?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
The current study was designed to examine the therapeutic role of hydroalcoholic extract of Cuscuta reflexa Roxb (CRE) and Peucedanum grande C.B. Clarke (PGE) on letrozole (1 mg/kg) induced polycystic ovary syndrome (PCOS) in female Wistar albino rats.
Methods
PCOS rats were treated with CRE (280 mg/kg), PGE (140 mg/kg) or CRE + PGE p.o. for 3 weeks. Vaginal smears for phase of estrous cycle determination, serum levels of sex androgens, lipid profile, oxidative stress parameters and histopathology of ovarian tissues were investigated.
Results
Diestrous cycle days treated with CRE (group III) or PGE (group IV) decreased significantly (p < 0.05) compared to PCOS control animals (group II). Moreover, weight of uteri in PCOS animals treated with the plant extracts also increased significantly (p < 0.05) compared to that of group II animals. Histopathological examination showed the protective effect of the CRE and PGE indicated by the disappearance of ovarian cyst.
Conclusion
The study demonstrated that the CRE and PGE either alone or in combination hold a significant effect in letrozole induced PCOS rat models and could be useful in the management of reproductive and metabolic disorders related to PCOS.
Introduction
Polycystic ovarian syndrome is the most common heterogenous endocrine disorder affecting 5–10% women of reproductive age with hyperandrogenism, hyperinsulinemia, low-grade systemic inflammation, and polycystic ovaries [Citation1]. It involves multisystem endocrinopathy with interlinked metabolic disturbances. Ovulatory dysfunction, enlarged polycystic ovaries and hyperandrogenism characterize the syndrome. Although the exact etiology of the syndrome is still unclear, evidence suggests a clear connection between PCOS and metabolic disturbances such as insulin resistance, obesity and type 2 diabetes [Citation2]. The spectrum of pathobiology of this disease is more prominent in adults as well as in children due to sedentary life style, food habits, cultural influences and also a genetic pre-disposition, that leads to obesity which in-turn have derailing effects on parameters related to fertility in women especially young [Citation3]. Various short term symptomatic therapies are being used to manage the syndrome but these therapies are associated with several adverse effects from mild to severe side effects such as mood swing, arthritis, breast tenderness, weight gain, irregular bleeding, bloating, fatigue, sexual dysfunction, osteoporosis, hot flashes, cardiovascular and thromboembolic problems, hepatic toxicity, etc. [Citation4]. The best and effective curative treatment is still an unmet medical need. Therefore, search for drug candidates from natural sources which shows no or minimal side effects is a priority.
Cuscuta reflexa Roxb. (Family: Convolvulaceae) and Peucedanum grande C.B. Clarke (family: Apiaceae) are used in conventional medicinal system of various Asian countries including China, India, Bangladesh, and Thailand for treating multiple disorders and a wide array of chemical compounds has been isolated with diverse medicinal properties [Citation5–7]. Several in-vitro and in-vivo studies have proved the promise of C. reflexa as hepatoprotective, anti-diabetic, anti-bacterial, anti-microbial, antioxidant, diuretic,anti-epileptic, anti-viral, anxiolytic and antitumor activities [Citation8–11]. P. grande have been indicated to exhibit nephroprotective and antiurolithiatic activities [Citation12,Citation13]. Furthermore, C. reflexa and P. grande are the rich sources of phytoconstituents including polyphenols, reducing sugars, flavonoids, saponins, glycosides, steroids, coumarins, aminoacids and a wide range of chemotypes [Citation12,Citation14]. We hypothesized that C. reflexa and P. grande may be beneficial in management of PCOS by virtue of the phytoconstituents present in the herbs that may be attributed with various pharmacological properties. In this context, hydroalcholic extracts of C. reflexa (CRE) and P. grande (PGE) were prepared and evaluated for their potential in the treatment of letrozole induced PCOS rat models.
Material and methods
Experimental animals
The experiment was conducted on healthy, cyclic, female Wistar albino rats weighing 190 ± 10g . Animals were acquired from Indian Institute of Integrative Medicine (IIIM), Jammu and acclimatized for two weeks. They were housed in sterilized polypropylene cages and maintained at standard conditions (22 ± 2°C, 55 ± 5% humidity and 12 h’ light and dark cycle). Animals were fed with standard rats feed and provided with water ad libitum. The study was approved by Institutional Animal Ethics Committee (IAEC) of Regional Research Institute of Unani Medicine (RRIUM), Srinagar (Reg. No-927/GO/Re/s/2006/CPCSEA) under the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA).
Drugs and reagents
All the drugs and chemicals used were of analytical grade. Letrozole was obtained from Sun Pharmaceuticals, Gujarat, India (Batch no. KFW013). Clomiphene citrate was obtained from Abbott India Limited, Nalgarh, Solan (Batch no- DAKB6004). Rat testosterone ELISA kit, estrogen kit, FSH kit, LH kit were obtained from sincere biotech, Beijing, China. Cholesterol kit from DiaSys Diagnostic India, triglycerides kit was obtained from Medsource Ozone Biomedical, India.
