworthy that all the tested carbohydrazides (1–6) are much stronger reducing agents than acetohydrazides (7–11) as well as antioxidant standards, such as AA and BHT. Furthermore, among the evaluated carbohydrazide-containing molecules the highest ferric ions reducing ability exhibited 8-(3,4-dichlorophenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazide (6), which at all concentrations was the most effective. In turn, in the acetohydrazide class the substances 11 (e.g. 2-[8-(4-chlorophenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide) and 9 (e.g. 2-[8-(4-methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide) proved to be good reducing agents with stronger effects than AA and BHT, the molecule 10 (e.g. 2-[8-(4-ethoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide) showed moderate reducing power (but comparable to that of both antioxidant standards), while the compounds 8 (e.g. 2-[8-(4-methylphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl]acetohydrazide) and 7 (e.g. 2-(4-oxo-8-phenyl-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-yl)acetohydrazide) demonstrated the lowest reducing abilities.
Conclusion
The investigated hydrazide-containing fused azaisocytosines (1–11) revealed two characteristic absorption bands in their UV spectra due to n → π* and π →π* transitions in their molecules, respectively. Among biologically important and bioavailable eleven molecules selected to antioxidant studies, three hydrazides (3, 6, 9) exhibited very good to excellent abilities to neutralise free radicals in different in vitro systems. It is noteworthy that they were found to be free radical scavengers with the potency better or comparable to that of commonly known antioxidants. Therefore, they are proposed as new antioxidants with prospective therapeutic applicability in a variety of disorders evoked or promoted by the oxidative stress.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kehrer JP, Klotz L-O. Free radicals and related reactive species as mediators of tissue injury and disease: implications for health. Toxicology. 2015;45(9):765–798.
- Shirinzadeh H, Ince E, Westwell AD, et al. Novel indole-based melatonin analogues substituted with triazole, thiadiazole and carbothioamides: studies on their antioxidant, chemopreventive and cytotoxic activities. J Enzyme Inhib Med Chem. 2016;31(6):1312–1321. doi: 10.3109/14756366.2015.1132209
- Kareem HS, Nordin N, Heidelberg T, et al. Conjugated oligo-aromatic compounds bearing a 3,4,5-trimethoxy moiety: investigation of their antioxidant activity correlated with a DFT study. Molecules. 2016;21:224–242. doi: 10.3390/molecules21020224
- Nagamallu R, Srinivasan B, Ningappa MB, et al. Synthesis of novel coumarin appended bis(formylpyrazole) derivatives: studies on their antimicrobial and antioxidant activities. Bioorg Med Chem Lett. 2016;26:690–694. doi: 10.1016/j.bmcl.2015.11.038
- Bonacorso HG, Cavinatto S, Moraes MC, et al. Synthesis, structure elucidation, antioxidant and antimicrobial activity of novel 2-(5-trifluoromethyl-1H-pyrazol-1-yl)-5-(5-trihalomethyl-1H-pyrazol-1-yl-1-carbonyl)pyridines. J Braz Chem Soc. 2015;26(11):2346–2361.
- Tana W, Lia Q, Lia W, et al. Synthesis and antioxidant property of novel 1,2,3-triazole-linked starch derivatives via ‘click chemistry’. Int J Biol Macromol. 2016;82:404–410. doi: 10.1016/j.ijbiomac.2015.10.007
- Amos RIJ, Gourlay BS, Yates BF, et al. Mechanistic investigation of the oxidation of hydrazides: implications for the activation of the TB drug isoniazid. Org Biomol Chem. 2013;11:170–176. doi: 10.1039/C2OB26419F
- Amos RIJ, Gourlay BS, Schiesser CH, et al. A mechanistic study on the oxidation of hydrazides: application to the tuberculosis drug isoniazid. Chem Commun. 2008;14:1695–1697. doi: 10.1039/b719570b
- Aleksandrova K, Belenichev I, Shkoda A, et al. Research of antioxidant properties of theophyllinyl-7-acetic acid derivatives. Oxid Antioxid Med Sci. 2014;3(3):187–194. doi: 10.5455/oams.191214.or.078
- Menteşe E, Ülker S, Kahveci B. Synthesis and study of α-glucosidase inhibitory, antimicrobial and antioxidant activities of some benzimidazole derivatives containing triazole, thiadiazole, oxadiazole, and morpholine rings. Chem Heterocycl Comp. 2015;50(12):1671–1682. doi: 10.1007/s10593-015-1637-1
- Menteşe E, Yılmaz F, Baltaş N, et al. Synthesis and antioxidant activities of some new triheterocyclic compounds containing benzimidazole, thiophene, and 1,2,4-triazole rings. J Enzyme Inhib Med Chem. 2015;30(3):435–441. doi: 10.3109/14756366.2014.943203
- Khan KM, Rani M, Ambreen N, et al. Acyl hydrazides: potent antioxidants. Lett Drug Design Discov. 2012;9(2):135–139. doi: 10.2174/157018012799079798
- Hadjipavlou-Litina D, Samadi A, Unzeta M. Analysis of the antioxidant properties of differently substituted 2- and 3-indolyl carbohydrazides and related derivatives. Eur J Med Chem. 2013;63:670–674. doi: 10.1016/j.ejmech.2013.03.014
- Szuster-Ciesielska A, Sztanke K, Kandefer-Szerszeń M. A novel fused 1,2,4-triazine aryl derivative as antioxidant and nonselective antagonist of adenosine A2A receptors in ethanol-activated liver stellate cells. Chem Biol Interact. 2012;195:18–24. doi: 10.1016/j.cbi.2011.10.004
- Kandefer-Szerszeń M, Szuster-Ciesielska A, Sztanke K, et al. 8-(4-Methoxyphenyl)-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazin-3-formic acid hydrazide used as a drug for liver diseases. Polish patent PL 2014;216264.