Plant material and extract preparation
C. reflexa Roxb. and P. grande C.B. Clarke plants were collected from high altitude regions of Sonamarg area Ganderbal District, Jammu and Kashmir, India and identified by botanist and pharmacognist of RRIUM/CCRUM. Plant samples were cleaned under the running tape water, shade dried and ground in a pulverizer separately. 200 g of the powdered materials of both the plant were packed in the soxhlet's apparatus and extracted in 50% hydroalcoholic (ethanol/distilled water, 1:1) for 72 h at 85°C. The extract thus obtained was then dried on a rotary evaporator. The yield percentage of extract was found to be 40% for C. reflexa and 23% for P. grande. Crude extracts were stored at −4°C until further analysis.
Fourier transform infrared
Fourier Transform Infrared (FT-IR) spectrum was developed using the Spectrum RX1, FT-IR (Perkin Elmer, USA) spectrum spectrometer, operating in 4000–400 cm−1 wavelength area, equipped with a potassium bromide (KBr) beam divider, DTGS sensor and nichrome. For the final range of 4 cm−1 resolution, a total of 100 scans were obtained. The dried powder extract was encapsulated in KBr pellets for the preparation of translucent sample discs.
Study design
The test was carried out by the induction method of [Citation15] and treatment schedule of Sasikala and Shamila with some modifications [Citation16]. Thirty-six female wistar rats were randomly divided into six groups of six animals each (). Group I served as plain control and received 1 ml of 0.5% CMC orally. The animals of group II to VI were orally administered with letrozole (1 mg/kg dissolved in 0.5% of CMC) for 21 consecutive days for induction of PCOS. Group II served as negative control and received no treatment while group III, group IV and group V were orally gavauged with hydroalcoholic extract of CRE, PGE and combination of CRE + PGE respectively once daily from 22nd day for 3 weeks. CRE and PGE were administered at the dose of 280 and 140 mg/kg body weight to the group III and IV respectively [Citation17]. Group VI served as positive control and orally received the metformin at the dose of 50 mg/kg body weight.
Table 1. Experimental design and grouping of rats with n = 6 in each group.
Determination of estrous cycle (vaginal smear)
Phase of estrous cycle was investigated by daily observations of vaginal smears for rats at 09.00 h for 21 days. The procedure adopted for collection of vaginal samples involved inserting about 50 µL of sterile normal saline into the vagina with the help of pipette tip. The normal saline was flushed in and out thrice against the vaginal wall and then aspirated in. About 10 µLof collected vaginal fluid were poured onto a clean glass slides, air dried and fixed with 70% ethanol, stained with methylene blue (Thomas Baker, USA) and examined by bright field microscopy (×100 magnification). Stained slides were evaluated under light microscope, with 40x objective lenses. Three types of cells were observed: little round ones were leucocytes, round and nucleated ones were epithelial cells and the irregular anucleated were cornified cells. The proportion among these three types of cells was used for determination of the phase of the estrous cycle.
Physical characteristics
Body weight of all the experimental groups were recorded on day O, day 21 after the letrozole administration and on day 42 after the CRE, PGE or CRE-PGE treatment. On Day42, before the animal dissection, standard measures of measurement were used to record the physical parameters including weight gain, body length; the abdominal circumference with thoracic circumference ratio (AC/TC), lee index, body mass index (BMI) and specific rate of body mass gain were reported.
Blood sampling
After 3 weeks of treatment with CRE or PGE, rats were euthanized by cervical dislocation and blood was collected by cardiac puncture. The blood samples were centrifuged (1000 rpm for 5 min, 25°C) and serum was separated and stored at −20°C until biochemical analysis.
Tissue collection and ovarian morphometry
Uteri and ovaries were dissected out from all the animals and freed from extra fats. Weight of ovaries, their diameter and ovarian organ index were recorded. Ovarian tissues were serially dehydrated in graded ethanol and xylene. Specimens were embedded in paraffin block and sections of approximately 5 µm thick were cut and stained with hematoxylin and eosin stain and visualized under light microscope. One ovary from each animal was fixed in 10% formalin for histological study. The ovarian follicle and corpus luteum at different stages of development were analyzed.
Serum hormone analysis
Serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), was measured using enzyme linked immunosorbent assay (ELISA) kits (Sincere Biotech Co., Ltd, China). Serum testosterone (TET) levels were measured using a testosterone ELISA Kit (Ela science, United States). The 17β-estradiol (EST) levels were measured using an Estradiol (Rat) ELISA Kit (BioVision, United States). All hormone levels were measured according to the supplier’s instructions.
Lipid profile
Total-cholesterol (TC), triglycerides (TG), low density lipid cholesterol (LDL) and high density lipid-cholesterol (HDL) were estimated using enzymatic kits procured from Sincere Biotech Co., Ltd, China.
Determination of superoxide dismutase activity
Superoxide dismutase (SOD) was measured by the procedure as described earlier [Citation18]. Cocktail containing sodium carbonate (1.0 mL, 50 mM), nitro blue tetrazolium (0.4 mL, 25 μm) and hydroxylamine hydrochloride (0.1 mM of 0.2 mL) that was freshly prepared. The reaction mixtures were mixed by turning the tubes upside down several times followed by the addition serum sample (0.1 mL, 1:10 v/v). The change in absorbance of samples was monitored at an optical density 560 nm.
Estimation of glutathione peroxidase assay (GPx)
GSH activity was determined by the procedure as discussed earlier [Citation19]. To the reaction mixture 10% of bovine serum albumin, 50 mM PBS (pH = 7.6), 2 mM NADPH, 20 mM GSSG were added. Absorbance was measured at 340 nm at a temperature of 25°C. The activity was calculated by using the molar coefficient for NADPH of 6.22 μ/mol−1 × cm−1 and expressed in U/mL of serum. The disappearance of NADPH at 340 nm was recorded at 25°C. Enzyme activity was calculated as nM NADPH oxidized/min/mL of serum using molar extinction coefficient of 6.22 × 103 M−1 cm−1.
Estimation of catalase
Catalase (CAT) was measured by the procedure as described elsewhere [Citation20]. Reaction mixture (1.5 mL) containing 0.01 M pH 7.0 phosphate buffer (1.0 mL), serum sample (0.1 mL) and hydrogen peroxide (H2O2; 2 M, 0.4 mL). The reaction was arrested by the adding dichromate-acetic acid reagent (2.0 mL, 5% potassium dichromate and glacial acid mixed in the ratio of 1:3). The optical density was measured at 620 nm and the activity was expressed as moles of H2O2consumed/min/mg protein.
Estimation of lipid peroxidation
Lipid peroxidation was measured by the modification of the procedure as reported elsewhere [Citation21]. Reaction mixture contained 125 µL of serum and 125 µL of 25% trichloroacetic acid (TBARS) was centrifuge at 2,000 rpm for 20 min. A 200 µL of protein free supernatant was mixed with 200 µL of 0.5% TBARS and heated at 95°C for 1 h. until pink coloration developed, the optical density of the end fraction product after cooling was recorded at 532 nm. The MDA concentration was calculated according to the following formula:
Statistical analysis
The results among different groups were analyzed by using one-way ANOVA, with Turkey Kramer multiple comparison test and Duncan comparison test. Statistical difference was considered significant at p < 0.05.
Results
FTIR analysis of CRE and PGE
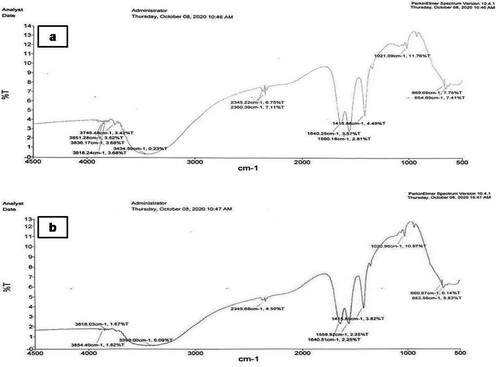
The FTIR of the samples presented in (a, b) were recorded in the mid IR range of 4000–450 cm−1 in the transmission mode on a digital Perkin Elmer 400 spectrophotometer. Each spectrum represented a collection of 12 numbers of scans. The FTIR spectrum of the CRE contains a broad peak of 3851.28, 3836.17, 3818.24 and 3746.48 cm−1 which corresponds to the O–H stretching frequencies, 3434.59 cm−1corresponds to either O–H Stretching and H-bond stretching, 2360.39 cm−1, 2345.22 corresponds to the C=N stretching frequency, 1640.25 cm−1 corresponds to the either C=C stretching or N–H bending, 1560.18 cm−1corresponds to the N-O asymmetric stretch frequencies, 1021.09 cm−1 corresponds to the C–N stretching frequency and 669.69 cm−1 corresponds to the –C≡C–H: C–H bend; C–Br stretch stretching frequencies.
The FTIR spectrum of the PGE contains a broad peak of 3854.04, 3818.03cm−1 corresponds to the O-H stretching frequencies, 3399.00 cm−1 corresponds to either N–H stretch, O–H stretch, H–bonded, 2345.68 cm−1, corresponds to the C=N stretching frequency, 1640.51 cm−1 corresponds to the either –C=C– stretch;N-H Bending frequencies, 1559.9 cm−1corresponds to the N–O asymmetric stretching frequency, 669.67 and 653.56cm−1 corresponds to the –C≡C–H: C–H bend and C–Br stretching frequencies.
Effect of CRE and PGE treatment on phases of estrous cycle
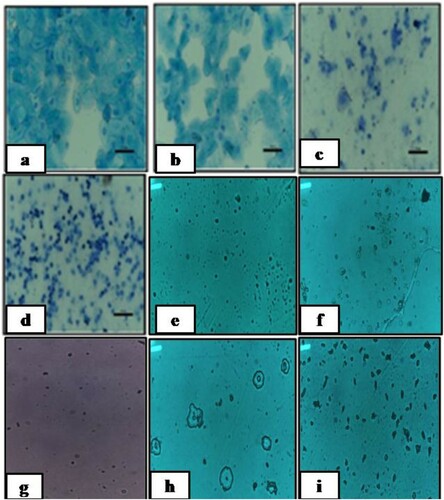
To examine the phase of estrous cycle in letrozole-induced PCOS rats, estrus cycle was monitored by preparing vaginal smears. As expected, rats with PCOS, microscopic observation of the vaginal smears revealed that the predominant cell type was leukocytes in letrozole-induced PCOS rats demonstrating estrous cycle arrest in diestrous phase (). Interestingly, some CRE or PGE treated rats with PCOS were observed to have regular cycles, similar to the placebo rats. Our results indicated that CRE or PGE did not lead to weight gain due to letrozole-induced PCOS but restored the regular cycle after letrozole-induced cycle arrest.