- Sztanke K, Pasternak K, Rzymowska J, et al. Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1,2,4-triazine aryl derivatives. Eur J Med Chem. 2008;43:1085–1094. doi: 10.1016/j.ejmech.2007.07.009
- Sztanke M, Tuzimski T, Janicka M, et al. Structure-retention behaviour of biologically active fused 1,2,4-triazinones – correlation with in silico molecular properties. Eur J Pharm Sci. 2015;68:114–126. doi: 10.1016/j.ejps.2014.12.011
- Sztanke K, Tkaczyński T. Synthesis of new derivatives of 8-phenyl-7,8-dihydro-4-oxoimidazo[2,1-c][1,2,4]triazine-3-acetic acid hydrazide. Acta Pol Pharm-Drug Res. 1998;55:251–252.
- Janicka M, Sztanke M, Sztanke K. Reversed-phase liquid chromatography with octadecylsilyl, immobilized artificial membrane and cholesterol columns in correlation studies with in silico biological descriptors of newly synthesized antiproliferative and analgesic active compounds. J Chromatogr A. 2013;1318:92–101. doi: 10.1016/j.chroma.2013.09.060
- Bartyzel A, Sztanke M, Sztanke K. An insight into the thermal behaviour of biologically active 8-aryl-4-oxo-4,6,7,8-tetrahydroimidazo[2,1-c][1,2,4]triazine-3-carbohydrazides. J Anal Appl Pyrolysis. 2016;121:138–145. doi: 10.1016/j.jaap.2016.07.014
- Sztanke K. New hydrazides of 8-aryl-6,7-dihydro-4H-imidazo[2,1-c][1,2,4]triazine-4-oxo-3-formic acids and methods for their manufacture. Polish patent PL 2009;201092.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5
- Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3
- Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307
- Barbuceanu SF, Ilies DC, Saramet G, et al. Synthesis and antioxidant activity evaluation of new compounds from hydrazinecarbothioamide and 1,2,4-triazole class containing diarylsulfone and 2,4-difluorophenyl moieties. Int J Mol Sci. 2014;15:10908–10925. doi: 10.3390/ijms150610908
- Mirkov SM, Djordjevic AN, Andric NL, et al. Nitric oxide-scavenging activity of polyhydroxylated fullerenol, C60(OH)24. Nitric Oxide. 2004;11:201–207. doi: 10.1016/j.niox.2004.08.003
- Borgohain M, Lakshmi SS, Duriarajan P. A comparative study of in-vitro anti-oxidant potential of phosphodiesterase-5 inhibitors. Int J Curr Pharm Clin Res. 2014;4:76–79.
- Merino-Montiel P, Maza S, Martos S, et al. Synthesis and antioxidant activity of O-alkyl selenocarbamates, selenoureas and selenohydantoins. Eur J Pharm Sci. 2013;48:582–592. doi: 10.1016/j.ejps.2012.12.016
- Hamada NMM, Abdo NYM. Synthesis, characterization, antimicrobial screening and free-radical scavenging activity of some novel substituted pyrazoles. Molecules. 2015;20:10468–10486. doi: 10.3390/molecules200610468
- Sankaran M, Kumarasamy C, Chokkalingam U, et al. Synthesis, antioxidant and toxicological study of novel pyrimido quinolone derivatives from 4-hydroxy-3-acyl quinolin-2-one. Bioorg Med Chem Lett. 2010;20:7147–7151. doi: 10.1016/j.bmcl.2010.09.018
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724
- Kiliç I, Yeşiloğlu Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim Acta A Mol Biomol Spectrosc. 2013;115:719–724. doi: 10.1016/j.saa.2013.06.110
- Chidan Kumar CS, Loh WS, Chandraju S, et al. Synthesis, structural and antioxidant studies of some novel N-ethyl phthalimide esters. PLoS One. 2015;10(3):e0119440. doi: 10.1371/journal.pone.0119440
- Apetrei CL, Tuchilus C, Aprotosoaie AC, et al. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules. 2011;16:7773–7788. doi: 10.3390/molecules16097773