Figure 2. Cytology of vaginal smears. a. Proestrous phase comprised of nucleated epithelial cells, b. Estrous phase comprised of cornified squamous epithelial cells, c. Metestrous phase comprised of leucocytes and cornified cells and d. Diestrous phase comprised of predominant leucocytes. e. Diestrous phase comprised of predominant leucocytes after 21 days of letrozole administration, f. Phase of Metaestrous-Diestrous cycle in letrozole induced PCOS rats treated with CRE, g. Phase of Metaestrous-Diestrous cycle in letrozole induced PCOS rats treated with PGE, h. Phase of Metaestrous cycle in letrozole induced PCOS rats treated with CRE and PGE and i. Phase of estrous cycle in letrozole induced PCOS rats treated with metformin.
Effect of CRE and PGE treatment on duration of different phases of estrous cycle
Letrozole administration to rats lead to prolonged diestrous phase from 6th day and estrous phase disappears on 15th day. After 21 days of letrozole administration, rats were found in diestrous phase. Treatment with CRE, PGE or CRE and PGE combination, showed significant (p < 0.05) difference in duration of diestrous phase of group III, IV and VI compared with group II (negative control) female rats. Contrary, to this no statistically significant difference was found among the CRE or PGE treated (group III, IV and VI) rats or with group I (standard) rats compared across the group ().
Table 2. Effect of CRE and PGE treatment on duration of diestrous phase of estrous cycle.
Effect of CRE and PGE treatment on physical characteristics of rats
Administration of letrozole to female rats resulted in a significant increase (P < 0.05) in final body weights of PCOS rats compared to group I (normal control) rats. However no significant difference in anthropometrical parameters such as weight gain, AC, TC, AC/TC ratio, body mass index (BMI), Lee index and specific rate of body mass gain which remained more or less unchanged. Body length increased significantly (P < 0.05) in the group II (PCOS control) rats compared to the group I (normal control) rats ().
Table 3. Effect of CRE and PGE treatment on rat physical parameters.
Effect of CRE and PGE treatment on ovarian morphometry
Letrozole administration to female rats showed a slight increase in the weight of ovaries in PCOS control rats. This increase ovarian tissue weight and size (diameter) and ovarian organ index among the different groups was not observed to be statistically significant ().
Table 4. Effect of CRE and PGE treatment on ovarian morphometry.
Effect of CRE and PGE treatment on hormonal levels
In the present study, hormonal imbalance was prominent in group II and significantly (p < 0.05) higher values of LH was observed in group II compared to Group I. Similarly, values of LH were elevated in group III & IV compared to group I. LH levels in group V & VI showed normalization trend towards healthy control group, as no significance could be appreciated between group I, group V & group VI. Furthermore, values of LH in the group III & group IV were significantly decreased compared to group II. From , it is clear that letrozole causes imbalance in hormone profile characteristic for PCOS. While treated with CRE-PGE combination and Metformin caused earlier and prominently significance (P > 0.01) normalization of hormonal profile near to values in healthy control group. For these results, it is explicit that combination use of CRE-PGE can better ameliorate the hormonal imbalance ( and ).
Figure 3. Box Plot analysis ofhormonal profilein different groups of PCOS induced female albino Wistar rats.
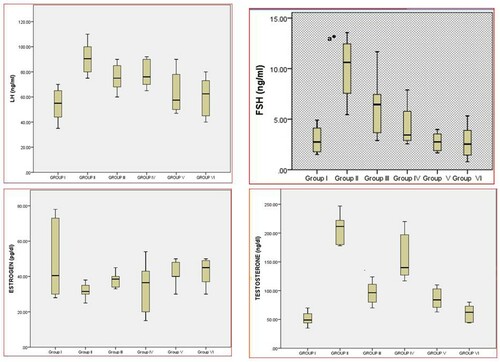
Table 5. Effect of CRE and PGE treatment on hormonal studies in control, PCOS induced and CRE or PGE treated female Wistar rats.
Effect of CRE and PGE treatment on lipid profile
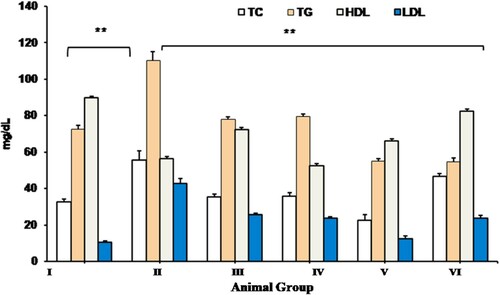
The mean value of serum TC, TGs and LDL in treatment groups (group III, IV and V rats) were found to be significantly (p < 0.05) reduced compared to group II (PCOS control) rats. Contrary to this serum HDL levels showed the opposite trend with significant (p < 0.05) elevation in CRE and PGE treated rats compared to letrozole induced group II PCOS control rats. No significant (p < 0.05) difference was observed in lipid profile among the CRE, PGE or CRE-PGE treated rats compared to metformin treated PCOS (group VI) rats. Additionally, statistically no significant (p < 0.05) difference was observed in the serum lipid profile in group VI animals (standard control group) compared to that of group I (negative control) rats ().
Figure 4. Effect of CRE and PGE treatment on Serum TC, TG, HDL and LDL. Values are expressed as mean + SEM, data were subjected to One Way ANNOVA with Turkeys HSD with Dunn’s compare to all groups **Indicates the significant difference (p < 0.05) across the group, Where TC; total cholesterol, TG; triglyceride, HDL; high density lipoprotein, LDL; low density lipoprotein.
Effect of CRE and PGE treatment on antioxidant activity and lipid peroxidation (TBARS) content
CRE-PGE treatment significantly reduced the oxidative stress markers (SOD, Gpx and CAT) in letrozole induced PCOS rats in groups III (p < 0.05), IV (p < 0.05) and VI (p < 0.01) respectively. Letrozole significantly increased the TBARS (P < 0.05) levels in PCOS compared to group I rats. No significant (p < 0.05) difference was observed in oxidative stress markers and TBARS content across CRE or PGE treated rats. Whereas, statistically no significant (p < 0.05) difference was observed in the mean values of oxidative stress markers and TBARS content in standard control group when compared to that of negative control (Group II) and synergistic group (Group V) ().
Table 6. Effect of CRE and PGE treatment on antioxidant activity and lipid peroxidation (TBARS) content.
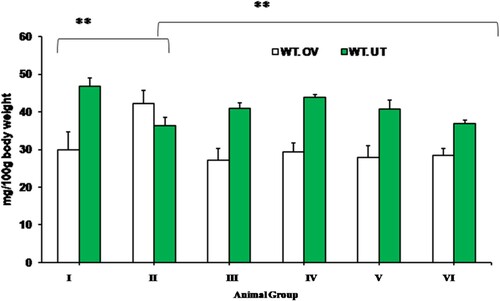
Effect of CRE and PGE on weight of ovary and uterus
A significant reduction (p < 0.05) in mean ovarian weight and significant increase in uterine weight in CRE or PGE treated PCOS rats was observed compared to that of negative control group. However, statistically no significant (p < 0.05) difference was observed across CRE, PGE or CRE-PGE treated rats as well as metformin treated rats when compared with each other ().
Figure 5. Effect of CRE and PGE on weight of ovary and uterus. Values are expressed as mean + SEM, data were subjected to One Way ANNOVA with Turkeys HSD with Dunn’s compare to all groups, **Indicates the significant difference (p < 0.05) across the group, Where WT.OV; weight of ovary, WT-UT; weight of uterus
.
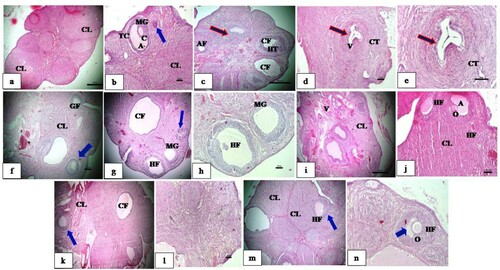
Effect of CRE and PGE treatment on histology of ovaries
The histological analysis of the ovarian section from plain control group ( a and b) showed normal cortex and medulla. Cortex shows numerous healthy growing follicles and a follicle with oocyte, healthy granulosa and theca layer, well developed corpus luteum with uniform round cells and eosinophilic cytoplasm. Negative control group ((c–e)) shows multiple follicular cysts, diminished granulosa layer, increased follicular antrum, hypertrophied theca, atretic follicles, atrophic corpus luteum and vacuolated granulosa and stroma. Histopathological changes after treatment with CRE (C. reflexa), PGE (P. grande) and their combination ((f–l)) shows developing healthy follicles in various stages of development. Reduction in size of cystic follicle was observed. Developing oocyte was also seen. Healthy stroma, normal granulosa and theca were observed. Thickened numerous blood vessels with congestion was observed in PGE (P. grande) treated groups. Standard control group ((M,N)) also shows formation of oocyte, healthy stroma, granulosa and theca, well developed corpus luteum along with some cystic follicles.
Figure 6. Effect CRE and PGE on rat ovary histology. a, b: Plain control group showing the presence of normal cyclic ovary, well grown healthy follicle, MG layer, theca cells and well developed CL. c–e: Negative control group supplemented with Letrozole showing many cystic follicles, thin MG layer, hyperplastic theca, atretic follicle and vacuolated stroma and granulosa. f: Letrozole supplemented with CRE showing numerous healthy growing follicles well developed CL and congestion of vessels. g depicts histopathological changes after treatment with PGE (P.grande) and h combination of CRE and PGE. i–l shows developing healthy follicles in various stages of development. Reduction in size of cystic follicle was observed. Developing oocyte was also seen. Healthy stroma, normal granulosa and theca were observed. Thickened numerous blood vessels with congestion was observed in PGE (P.grande) treated groups. Standard control group. Figure m–n also shows formation of oocyte, healthy stroma, granulosa and theca, well developed corpus luteum along with some cystic follicles. Secondary follicles with secondary oocytes appeared normal – Blue arrow. Multiple cystic conditions are noticed in the medullary region of ovary – Red arrow. Where, CL: Corpus Luteum, MG: Membranagranulosa, TC: Theca, A: Antrum, CF: Cystic follicle, V: Vacuole, GF: Growing follicle.
Discussion
FT-IR spectral analysis of C. reflexa and P. grande revealed the occurrence of various functional groups through the absorption bands of the phytocompounds in it. Spectral data confirmed the existence of bioactive functional groups like alcohols (O–H stretch), phenols (H–bonded),nitriles (C=N Stretch), alkenes (–C=C– stretch), amides (N-H Bend), nitro compounds (N–O asymmetric stretch), aromatics (C–C stretch in a ring), aliphatic amines (C–N stretch), alkynes (–C≡C–H: C–H) and alkyl halides(C–Br stretch) in hydroalcoholic extract of C. reflexa and P. grande. The analyzed functional groups give the probable identification of compounds present in hydroalcoholic extract and support the data analyzed in earlier findings [Citation11,Citation22,Citation23]. The outcomes from FTIR characterization demonstrate that the ameliorative effects property of C. reflexa and P. grande extract may be due to the presence of phenolic or aromatic compoundspresent in the extracts. However, further studies like fractionation, isolation and characterization of compounds in the extracts can insightsa bout the lead molecule for the development of new therapeutic drug. The present study was undertaken to evaluate the effect of C. reflexa (CRE) commonly known as Aftimoon and P.grande (PGE) commonly known as Duqu against letrozole induced PCOS in female Wistar rats. The efficacy of letrozole (an aromatase inhibitor) in establishing PCOS in rats is well documented [Citation15]. In contrast with normal control rats, letrozole administration expanded the diestrous condition, indicating that the model imitated anovulation, which is typical of PCOS.
Letrozole disrupts estrous cycle in rats linked with alterations in the circulating concentrations of the sex hormones and gonadotrophins. These hormones control ovarian functions such as follicular maturation and ovulation. Any imbalance in these hormones would have led to irregular estrous cycle and an altered ovarian function [Citation24]. The PCOS-induced letrozole model is a successful system, as animals have established many human PCOS properties, including hyperandrogenism and dysfunctional follicles, hyperglycemia and oxidative stress.
Treatment with hydroalcoholic extract of CRE, PGE or combination of CRE and PGE for 21 days resulted in significant reduction (p < 0.05) in duration of diestrous phase compared to that of negative control group. The diestrous phase in the treatment groups reduced by average 5 days compared to letrozole induced PCOS untreated group (group II). Our findings are in concordance with the observations reported previously [Citation15]. Similarly, letrozole induced PCOS in rats is associated with prolonged estrus cycle [Citation25,Citation26]. The results of the study indicated that CRE and PGE ameliorated reproductive and metabolic problems in rats with PCOS after 3 weeks of therapy. This therapeutic effect was characterized by restoration of estrus cyclicity (). In the present study, the ameliorative effects of CRE and PGE were significantly low compared to metformin, which is in clinical use for treatment of PCOS. However, the use of metformin for management of PCOS is restricted owing some major limitations including gastrointestinal symptoms of nausea, diarrhoea, flatulence, bloating, anorexia, metallic taste and abdominal pain [Citation27]. The CRE, PGE or CRE-PGE treated groups, did not show any significant increase in body weight which is indicative of the fact that CRE or PGE may possibly have down regulated the expression levels of adipogenic genes thereby reduced the weight of adipose tissues [Citation28]. No significant increase in the weight, diameter and organ index in ovarian tissues were observed. This insignificant increase in anthropometrical parameters may be attributed to the fast metabolic response of the rats, which point out that other genetic and/environmental factors or prolonged exposure to aromatase inhibitor such as letrozole, the provoking drug was necessary to achieve the full range of ovarian pathology.
Letrozole, arrested the aromatase activity, thus further elevating the level of ovarian androgens, leading to hyperandrogenism, a defining characteristic of PCOS. Hyperandrogenismis the predominant endocrinopathy in the women of reproductive age. Treatment with CRE and PGE significantly (p < 0.05) reversed the levels of hormones such as FSH, LH and TET while as EST showed the opposite trend in PCOS rats. It appears that the PGE extracts are more effective in the modulation of circulating androgen profile than CRE and combination of CRE and PGE proved to be significantly better in reversing the androgen levels (hypoandrogenic) compared to CRE or PGE, when used alone thereby indicating the positive interaction between the tested plant extracts. This effect could be related to its active principles (mostly flavonoid compound) in these plant materials [Citation12,Citation29,Citation30].
Dyslipidemia is common in PCOS characterized by elevated TGs and low HDL cholesterol. Dyslipidemia in PCOS occurs independent of BMI. However, obesity and insulin resistance in PCOS has synergistic harmful effect that is equivalent to that seen in type-2 diabetes mellitus. In PCOS, dyslipidemia has multiple causes. Among these, insulin resistance plays a key role by stimulating the lipolysis and altering expression of lipoprotein lipase and hepatic lipase [Citation31]. Letrozole administration increased the circulating lipid parameters including TC, TG and LDL in PCOS and lowered the HDL levels. The tested plant extracts either alone or in combination provided strong evidence of their anti-hyperlipidemic effects by reversing the dyslipidemic status in the sera of letrozole induced PCOS rats. The hypolipidimic effects of these plants are well documented in the literature [Citation32,Citation33]. In the current study CRE and PGE effectively reduced the lipid profile in rodents with hyperlipidemia complications.
It has been reported that the antioxidant enzymes decline in patients with PCOS [Citation34]. In several studies, oxidative stress has been established as one of the major PCOS pathologic factors [Citation35]. Enhanced oxidant levels can alter the stereo diagnosis in ovaries, that ultimately leads to increased production of androgen and polycystic ovaries. In the current study, the letrozole induced PCOS rats showed higher oxidative stress markers. Letrozole administration to rats significantly declined the SOD, CAT, and GPx activity in the sera of PCOS rats and subsequent treatment with CRE or PGE regained their activities. Lipid peroxidation is probably considered one of the biggest markers for oxidative tissue damage, since it provokes free radical damage to the cell membrane components resulting in cell necrosis and inflammation [Citation35]. TBARS is produced as a lipid peroxidation by-product. In our study, the formation of TBARS in polycystic ovaries risen dramatically. However, treatment with CRE or PGE regularized its level as well as oxidative stress markers including SOD, GSH, CAT and TBRS in PCOS. Presence of flavonoids in several plants has the ability to increase serum FSH and reduce serum levels of TET, LH and insulin by partly inhibiting the activation of JAK2/STAT3 pathway and partially up-regulating the IL-6 expression in ovaries of PCOS rats [Citation36].
Both plant extracts reduced the circulating lipids levels in PCOS rats to that of plain control group because the conversion of androgen substrates into estrogens was blocked by letrozole. Treatment with Aftimoon (C. reflexa) and Duqu (P. grande) resulted in significant reduction in testosterone levels in both the doses. These findings agree with previous studies [Citation25,Citation26]. Moreover, extract of raspberry fruit caused a substantial reduction in testosterone levels and preserved ovarian function in rats of PCOS due to its antioxidant capacity [Citation37]. In PCOS rats, Vitex agnus-castus fruit extract also has an antiandrogenic effect by increasing the mechanism of aromatase (increasing the conversion of testosterone to estradiol) leading to low testosterone levels [Citation38].
Ovarian morphologic changes in letrozole treated PCOS rats include development of cysts with thin granulosa layer, abundance of connective tissues and subcapsular cysts, hyperplasia of theca interna and vacuolation of granulose [Citation39]. These findings are indicative of biologically active levels of FSH, raised LH, and lack of interplay between granulosa and theca cells and result could be ascribed to elevated luteinizing hormone (LH) level stimulating the ovary to secrete androgens. Treatment with CRE or PGE resulted in significant recovery of ovarian histological sections, with well-developed antral follicles, thickened granulosa cell layer, well-defined theca layer and well developed corpora lutea. Thus, these physical, biochemical and histological results clearly demonstrate that hydroalcholic extracts of C. reflexa and P. grande can exert beneficial effects in PCOS in the individual having decreased aromatase activity and elevated plasma testosterone.
Conclusion
It is to be noted that the current study is the first of its kind in which ameliorative effects of CRE in PCOS rat models has been established. It can be concluded that the hydroalcoholic extract of CRE and PGE exerted significant protective effect against letrozole induced PCOS in rat models. The study demonstrated good anti-androgenic effect of CRE and PGE as well as significant recovery of serum FSH, LH, EST and TET levels. Notable recovery of ovarian tissues and, normalization of lipid profile, antioxidant level and attenuation of the lengthened diestrous phase emphasize its possible role in the management of gynecological problems. The findings of this study can provide base line data for designing further research on C. reflexa and P. grande.
Acknowledgment
The authors are highly thankful to the Professor and Head, Division of Veterinary Biochemistry, Faculty of Veterinary Sciences& Animal Husbandry, SKAUST-Kashmir, Srinagar-190006, Jammu & Kashmir, India, for providing the necessary facilities required for smooth conducting of this research work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520.
- Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937.
- Rosiek A, Maciejewska N, Leksowski K, et al. Effect of television on obesity and excess of weight and consequences of health. Int J Environ Res Public Health. 2015;12(8):9408–9426.
- Andrade RJ, Lucena MI, Fernández MC, et al. Fulminant liver failure associated with flutamide therapy for hirsutism. Lancet. 1999;353(9157):983.
- Singh S, Sharma A. Studies on ethnomedicinal plant of Baghicha Jashpur Chattisgarh. J Sci Lett. 2017;2:48–55.
- Saini P, Mithal R, Menghani E. A parasitic medicinal plant Cuscuta reflexa: an overview. Int J Sci Eng Res. 2015;6:951–959.
- Dokuparthi SK, Banerjee N, Kumar A, et al. Phytochemical investigation and evaluation of antimutagenic activity of the extract of Cuscuta reflexa Roxb by Ames test. Int J Pharm Sci Res. 2014;5(8):3430.
- Borole S, Oswal RJ, Antre RV, et al. Evaluation of anti-epileptic activity of Cuscuta reflexa Roxb. Res J Pharm Biol Chem Sci. 2011;2(1):657–663.
- Paudel P, Satyal P, Maharjan S, et al. Volatile analysis and antimicrobial screening of the parasitic plant Cuscuta reflexa Roxb. from Nepal. Nat Prod Res. 2014;28(2):106–110.
- Noureen S, Noreen S, Ghumman SA, et al. The genus Cuscuta (Convolvolaceac): An updated review on indigenous uses, phytochemistry, and pharmacology. Iran J Basic Med Sci. 2019;22(11):1225–1252.
- Rath D, Kar DM, Panigrahi SK, et al. Antidiabetic effects of Cuscuta reflexa Roxb. in streptozotocin induced diabetic rats. J Ethnopharmacol. 2016;192:442–449.
- Aslam M, Ahmad ST, Dayal R, et al. Nephroprotective action of Peucedanum grande against cadmium chloride induced renal toxicity in Wistar rats. EXCLI J. 2012;11:444–452.
- Kumar BN, Wadud A, Jahan N, et al. Antilithiatic effect of Peucedanum grande C. B. Clarke in chemically induced urolithiasis in rats. J Ethnopharmacol. 2016;194:1122–1129.
- Sahadevan SV, Ramanathan K, Devi BP. Cuscuta reflexa ROXB. – A wonderful miracle plant in ethnomedicine. Indian J Nat Sci. 2011;2:676–683. ISSN: 0976–0997
- Kafali H, Iriadam M, Ozardalı I, et al. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103–108.
- Sasikala S, Shamila S. A novel ayurvedic medicine-Asokarishtam in the treatment of letrozole induced PCOS in rat. J Cell Tissue Res. 2009;9(2):1903.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175.
- Tappel A. [53] Glutathione peroxidase and hydroperoxides. Meth Enzymol. 1978: 506–513. Elsevier
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–394.treatment of letrozole induced PCOS in rat. J Cell Tissue Res. 2009;9(2):1903.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27.
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175.
- Todorova I, Simeonova G, Kyuchukova D, et al. Reference values of oxidative stress parameters (MDA, SOD, CAT) in dogs and cats. Comp Clin Path. 2005;13(4):190–194.
- Tanruean K, Poolprasert P, Kumla J, et al. Bioactive compounds content and their biological properties of acetone extract of Cuscuta reflexa Roxb. grown on various host plants. Nat Prod Res. 2019;33(4):544–547.
- Bao X, Wang Z, Fang J, et al. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med. 2002;68(3):237–243.
- Adams J, Polson DW, Abdulwahid N, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;326(8469-8470):1375–1379.
- Yang H, Lee SY, Lee SR, et al. Therapeutic effect of Ecklonia cava extract in letrozole-induced polycystic ovary syndrome rats. Front Pharmacol. 2018;9:1325.
- Rajan RK, Balaji B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm Biol. 2017;55(1):242–251.
- Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2010;1(3):117–128.
- Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1):41.
- Khare CP. Indian medicinal plants: an illustrated dictionary. New York: Springer Science & Business Media; 2008.
- Vijikumar S. Cuscuta reflexa Roxb—A wonderful miracle plant in ethnomedicine. Indian J Nat Sci Int Bimon. 2011;976:997.
- Sangameswaran B, Jayakar B. Anti-diabetic, anti-hyperlipidemic and spermatogenic effects of Amaranthus spinosus Linn. on streptozotocin-induced diabetic rats. J Nat Med. 2007;62(1):79–82.
- Abasian Z, Mohammadi M, Hosseini M, et al. A review on role of medicinal plants in polycystic ovarian syndrome: pathophysiology, neuroendocrine signaling, therapeutic status and future prospects. Middle East Fertil Soc J. 2018;23(4):255–262.
- Virshette S, Patil M, Shaikh JR. A review on pharmacological properties and phytoconstituents of indigenous carminative agents. J Pharmacogn Phytochem. 2020;9(3):142–145.
- Liu J, Zhang D. The role of oxidative stress in the pathogenesis of polycystic ovary syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban – J Sichuan Univ Med Sci Ed. 2012;43(2):187–190.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014, 1–31.
- Zhou Y, Lv L, Liu Q, et al. Total flavonoids extracted from Nervilia Fordii function in polycystic ovary syndrome through IL-6 mediated JAK2/STAT3 signaling pathway. Biosci Rep. 2019;39(1).
- Bardei K. The effects of hydro-alcoholic extract of raspberry fruit on ovarian follicles and serum parameters in poly cystic ovary syndrome-induced rat. Armaghane Danesh. 2015;19(11):955–968.
- Jelodar G, Askari K. Effect of Vitex agnus-castus fruits hydroalcoholic extract on sex hormones in rat with induced polycystic ovary syndrome (PCOS). Physiol Pharmacol. 2012;16(1):62–69.
- Baravalle C, Salvetti NR, Mira GA, et al. Microscopic characterization of follicular structures in letrozole-induced polycystic ovarian syndrome in the rat. Arch Med Res. 2006;37(7):830–839.